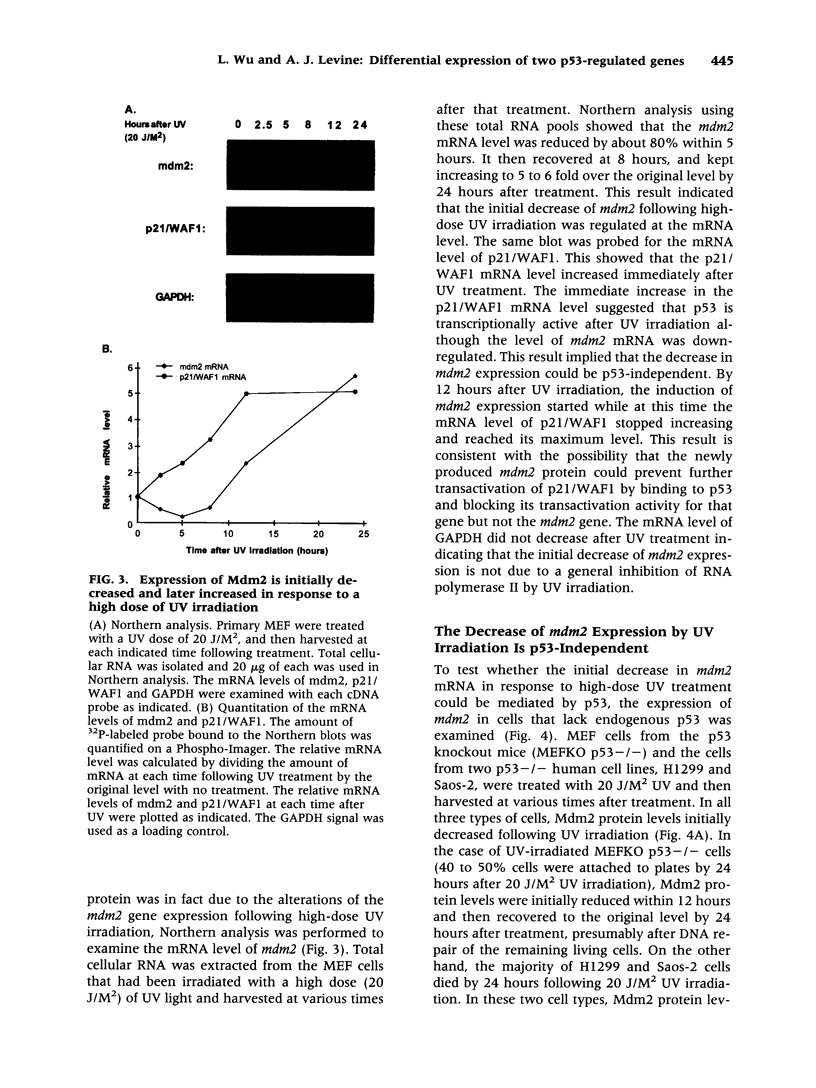
Abstract
BACKGROUND: DNA damage in mammalian cells stabilizes the p53 protein which then functions as a cell cycle checkpoint by leading to growth arrest or apoptosis. p53 is a transcription factor and positively regulates the expression of the p21/WAF-1 gene and the mdm2 gene. After high-dose UV irradiation, p53 increases the expression of the p21/WAF-1 gene immediately (2 to 5 hours after irradiation) while the induction of the mdm2 gene is delayed (8 to 12 hours after irradiation). Experiments presented here explore this differential expression of two different p53-regulated genes. MATERIALS AND METHODS: IP-Western (protein) and Northern (mRNA) blot experiments are used to follow mdm2 and p21/WAF-1 expression in primary rat or mouse cells after a low-dose (4 J/m2) or a high-dose (20 J/M2) of UV irradiation. Northern blot and nuclear run-on experiments are employed to study mRNA stability as well as transcription rates of selected genes. RESULTS: After high-dose UV irradiation, p53 is rapidly stabilized and the expression of p21/WAF1 is immediately increased. By contrast, both protein and mRNA levels of mdm2 first decrease in a p53-independent manner, and later increase in a p53-dependent manner. The initial decline of mdm2 expression following high-dose UV irradiation is UV-dosage dependent and regulated at the level of transcription. CONCLUSION: p53 regulates two genes, p21/WAF1 (blocks cell cycle progression) and mdm2 (reverses p53 activity), that mediate opposite actions. This process is regulated in a temporal fashion after high-dose UV irradiation, so that cell cycle progression can be halted while DNA repair continues prior to reversal of p53-mediated arrest by mdm2.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak Y., Gottlieb E., Juven-Gershon T., Oren M. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dev. 1994 Aug 1;8(15):1739–1749. doi: 10.1101/gad.8.15.1739. [DOI] [PubMed] [Google Scholar]
- Barak Y., Oren M. Enhanced binding of a 95 kDa protein to p53 in cells undergoing p53-mediated growth arrest. EMBO J. 1992 Jun;11(6):2115–2121. doi: 10.1002/j.1460-2075.1992.tb05270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueso-Ramos C. E., Yang Y., deLeon E., McCown P., Stass S. A., Albitar M. The human MDM-2 oncogene is overexpressed in leukemias. Blood. 1993 Nov 1;82(9):2617–2623. [PubMed] [Google Scholar]
- Cahilly-Snyder L., Yang-Feng T., Francke U., George D. L. Molecular analysis and chromosomal mapping of amplified genes isolated from a transformed mouse 3T3 cell line. Somat Cell Mol Genet. 1987 May;13(3):235–244. doi: 10.1007/BF01535205. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Oliner J. D., Zhan Q., Fornace A. J., Jr, Vogelstein B., Kastan M. B. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2684–2688. doi: 10.1073/pnas.91.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lin J., Levine A. J. Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol Med. 1995 Jan;1(2):142–152. [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wu X., Lin J., Levine A. J. mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol Cell Biol. 1996 May;16(5):2445–2452. doi: 10.1128/mcb.16.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakharzadeh S. S., Trusko S. P., George D. L. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991 Jun;10(6):1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A. The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol Cell Biol. 1993 Jan;13(1):301–306. doi: 10.1128/mcb.13.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. N., Roe A. E., Donehower L. A., Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995 Nov 9;378(6553):206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- Juven T., Barak Y., Zauberman A., George D. L., Oren M. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene. 1993 Dec;8(12):3411–3416. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Ladanyi M., Cha C., Lewis R., Jhanwar S. C., Huvos A. G., Healey J. H. MDM2 gene amplification in metastatic osteosarcoma. Cancer Res. 1993 Jan 1;53(1):16–18. [PubMed] [Google Scholar]
- Leach F. S., Tokino T., Meltzer P., Burrell M., Oliner J. D., Smith S., Hill D. E., Sidransky D., Kinzler K. W., Vogelstein B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993 May 15;53(10 Suppl):2231–2234. [PubMed] [Google Scholar]
- Lu X., Lane D. P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993 Nov 19;75(4):765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Martin K., Trouche D., Hagemeier C., Sørensen T. S., La Thangue N. B., Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995 Jun 22;375(6533):691–694. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna R., Wagner D. S., Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995 Nov 9;378(6553):203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- Murphy M., Hinman A., Levine A. J. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 1996 Dec 1;10(23):2971–2980. doi: 10.1101/gad.10.23.2971. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Perry M. E., Piette J., Zawadzki J. A., Harvey D., Levine A. J. The mdm-2 gene is induced in response to UV light in a p53-dependent manner. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11623–11627. doi: 10.1073/pnas.90.24.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberger G., Liu L., Ichimura K., Schmidt E. E., Collins V. P. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993 Jun 15;53(12):2736–2739. [PubMed] [Google Scholar]
- Sheikh M. S., Shao Z. M., Hussain A., Fontana J. A. The p53-binding protein MDM2 gene is differentially expressed in human breast carcinoma. Cancer Res. 1993 Jul 15;53(14):3226–3228. [PubMed] [Google Scholar]
- Watanabe T., Hotta T., Ichikawa A., Kinoshita T., Nagai H., Uchida T., Murate T., Saito H. The MDM2 oncogene overexpression in chronic lymphocytic leukemia and low-grade lymphoma of B-cell origin. Blood. 1994 Nov 1;84(9):3158–3165. [PubMed] [Google Scholar]
- Wu X., Bayle J. H., Olson D., Levine A. J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993 Jul;7(7A):1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- Xiao Z. X., Chen J., Levine A. J., Modjtahedi N., Xing J., Sellers W. R., Livingston D. M. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995 Jun 22;375(6533):694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]








