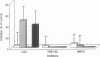Abstract
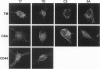
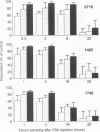
BACKGROUND: Chondroitin-4-sulfate (CSA) was recently described as a Plasmodium falciparum cytoadherence receptor present on Saimiri brain microvascular and human lung endothelial cells. MATERIALS AND METHODS: To specifically study chondroitin-4-sulfate-mediated cytoadherence, a parasite population was selected through panning of the Palo-Alto (FUP) 1 P. falciparum isolate on monolayers of Saimiri brain microvascular endothelial cells (SBEC). Immunofluorescence showed this SBEC cell line to be unique for its expression of CSA-proteoglycans, namely CD44 and thrombomodulin, in the absence of CD36 and ICAM-1. RESULTS: The selected parasite population was used to monitor cytoadherence inhibition/dissociating activities in Saimiri sera collected at different times after intramuscular injection of 50 mg CSA/kg of body weight. Serum inhibitory activity was detectable 30 min after injection and persisted for 8 hr. Furthermore, when chondroitin-4-sulfate was injected into monkeys infected with Palo-Alto (FUP) 1 P. falciparum, erythrocytes containing P. falciparum mature forms were released into the circulation. The cytoadherence phenotype of circulating infected red blood cells (IRBC) was determined before and 8 hr after inoculation of CSA. Before inoculation, in vitro cytoadherence of IRBCs was not inhibited by CSA. In contrast, in vitro cytoadherence of circulating infected erythrocytes obtained 8 hr after CSA inoculation was inhibited by more than 90% by CSA. CONCLUSIONS: In the squirrel monkey model for infection with P. falciparum, chondroitin-4-sulfate impairs in vitro and in vivo cytoadherence of parasitized erythrocytes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988 Jul;39(1):3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Bowman D., Carrington W., Fogarty K., McNeil J. A., Fay F. S., Lawrence J. B. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 1993 Feb 26;259(5099):1330–1335. doi: 10.1126/science.8446902. [DOI] [PubMed] [Google Scholar]
- Conte A., de Bernardi M., Palmieri L., Lualdi P., Mautone G., Ronca G. Metabolic fate of exogenous chondroitin sulfate in man. Arzneimittelforschung. 1991 Jul;41(7):768–772. [PubMed] [Google Scholar]
- David P. H., Hommel M., Miller L. H., Udeinya I. J., Oligino L. D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J. 1995 Jul;9(10):946–955. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- Fried M., Duffy P. E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996 Jun 7;272(5267):1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- Gay F., Robert C., Pouvelle B., Peyrol S., Scherf A., Gysin J. Isolation and characterization of brain microvascular endothelial cells from Saimiri monkeys. An in vitro model for sequestration of Plasmodium falciparum-infected erythrocytes. J Immunol Methods. 1995 Jul 17;184(1):15–28. doi: 10.1016/0022-1759(95)00070-q. [DOI] [PubMed] [Google Scholar]
- Groux H., Perraut R., Garraud O., Poingt J. P., Gysin J. Functional characterization of the antibody-mediated protection against blood stages of Plasmodium falciparum in the monkey Saimiri sciureus. Eur J Immunol. 1990 Oct;20(10):2317–2323. doi: 10.1002/eji.1830201022. [DOI] [PubMed] [Google Scholar]
- Gysin J., Aikawa M., Tourneur N., Tegoshi T. Experimental Plasmodium falciparum cerebral malaria in the squirrel monkey Saimiri sciureus. Exp Parasitol. 1992 Dec;75(4):390–398. doi: 10.1016/0014-4894(92)90252-6. [DOI] [PubMed] [Google Scholar]
- Gysin J., Fandeur T. Saimiri sciureus (karyotype 14-7): an alternative experimental model of Plasmodium falciparum infection. Am J Trop Med Hyg. 1983 May;32(3):461–467. doi: 10.4269/ajtmh.1983.32.461. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Gilladoga A. D. Molecular studies related to the pathogenesis of cerebral malaria. Blood. 1989 Dec;74(8):2603–2618. [PubMed] [Google Scholar]
- Maruyama I., Bell C. E., Majerus P. W. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985 Aug;101(2):363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F. The molecular basis for the cytoadherence of Plasmodium falciparum-infected erythrocytes to endothelium. Semin Cell Biol. 1993 Oct;4(5):297–303. doi: 10.1006/scel.1993.1036. [DOI] [PubMed] [Google Scholar]
- Robert C., Peyrol S., Pouvelle B., Gay-Andrieu F., Gysin J. Ultrastructural aspects of Plasmodium falciparum-infected erythrocyte adherence to endothelial cells of Saimiri brain microvasculature. Am J Trop Med Hyg. 1996 Feb;54(2):169–177. doi: 10.4269/ajtmh.1996.54.169. [DOI] [PubMed] [Google Scholar]
- Robert C., Pouvelle B., Meyer P., Muanza K., Fujioka H., Aikawa M., Scherf A., Gysin J. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res Immunol. 1995 Jul-Aug;146(6):383–393. doi: 10.1016/0923-2494(96)81042-x. [DOI] [PubMed] [Google Scholar]
- Rogerson S. J., Brown G. V. Chondroitin sulphate A as an adherence receptor for Plasmodium falciparum-infected erythrocytes. Parasitol Today. 1997 Feb;13(2):70–75. doi: 10.1016/s0169-4758(96)10081-8. [DOI] [PubMed] [Google Scholar]
- Rogerson S. J., Chaiyaroj S. C., Ng K., Reeder J. C., Brown G. V. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995 Jul 1;182(1):15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz P., Schwärzler C., Günthert U. CD44 isoforms during differentiation and development. Bioessays. 1995 Jan;17(1):17–24. doi: 10.1002/bies.950170106. [DOI] [PubMed] [Google Scholar]
- Sein K. K., Brown A. E., Maeno Y., Smith C. D., Corcoran K. D., Hansukjariya P., Webster H. K., Aikawa M. Sequestration pattern of parasitized erythrocytes in cerebrum, mid-brain, and cerebellum of Plasmodium coatneyi-infected rhesus monkeys (Macaca mulatta). Am J Trop Med Hyg. 1993 Oct;49(4):513–519. doi: 10.4269/ajtmh.1993.49.513. [DOI] [PubMed] [Google Scholar]
- Sein K. K., Maeno Y., Thuc H. V., Anh T. K., Aikawa M. Differential sequestration of parasitized erythrocytes in the cerebrum and cerebellum in human cerebral malaria. Am J Trop Med Hyg. 1993 Apr;48(4):504–511. doi: 10.4269/ajtmh.1993.48.504. [DOI] [PubMed] [Google Scholar]
- Sugiura N., Sakurai K., Hori Y., Karasawa K., Suzuki S., Kimata K. Preparation of lipid-derivatized glycosaminoglycans to probe a regulatory function of the carbohydrate moieties of proteoglycans in cell-matrix interaction. J Biol Chem. 1993 Jul 25;268(21):15779–15787. [PubMed] [Google Scholar]
- Turner G. D., Morrison H., Jones M., Davis T. M., Looareesuwan S., Buley I. D., Gatter K. C., Newbold C. I., Pukritayakamee S., Nagachinta B. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994 Nov;145(5):1057–1069. [PMC free article] [PubMed] [Google Scholar]