Abstract
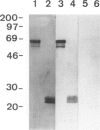
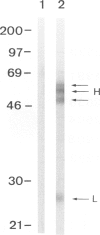
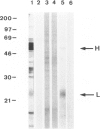
BACKGROUND: Bm2325, a major IgE-inducing antigen of the filarial parasite Brugia malayi has been implicated in the pathology of tropical pulmonary eosinophilia (TPE), a pulmonary syndrome thought to result from hypersensitivity to microfilariae. MATERIALS AND METHODS: Affinity-purified IgE to Bm2325 from patients with TPE was used to identify a complementary DNA (cDNA) from a B. malayi expression library. Sequence analysis of the cDNA revealed a hitherto unknown parasite protein. Immunoblotting of the recombinant filarial protein using sera of patients with TPE determined its IgE-binding capacity. Reactivity to human lung epithelial cell proteins was analyzed using murine anti-Bm2325 antibodies and serum from patients with TPE. RESULTS: The predicted protein is a homolog of the entire precursor of the gamma-glutamyl transpeptidase (gamma-GT), a key enzyme in the synthesis and degradation of glutathione. The filarial precursor encodes both the heavy (H) and the light (L) chain subunits and shares structural similarities with the mammalian enzymes. The Bm2325 allergen was identified as the homolog of the enzyme light chain subunit. Murine antibodies against the recombinant parasite gamma-GT cross-reacted with the human enzyme present in human airway epithelial cells, and human gamma-GT is a target of antibodies present in the serum of patients with TPE. CONCLUSION: Molecular mimicry between the parasite gamma-GT homolog and the host membrane-bound gamma-GT present in lung epithelial cells likely contributes to the pathogenesis observed in tropical pulmonary eosinophilia.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardehle G., Klonisch T., Schott H. H., Stirm S., Zahner H. Isolation of pure sheaths of Litomosoides carinii microfilariae. Parasitol Res. 1987;74(2):188–190. doi: 10.1007/BF00536032. [DOI] [PubMed] [Google Scholar]
- Bourdon M. A., Krusius T., Campbell S., Schwartz N. B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clos J., Brandau S. pJC20 and pJC40--two high-copy-number vectors for T7 RNA polymerase-dependent expression of recombinant genes in Escherichia coli. Protein Expr Purif. 1994 Apr;5(2):133–137. doi: 10.1006/prep.1994.1020. [DOI] [PubMed] [Google Scholar]
- Coyle A. J., Wagner K., Bertrand C., Tsuyuki S., Bews J., Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med. 1996 Apr 1;183(4):1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Finidori J., Laperche Y., Haguenauer-Tsapis R., Barouki R., Guellaen G., Hanoune J. In vitro biosynthesis and membrane insertion of gamma-glutamyl transpeptidase. J Biol Chem. 1984 Apr 25;259(8):4687–4690. [PubMed] [Google Scholar]
- Forman H. J., Skelton D. C. Protection of alveolar macrophages from hyperoxia by gamma-glutamyl transpeptidase. Am J Physiol. 1990 Aug;259(2 Pt 1):L102–L107. doi: 10.1152/ajplung.1990.259.2.L102. [DOI] [PubMed] [Google Scholar]
- Gardell S. J., Tate S. S. Latent proteinase activity of gamma-glutamyl transpeptidase light subunit. J Biol Chem. 1979 Jun 25;254(12):4942–4945. [PubMed] [Google Scholar]
- Garraud O., Nkenfou C., Bradley J. E., Perler F. B., Nutman T. B. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J Immunol. 1995 Aug 1;155(3):1316–1325. [PubMed] [Google Scholar]
- Glass D. B., el-Maghrabi M. R., Pilkis S. J. Synthetic peptides corresponding to the site phosphorylated in 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase as substrates of cyclic nucleotide-dependent protein kinases. J Biol Chem. 1986 Feb 25;261(6):2987–2993. [PubMed] [Google Scholar]
- Hussein A. S., Walter R. D. Purification and characterization of gamma-glutamyl transpeptidase from Ascaris suum. Mol Biochem Parasitol. 1996 Apr;77(1):41–47. doi: 10.1016/0166-6851(96)02573-x. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Fujii J., Taniguchi N., Meister A. Human gamma-glutamyl transpeptidase mutants involving conserved aspartate residues and the unique cysteine residue of the light subunit. J Biol Chem. 1995 May 26;270(21):12471–12475. [PubMed] [Google Scholar]
- Kamata I., Yamada M., Uchikawa R., Matsuda S., Arizono N. Cysteine protease of the nematode Nippostrongylus brasiliensis preferentially evokes an IgE/IgG1 antibody response in rats. Clin Exp Immunol. 1995 Oct;102(1):71–77. doi: 10.1111/j.1365-2249.1995.tb06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kugelman A., Choy H. A., Liu R., Shi M. M., Gozal E., Forman H. J. gamma-Glutamyl transpeptidase is increased by oxidative stress in rat alveolar L2 epithelial cells. Am J Respir Cell Mol Biol. 1994 Nov;11(5):586–592. doi: 10.1165/ajrcmb.11.5.7946387. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobos E., Altmann M., Mengod G., Weiss N., Rudin W., Karam M. Identification of an Onchocerca volvulus cDNA encoding a low-molecular-weight antigen uniquely recognized by onchocerciasis patient sera. Mol Biochem Parasitol. 1990 Feb;39(1):135–145. doi: 10.1016/0166-6851(90)90016-f. [DOI] [PubMed] [Google Scholar]
- Lobos E., Ondo A., Ottesen E. A., Nutman T. B. Biochemical and immunologic characterization of a major IgE-inducing filarial antigen of Brugia malayi and implications for the pathogenesis of tropical pulmonary eosinophilia. J Immunol. 1992 Nov 1;149(9):3029–3034. [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. III. Evidence that the amino terminus of the heavy subunit is the membrane binding segment. J Biochem. 1983 May;93(5):1427–1433. doi: 10.1093/oxfordjournals.jbchem.a134278. [DOI] [PubMed] [Google Scholar]
- Nash B., Tate S. S. In vitro translation and processing of rat kidney gamma-glutamyl transpeptidase. J Biol Chem. 1984 Jan 10;259(1):678–685. [PubMed] [Google Scholar]
- Neva F. A., Ottesen E. A. Tropical (filarial) eosinophilia. N Engl J Med. 1978 May 18;298(20):1129–1131. doi: 10.1056/NEJM197805182982006. [DOI] [PubMed] [Google Scholar]
- Nutman T. B., Vijayan V. K., Pinkston P., Kumaraswami V., Steel C., Crystal R. G., Ottesen E. A. Tropical pulmonary eosinophilia: analysis of antifilarial antibody localized to the lung. J Infect Dis. 1989 Dec;160(6):1042–1050. doi: 10.1093/infdis/160.6.1042. [DOI] [PubMed] [Google Scholar]
- Ottesen E. A. Immunological aspects of lymphatic filariasis and onchocerciasis in man. Trans R Soc Trop Med Hyg. 1984;78 (Suppl):9–18. doi: 10.1016/0035-9203(84)90309-2. [DOI] [PubMed] [Google Scholar]
- Ottesen E. A., Nutman T. B. Tropical pulmonary eosinophilia. Annu Rev Med. 1992;43:417–424. doi: 10.1146/annurev.me.43.020192.002221. [DOI] [PubMed] [Google Scholar]
- Papandrikopoulou A., Frey A., Gassen H. G. Cloning and expression of gamma-glutamyl transpeptidase from isolated porcine brain capillaries. Eur J Biochem. 1989 Aug 15;183(3):693–698. doi: 10.1111/j.1432-1033.1989.tb21100.x. [DOI] [PubMed] [Google Scholar]
- Pellé R., Murphy N. B. Northern hybridization: rapid and simple electrophoretic conditions. Nucleic Acids Res. 1993 Jun 11;21(11):2783–2784. doi: 10.1093/nar/21.11.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston P., Vijayan V. K., Nutman T. B., Rom W. N., O'Donnell K. M., Cornelius M. J., Kumaraswami V., Ferrans V. J., Takemura T., Yenokida G. Acute tropical pulmonary eosinophilia. Characterization of the lower respiratory tract inflammation and its response to therapy. J Clin Invest. 1987 Jul;80(1):216–225. doi: 10.1172/JCI113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E., Heisterkamp N., Groffen J. Cloning and nucleotide sequence of human gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8840–8844. doi: 10.1073/pnas.85.23.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. K., Ikeda Y., Fujii J., Taniguchi N., Meister A. Different sites of acivicin binding and inactivation of gamma-glutamyl transpeptidases. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2360–2364. doi: 10.1073/pnas.92.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spry C. J., Kumaraswami V. Tropical eosinophilia. Semin Hematol. 1982 Apr;19(2):107–115. [PubMed] [Google Scholar]
- Suzuki H., Kumagai H., Echigo T., Tochikura T. DNA sequence of the Escherichia coli K-12 gamma-glutamyltranspeptidase gene, ggt. J Bacteriol. 1989 Sep;171(9):5169–5172. doi: 10.1128/jb.171.9.5169-5172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Khadse V., Wellner D. Renal gamma-glutamyl transpeptidases: structural and immunological studies. Arch Biochem Biophys. 1988 May 1;262(2):397–408. doi: 10.1016/0003-9861(88)90390-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. F., Jr, Madden K. B., Svetić A., Cheever A., Trotta P. P., Gause W. C., Katona I. M., Finkelman F. D. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992 Jun;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- Wetmore L. A., Gerard C., Drazen J. M. Human lung expresses unique gamma-glutamyl transpeptidase transcripts. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7461–7465. doi: 10.1073/pnas.90.16.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree R., Hoffman D. R., van Dijk W., Brodard V., Mahieu K., Koeleman C. A., Grande M., van Leeuwen W. A., Aalberse R. C. Lol p XI, a new major grass pollen allergen, is a member of a family of soybean trypsin inhibitor-related proteins. J Allergy Clin Immunol. 1995 May;95(5 Pt 1):970–978. doi: 10.1016/s0091-6749(95)70097-8. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]