Abstract
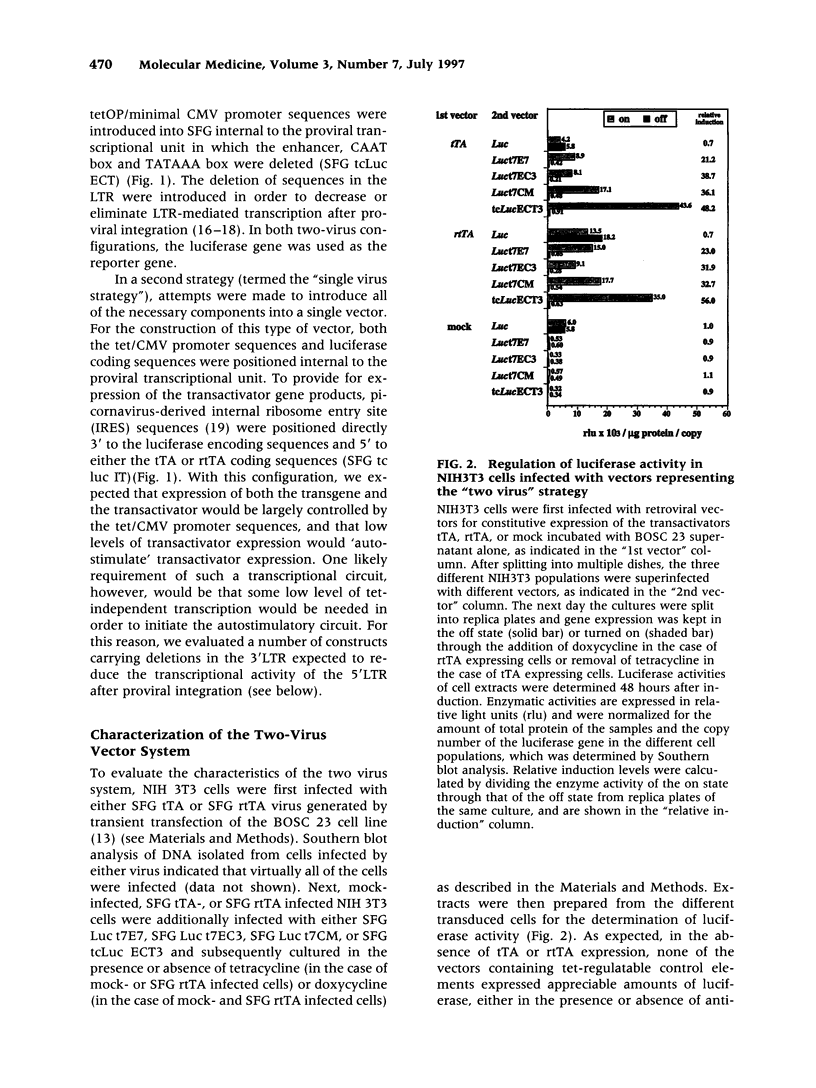
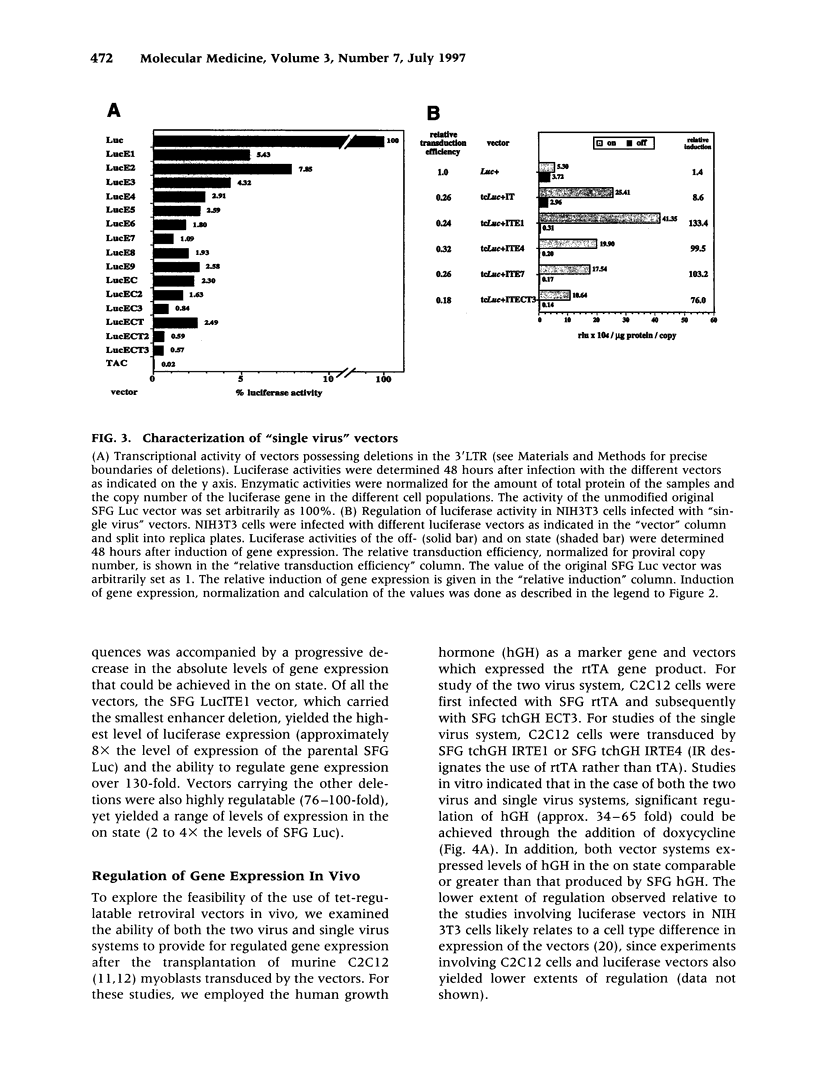
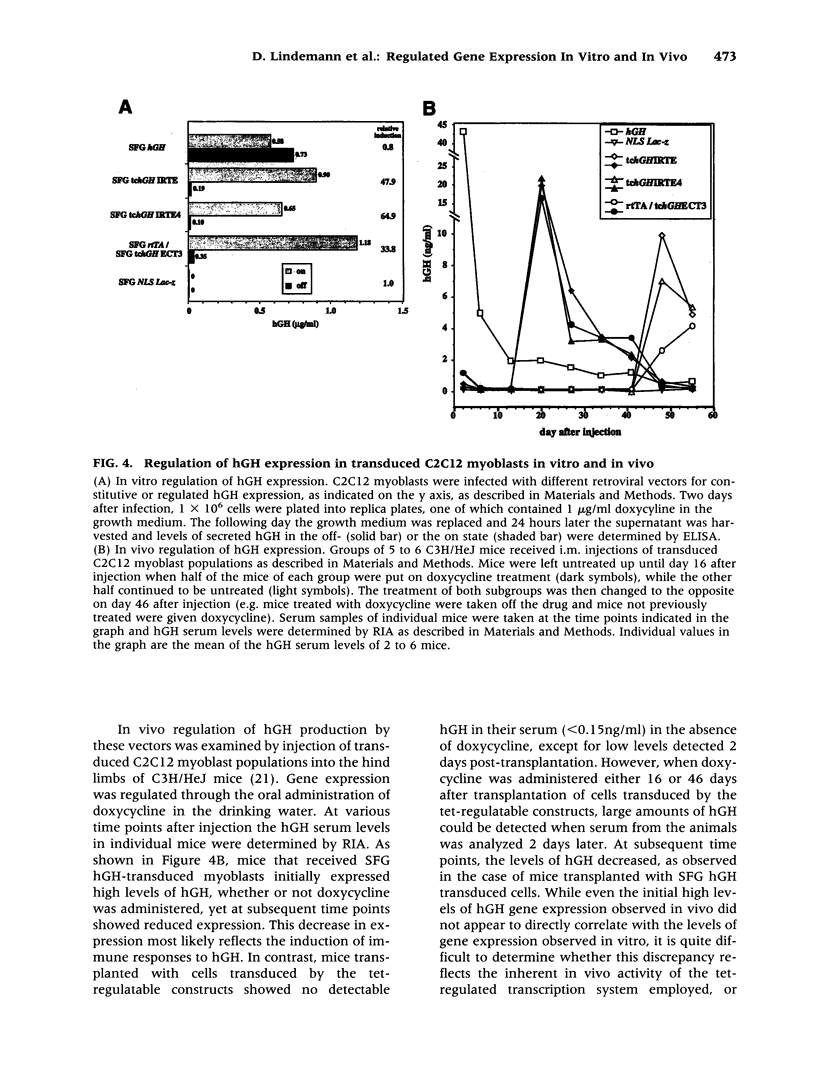
BACKGROUND: Several plasmid DNA-based mammalian expression systems have recently been developed which make it possible to manipulate gene expression via the administration of exogenous agents. In order to extend the application of these systems, we have developed retroviral vectors which allow for the controlled expression of inserted genes both in vitro and in vivo. MATERIALS AND METHODS: Two vector strategies which make use of the tetracycline-regulated gene expression system described by Gossen and Bujard were evaluated. In a first strategy, one virus was generated which encoded the tTA or rtTA transactivator gene product, and a second virus was generated in which expression of the gene of interest was dependent upon tetracycline-responsive transcriptional control elements placed either within the viral LTR or within the proviral transcriptional unit. In a second vector strategy, both components of the tet-regulatable system were incorporated into a single proviral genome in such a way that expression of both the transgene and the transactivator gene product were under control of tet-regulatable control elements. RESULTS: Both vector strategies resulted in the ability to regulate the expression of inserted genes. In one single virus configuration, gene expression could be regulated over 100X and the level of gene expression in the induced state was comparable to or greater than that achieved with standard LTR-based vectors. The use of different deletions in the viral LTR made it possible to generate a number of vectors which provide for a four-fold range of levels of expression of inserted genes in the induced state. Studies in mice with transduced cells demonstrated that gene expression could be induced in vivo by manipulation of tetracycline for at least 48 days. CONCLUSIONS: The availability of highly transmissible, regulatable retroviral vectors should greatly facilitate studies in which it is of interest to manipulate the expression of specific genes in vitro or in vivo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackland-Berglund C. E., Leib D. A. Efficacy of tetracycline-controlled gene expression is influenced by cell type. Biotechniques. 1995 Feb;18(2):196–200. [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Büeler H., Mulligan R. C. Induction of antigen-specific tumor immunity by genetic and cellular vaccines against MAGE: enhanced tumor protection by coexpression of granulocyte-macrophage colony-stimulating factor and B7-1. Mol Med. 1996 Sep;2(5):545–555. [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J., Pan L. C., Pavlath G. K., Travis M. A., Lanctot A. M., Blau H. M. Systemic delivery of human growth hormone by injection of genetically engineered myoblasts. Science. 1991 Dec 6;254(5037):1509–1512. doi: 10.1126/science.1962213. [DOI] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth P. A., St Onge L., Böger H., Gruss P., Gossen M., Kistner A., Bujard H., Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bonin A. L., Freundlieb S., Bujard H. Inducible gene expression systems for higher eukaryotic cells. Curr Opin Biotechnol. 1994 Oct;5(5):516–520. doi: 10.1016/0958-1669(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Freundlieb S., Bender G., Müller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995 Jun 23;268(5218):1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Nolan G. P., Blau H. M. Rapid retroviral delivery of tetracycline-inducible genes in a single autoregulatory cassette. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5185–5190. doi: 10.1073/pnas.93.11.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. J., Scuric Z., Anderson W. F. Novel retroviral vector transferring a suicide gene and a selectable marker gene with enhanced gene expression by using a tetracycline-responsive expression system. J Virol. 1996 Nov;70(11):8138–8141. doi: 10.1128/jvi.70.11.8138-8141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida A., Chen S. T., Friedmann T., Yee J. K. Inducible gene expression by retrovirus-mediated transfer of a modified tetracycline-regulated system. J Virol. 1996 Sep;70(9):6054–6059. doi: 10.1128/jvi.70.9.6054-6059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Hallewell R. A., Baxter J. D., Goodman H. M. Human growth hormone: complementary DNA cloning and expression in bacteria. Science. 1979 Aug 10;205(4406):602–607. doi: 10.1126/science.377496. [DOI] [PubMed] [Google Scholar]
- Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- No D., Yao T. P., Evans R. M. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory D. S., Neugeboren B. A., Mulligan R. C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W., Baur I., Boyce F. M., Breakefield X. O., Reeves S. A. Self-contained, tetracycline-regulated retroviral vector system for gene delivery to mammalian cells. J Virol. 1996 Jan;70(1):62–67. doi: 10.1128/jvi.70.1.62-67.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Clackson T., Natesan S., Pollock R., Amara J. F., Keenan T., Magari S. R., Phillips T., Courage N. L., Cerasoli F., Jr A humanized system for pharmacologic control of gene expression. Nat Med. 1996 Sep;2(9):1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- Rivière I., Brose K., Mulligan R. C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yee J. K., Moores J. C., Jolly D. J., Wolff J. A., Respess J. G., Friedmann T. Gene expression from transcriptionally disabled retroviral vectors. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5197–5201. doi: 10.1073/pnas.84.15.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]





