Abstract
BACKGROUND: Prion diseases belong to a group of neurodegenerative disorders affecting humans and animals. The human diseases include kuru, Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome (GSS), and fatal familial insomnia (FFI). The pathogenic mechanisms of the prion diseases are not yet understood. Monoclonal antibodies provide valuable tools in the diagnosis, as well as in the basic research, of several diseases; however, monospecific antisera or monoclonal antibodies (mAbs) against human prion proteins were, until now, not available. MATERIALS AND METHODS: We have developed an immunization protocol based on nucleic acid injection into nontolerant PrP0/0 mice. DNA or RNA coding for different human prion proteins including the mutated sequences associated with CJD, GSS, and FFI were injected into muscle tissue. Mice were primarily inoculated with DNA plasmids encoding the prion protein (PRNP) gene and boosted either with DNA, RNA, or recombinant Semliki Forest Virus particles expressing PRNP. Hybridomas were then prepared. RESULTS: Different mAbs against human prion proteins were obtained, and their binding behavior was analyzed by peptide enzyme-linked immunosorbent assay, Western blot, immunofluorescence, and immunoprecipitation. Their cross-reactivity with prion protein from other species was also determined. Our mAbs are directed against four different linear epitopes and may also recognize discontinuous regions of the native prion protein. CONCLUSIONS: These antibodies should allow us to address questions concerning the nature of the prion protein as well as the initiation and progression of prion diseases. Moreover, these mAbs can now be used for the diagnosis of prion diseases of humans and animals.
Full text
PDF




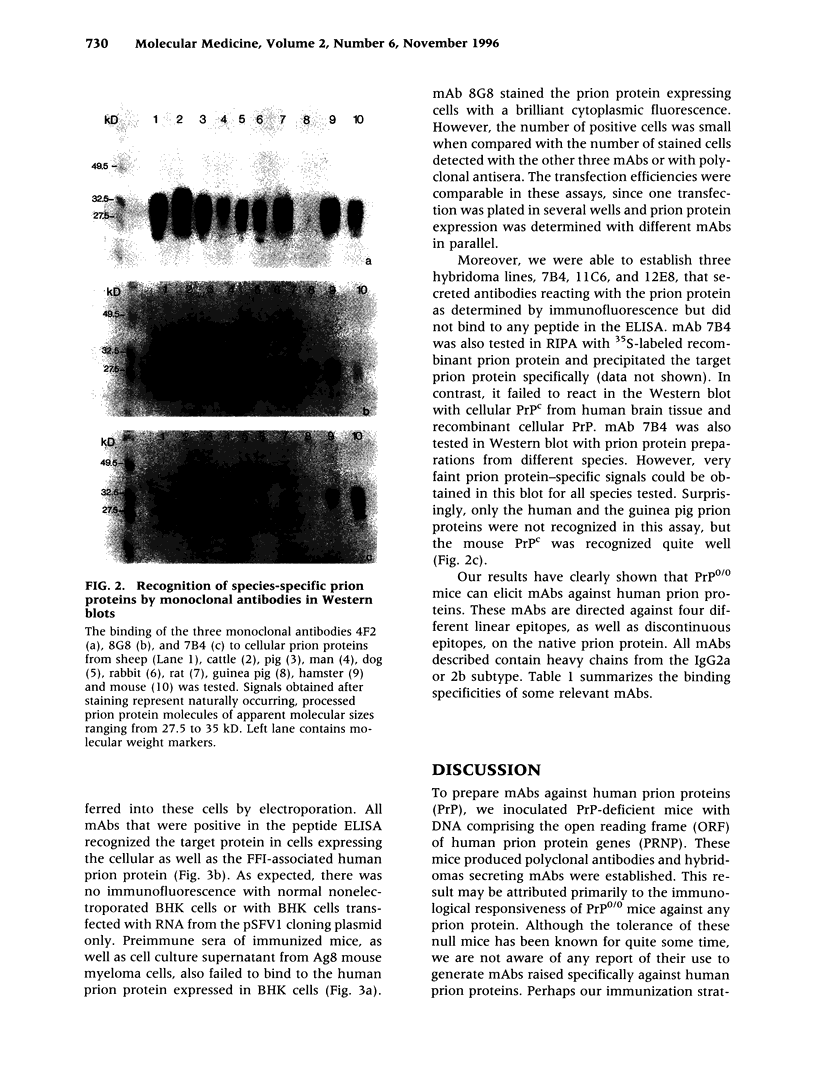
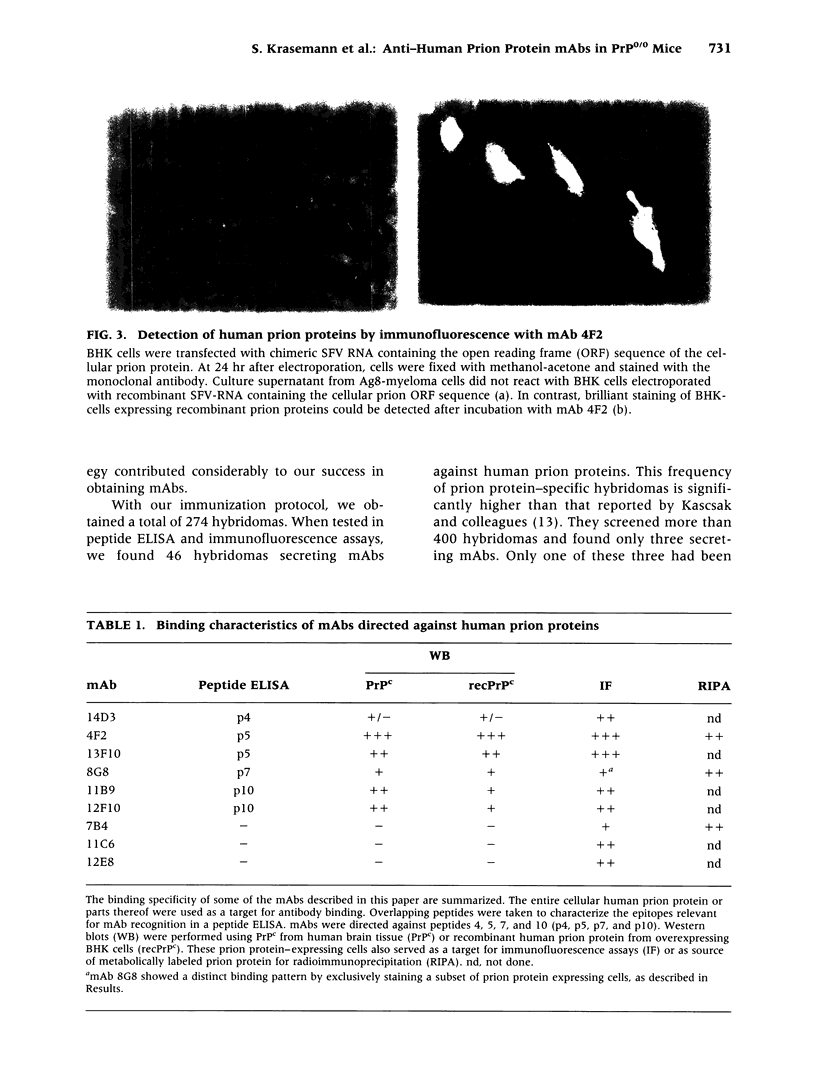



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Vincent M. T., Kent S. B., Hood L. E., Prusiner S. B. Characterization of prion proteins with monospecific antisera to synthetic peptides. J Immunol. 1988 Feb 15;140(4):1188–1193. [PubMed] [Google Scholar]
- Berglund P., Sjöberg M., Garoff H., Atkins G. J., Sheahan B. J., Liljeström P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Biotechnology (N Y) 1993 Aug;11(8):916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- Brown P., Cervenáková L., Goldfarb L. G., McCombie W. R., Rubenstein R., Will R. G., Pocchiari M., Martinez-Lage J. F., Scalici C., Masullo C. Iatrogenic Creutzfeldt-Jakob disease: an example of the interplay between ancient genes and modern medicine. Neurology. 1994 Feb;44(2):291–293. doi: 10.1212/wnl.44.2.291. [DOI] [PubMed] [Google Scholar]
- Brown P., Coker-Vann M., Pomeroy K., Franko M., Asher D. M., Gibbs C. J., Jr, Gajdusek D. C. Diagnosis of Creutzfeldt-Jakob disease by Western blot identification of marker protein in human brain tissue. N Engl J Med. 1986 Feb 27;314(9):547–551. doi: 10.1056/NEJM198602273140904. [DOI] [PubMed] [Google Scholar]
- Brown P., Goldfarb L. G., Gajdusek D. C. The new biology of spongiform encephalopathy: infectious amyloidoses with a genetic twist. Lancet. 1991 Apr 27;337(8748):1019–1022. doi: 10.1016/0140-6736(91)92670-w. [DOI] [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993 Jul 2;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H. P., DeArmond S. J., Prusiner S. B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992 Apr 16;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Davis H. L., Michel M. L., Mancini M., Schleef M., Whalen R. G. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine. 1994 Dec;12(16):1503–1509. doi: 10.1016/0264-410x(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Groth D., Prusiner S. B. Immunoaffinity purification and neutralization of scrapie prion infectivity. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6617–6621. doi: 10.1073/pnas.85.18.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon R., Prusiner S. B. Prion liposomes. Biochem J. 1990 Feb 15;266(1):1–14. doi: 10.1042/bj2660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Gajdusek D. C., Asher D. M., Alpers M. P., Beck E., Daniel P. M., Matthews W. B. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science. 1968 Jul 26;161(3839):388–389. doi: 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Krasemann S., Zerr I., Weber T., Poser S., Kretzschmar H., Hunsmann G., Bodemer W. Prion disease associated with a novel nine octapeptide repeat insertion in the PRNP gene. Brain Res Mol Brain Res. 1995 Dec 1;34(1):173–176. doi: 10.1016/0169-328x(95)00175-r. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D., Serban A., Koehler R., Foster D., Torchia M., Burton D., Yang S. L., DeArmond S. J. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10608–10612. doi: 10.1073/pnas.90.22.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Hsiao K. K. Human prion diseases. Ann Neurol. 1994 Apr;35(4):385–395. doi: 10.1002/ana.410350404. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Devine-Gage E. A., Jenkins E. C., Kascsak R. J., Brown W. T., Krawczun M. S., Silverman W. P. Localization of a human gene homologous to the PrP gene on the p arm of chromosome 20 and detection of PrP-related antigens in normal human brain. Biochem Biophys Res Commun. 1986 Oct 30;140(2):758–765. doi: 10.1016/0006-291x(86)90796-5. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Donnelly J. J., Parker S. E., Rhodes G. H., Felgner P. L., Dwarki V. J., Gromkowski S. H., Deck R. R., DeWitt C. M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Xiang Z. Q., Spitalnik S., Tran M., Wunner W. H., Cheng J., Ertl H. C. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994 Feb 15;199(1):132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- d'Albis A., Couteaux R., Janmot C., Mira J. C. Myosin isoform transitions in regeneration of fast and slow muscles during postnatal development of the rat. Dev Biol. 1989 Oct;135(2):320–325. doi: 10.1016/0012-1606(89)90182-6. [DOI] [PubMed] [Google Scholar]