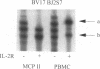
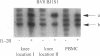
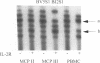
Abstract
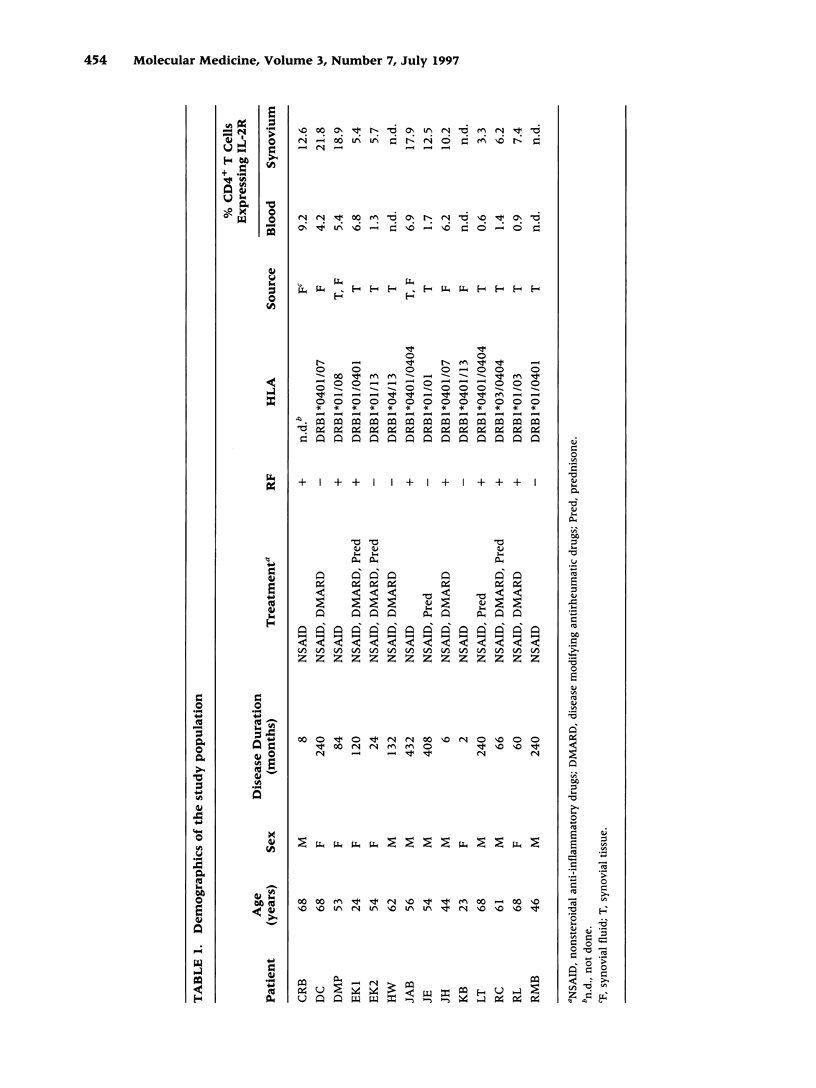
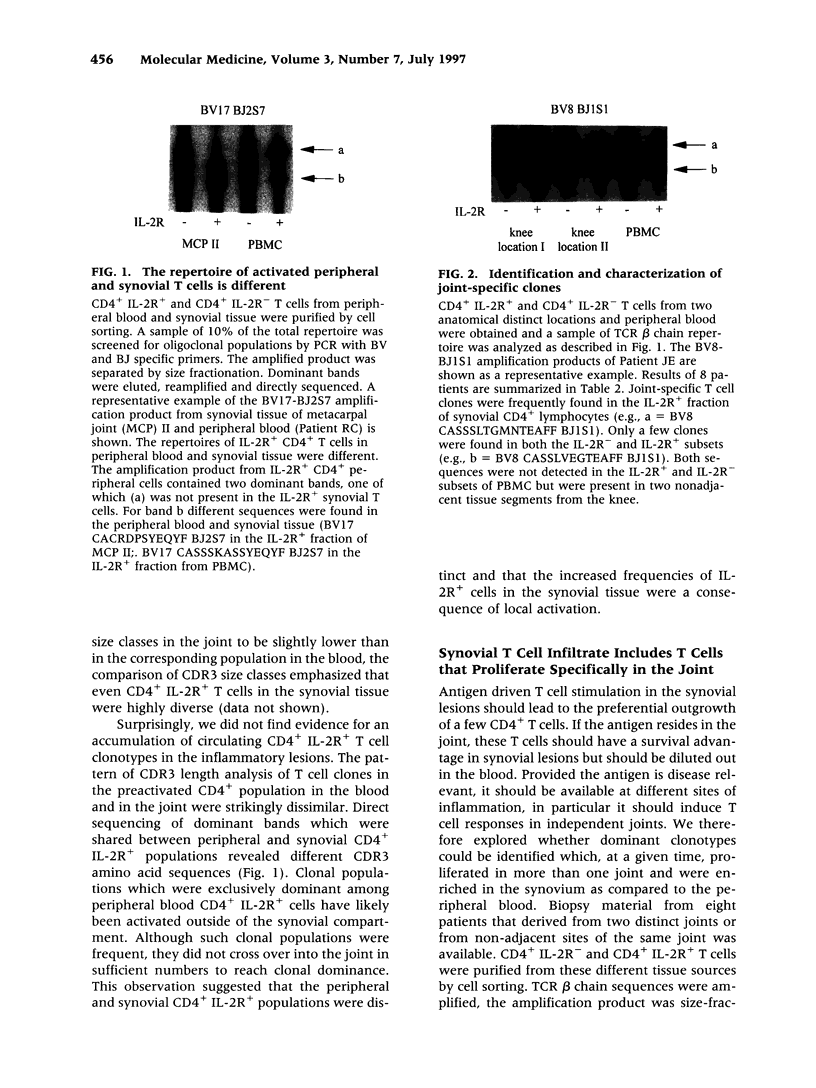
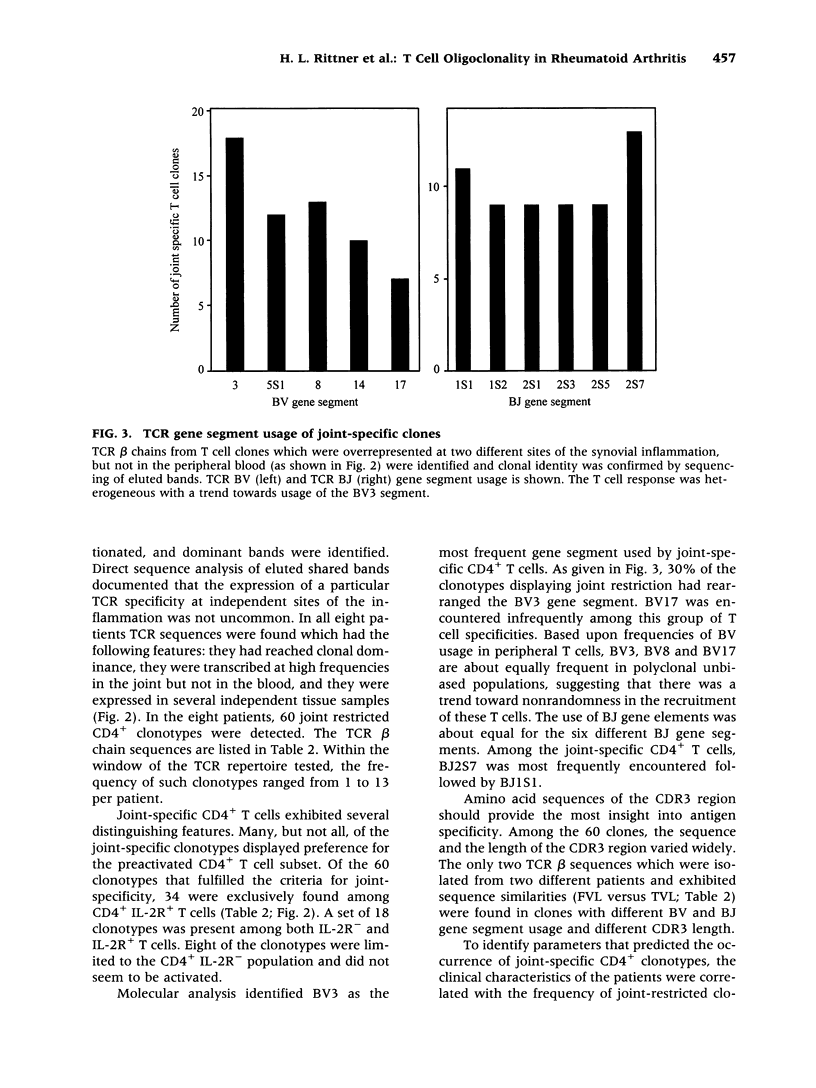
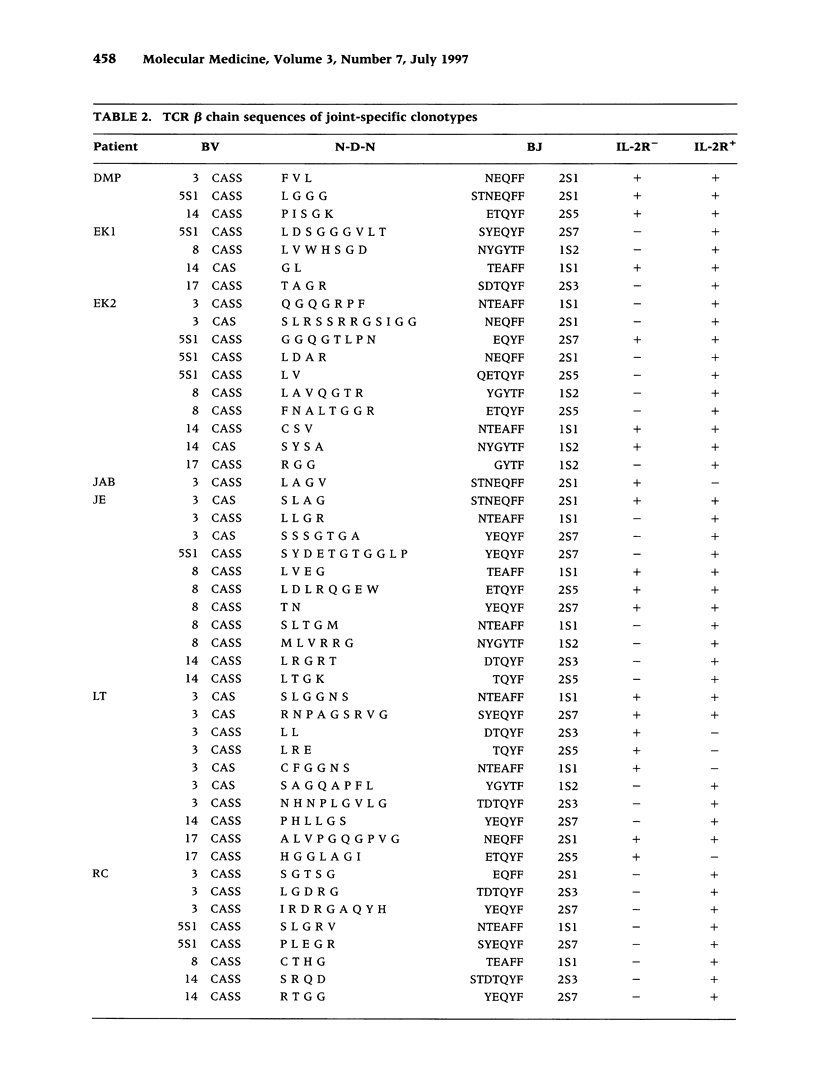
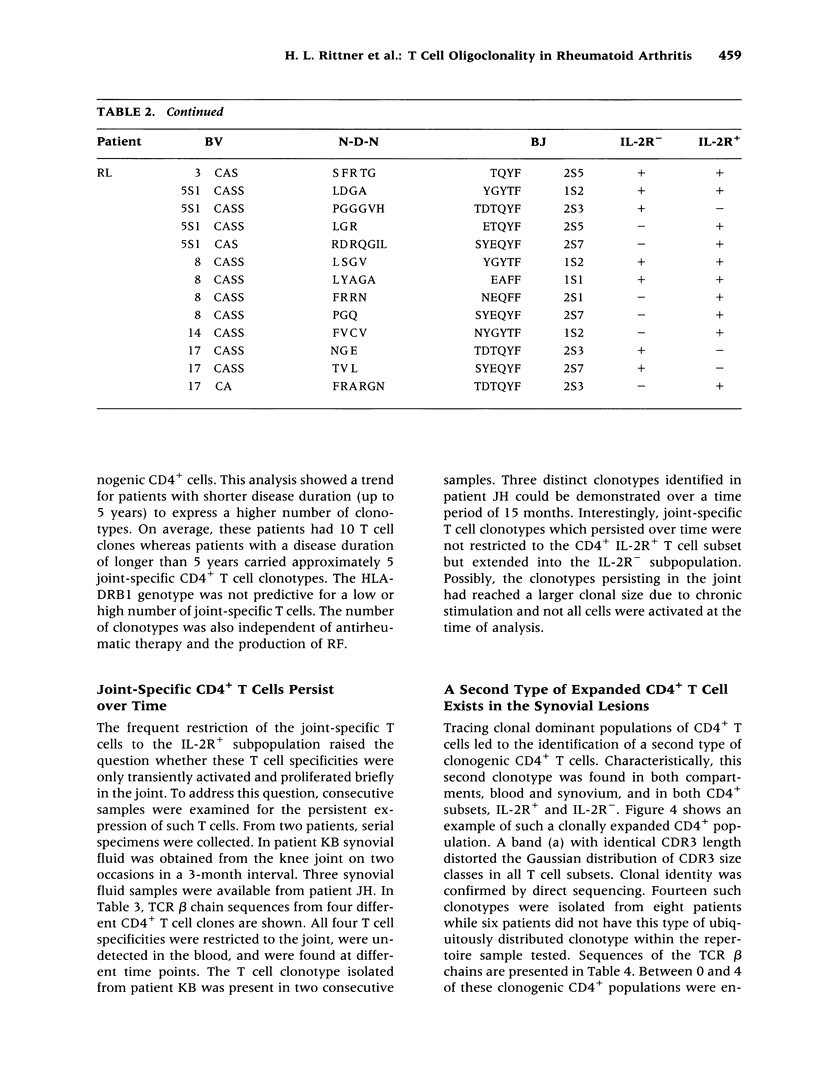
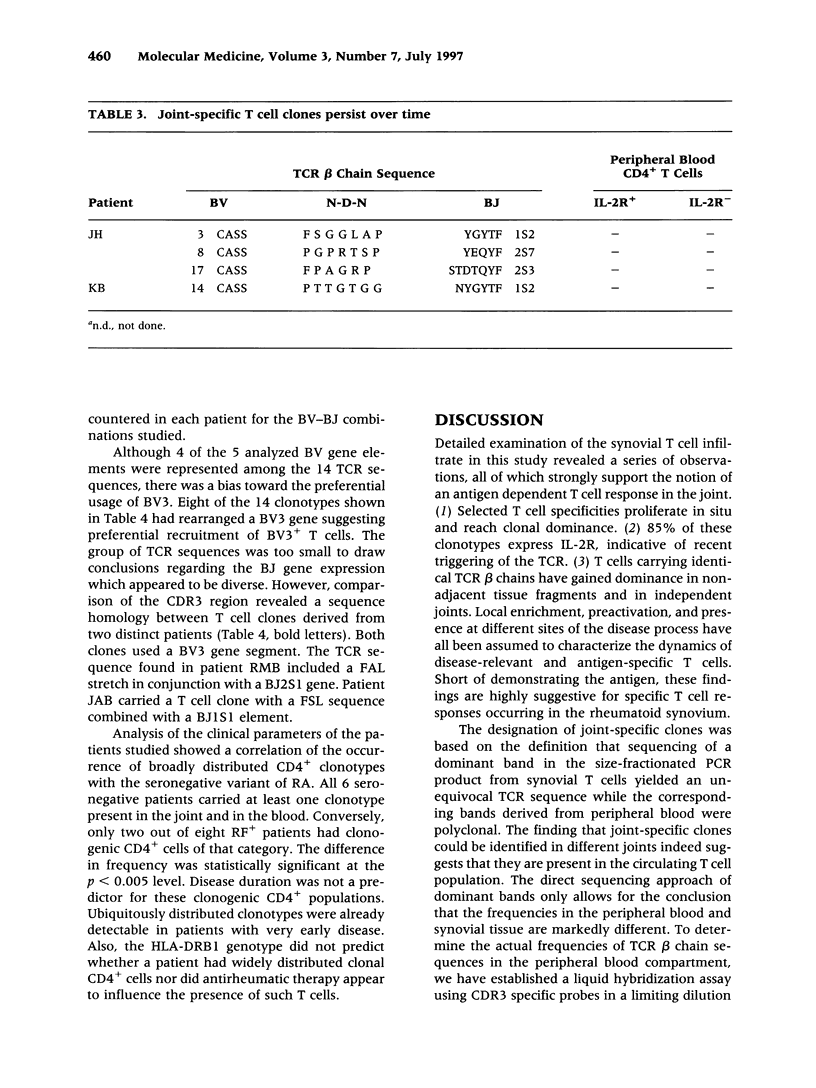
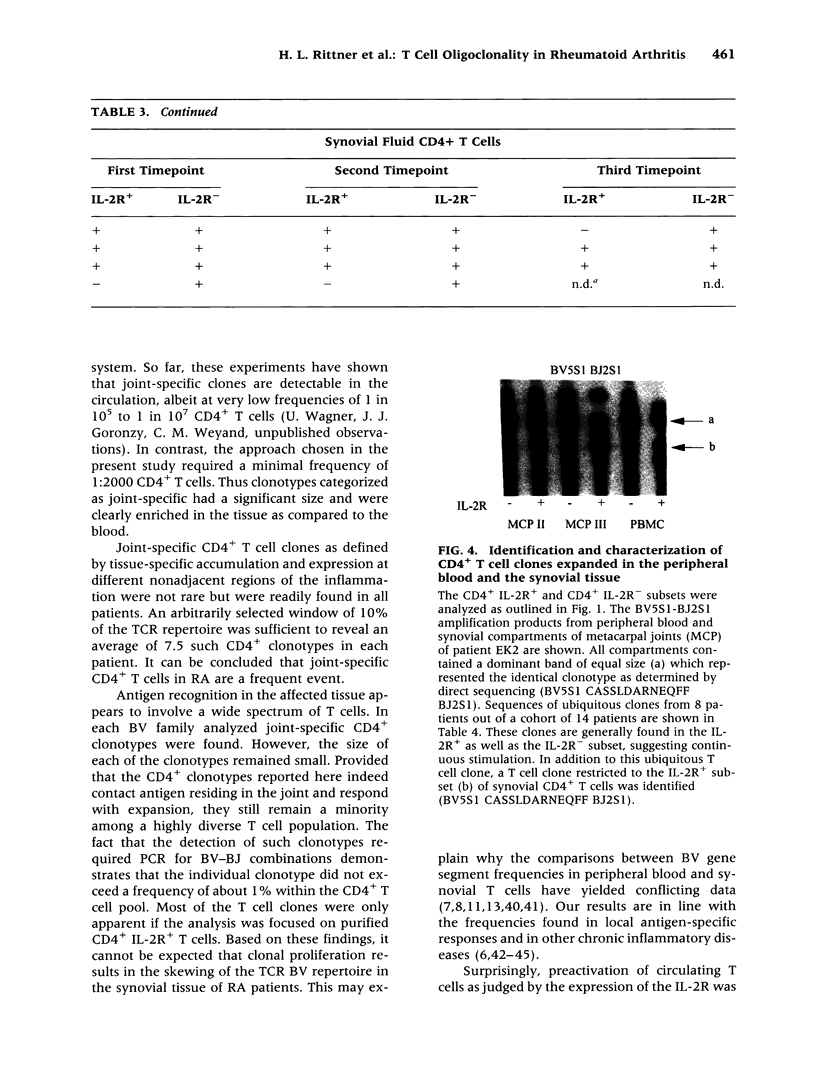
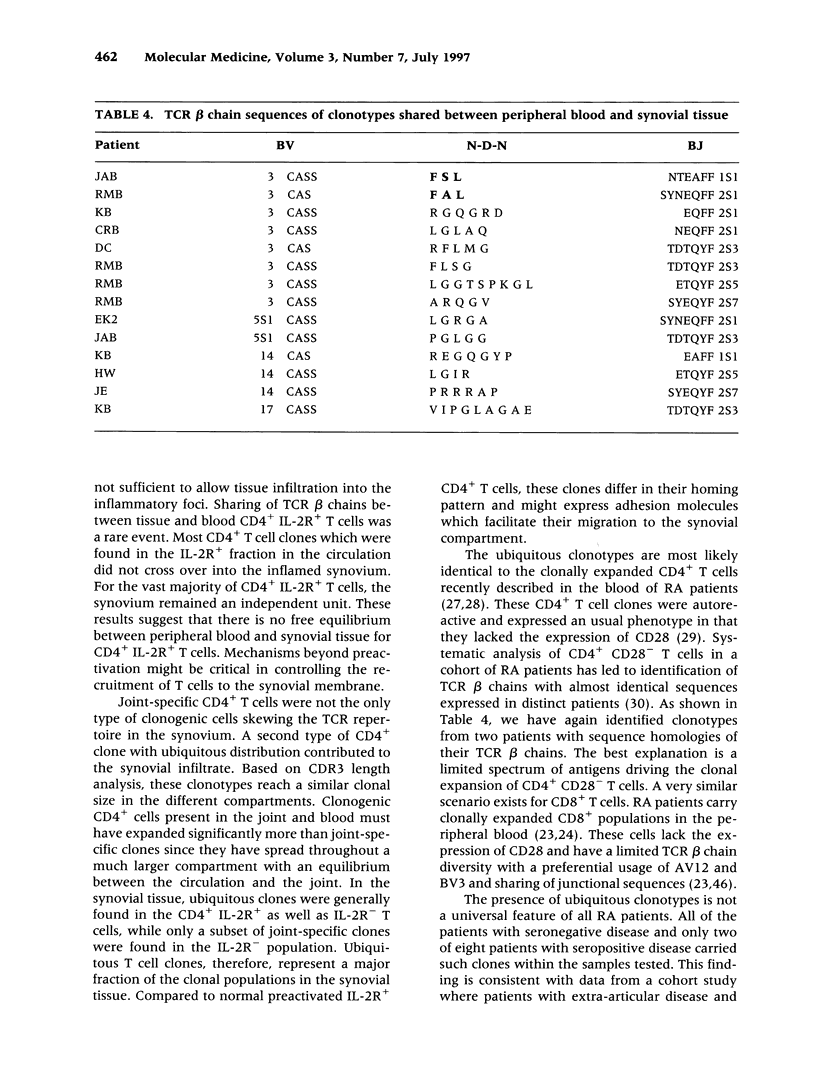
BACKGROUND: The synovial T cell infiltrate in rheumatoid arthritis (RA) is diverse but contains clonally expanded CD4+ populations. Recent reports have emphasized that RA patients have a tendency to develop CD4+ T cell oligoclonality which also manifests in the peripheral blood. Clonal dominance in the tissue may thus result from antigen specific stimulation in the synovial membrane or may reflect the infiltration of expanded clonotypes present throughout the lymphoid system. We have explored to what extent clonal populations amongst tissue CD4+ T cells display joint specificity as defined by their restriction to the joint, their persistence over time, and their expression of markers indicative for local activation. MATERIALS AND METHODS: Matched samples of peripheral blood and synovial fluid or synovial tissue were collected from 14 patients with active RA and CD4+ IL-2R+ and CD4+ IL-2R- T cells from both compartments were purified. Clonal populations of CD4+ T cells were detected by RT-PCR amplification of T cell receptor (TCR) transcripts with BV and BJ specific primers followed by size fractionation and direct sequencing of dominant size classes of TCR transcripts. RESULTS: Clonal CD4+ T cells were detected in the synovial fluid and synovial tissue of all patients. All patients carried synovial clonotypes that were undetectable in the blood but were present in independent joints or at several non-adjacent areas of the same joint. These joint restricted CD4+ clonotypes were generally small in size, were preferentially found in the IL-2R+ subpopulation, and persisted over time. A second type of clonogenic T cells in the synovial infiltrate had an unrestricted tissue distribution and was present at similar frequencies amongst activated and nonactivated T cells in the blood and affected joints. Ubiquitous clonotypes isolated from two different patients expressed sequence homologies of the TCR beta chain. CONCLUSIONS: Two types of expanded CD4+ clonotypes contribute to the T cell infiltrate in rheumatoid synovitis. Differences in the distribution pattern and in molecular features suggest that distinct mechanisms are supporting the clonal outgrowth of these two groups of clonotypes. Clonally expanded T cells restricted to the joint but present in several independent joints appear to respond to locally residing antigens. Clonogenic cells with an unrestricted distribution pattern and widespread activation in the blood and tissue may react to a different class of antigens which appear to be shared by multiple patients. T cell recognition in RA may be involved at several different levels and may be related to more than one pathomechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Ameline J., Lim A., Davodeau F., Peyrat M. A., Berthelot J. M., Semana G., Pannetier C., Gaschet J., Vie H., Even J. Selection of T cells reactive against autologous B lymphoblastoid cells during chronic rheumatoid arthritis. J Immunol. 1996 Nov 15;157(10):4697–4706. [PubMed] [Google Scholar]
- DerSimonian H., Sugita M., Glass D. N., Maier A. L., Weinblatt M. E., Rème T., Brenner M. B. Clonal V alpha 12.1+ T cell expansions in the peripheral blood of rheumatoid arthritis patients. J Exp Med. 1993 Jun 1;177(6):1623–1631. doi: 10.1084/jem.177.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Eichelberger M., Carding S. R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Duby A. D., Sinclair A. K., Osborne-Lawrence S. L., Zeldes W., Kan L., Fox D. A. Clonal heterogeneity of synovial fluid T lymphocytes from patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6206–6210. doi: 10.1073/pnas.86.16.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. E., Ricalton N. S., Meyer A. C., West S. G., Kaplan H., Behrendt C., Kotzin B. L. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995 Apr 1;154(7):3538–3547. [PubMed] [Google Scholar]
- González-Quintial R., Baccalá R., Pope R. M., Theofilopoulos A. N. Identification of clonally expanded T cells in rheumatoid arthritis using a sequence enrichment nuclease assay. J Clin Invest. 1996 Mar 1;97(5):1335–1343. doi: 10.1172/JCI118550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J. J., Bartz-Bazzanella P., Hu W., Jendro M. C., Walser-Kuntz D. R., Weyand C. M. Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J Clin Invest. 1994 Nov;94(5):2068–2076. doi: 10.1172/JCI117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J. J., Oppitz U., Weyand C. M. Clonal heterogeneity of superantigen reactivity in human V beta 6+ T cell clones. Limited contributions of V beta sequence polymorphisms. J Immunol. 1992 Jan 15;148(2):604–611. [PubMed] [Google Scholar]
- Goronzy J. J., Weyand C. M. Interplay of T lymphocytes and HLA-DR molecules in rheumatoid arthritis. Curr Opin Rheumatol. 1993 Mar;5(2):169–177. doi: 10.1097/00002281-199305020-00008. [DOI] [PubMed] [Google Scholar]
- Goronzy J. J., Weyand C. M. T cells in rheumatoid arthritis. Paradigms and facts. Rheum Dis Clin North Am. 1995 Aug;21(3):655–674. [PubMed] [Google Scholar]
- Gorski J., Yassai M., Zhu X., Kissela B., Kissella B [corrected to Kissela B. ]., Keever C., Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994 May 15;152(10):5109–5119. [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hingorani R., Monteiro J., Furie R., Chartash E., Navarrete C., Pergolizzi R., Gregersen P. K. Oligoclonality of V beta 3 TCR chains in the CD8+ T cell population of rheumatoid arthritis patients. J Immunol. 1996 Jan 15;156(2):852–858. [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Masuko K., Nakai Y., Kato T., Hasanuma T., Yoshino S. I., Mizushima Y., Nishioka K., Yamamoto K. High frequencies of identical T cell clonotypes in synovial tissues of rheumatoid arthritis patients suggest the occurrence of common antigen-driven immune responses. Arthritis Rheum. 1996 Mar;39(3):446–453. doi: 10.1002/art.1780390312. [DOI] [PubMed] [Google Scholar]
- Jenkins R. N., Nikaein A., Zimmermann A., Meek K., Lipsky P. E. T cell receptor V beta gene bias in rheumatoid arthritis. J Clin Invest. 1993 Dec;92(6):2688–2701. doi: 10.1172/JCI116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone E. C., Minden M., Klock R., Poplonski L., Zalcberg J., Takadera T., Mak T. W. Structure of T cell antigen receptor beta chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Dec;31(12):1555–1557. doi: 10.1002/art.1780311213. [DOI] [PubMed] [Google Scholar]
- Kohem C. L., Brezinschek R. I., Wisbey H., Tortorella C., Lipsky P. E., Oppenheimer-Marks N. Enrichment of differentiated CD45RBdim,CD27- memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1996 May;39(5):844–854. doi: 10.1002/art.1780390518. [DOI] [PubMed] [Google Scholar]
- Kouskoff V., Korganow A. S., Duchatelle V., Degott C., Benoist C., Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996 Nov 29;87(5):811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Sun G. R., Tumang J. R., Crow M. K., Friedman S. M. CDR3 sequence motifs shared by oligoclonal rheumatoid arthritis synovial T cells. Evidence for an antigen-driven response. J Clin Invest. 1994 Dec;94(6):2525–2531. doi: 10.1172/JCI117624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H. X., Haynes B. F. Role of adhesion molecules in the pathogenesis of rheumatoid arthritis. Rheum Dis Clin North Am. 1995 Aug;21(3):715–740. [PubMed] [Google Scholar]
- Martinez-Taboada V., Hunder N. N., Hunder G. G., Weyand C. M., Goronzy J. J. Recognition of tissue residing antigen by T cells in vasculitic lesions of giant cell arteritis. J Mol Med (Berl) 1996 Nov;74(11):695–703. doi: 10.1007/s001090050074. [DOI] [PubMed] [Google Scholar]
- McCoy J. P., Jr, Overton W. R., Schroeder K., Blumstein L., Donaldson M. H. Immunophenotypic analysis of the T cell receptor V beta repertoire in CD4+ and CD8+ lymphocytes from normal peripheral blood. Cytometry. 1996 Jun 15;26(2):148–153. doi: 10.1002/(SICI)1097-0320(19960615)26:2<148::AID-CYTO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- McInnes I. B., al-Mughales J., Field M., Leung B. P., Huang F. P., Dixon R., Sturrock R. D., Wilkinson P. C., Liew F. Y. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996 Feb;2(2):175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Melancon-Kaplan J., Young S. M., Pirmez C., Kino H., Convit J., Rea T. H., Bloom B. R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J., Hingorani R., Choi I. H., Silver J., Pergolizzi R., Gregersen P. K. Oligoclonality in the human CD8+ T cell repertoire in normal subjects and monozygotic twins: implications for studies of infectious and autoimmune diseases. Mol Med. 1995 Sep;1(6):614–624. [PMC free article] [PubMed] [Google Scholar]
- Nanki T., Kohsaka H., Mizushima N., Ollier W. E., Carson D. A., Miyasaka N. Genetic control of T cell receptor BJ gene expression in peripheral lymphocytes of normal and rheumatoid arthritis monozygotic twins. J Clin Invest. 1996 Oct 1;98(7):1594–1601. doi: 10.1172/JCI118953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Nepom B. S. Prediction of susceptibility to rheumatoid arthritis by human leukocyte antigen genotyping. Rheum Dis Clin North Am. 1992 Nov;18(4):785–792. [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Pannetier C., Cochet M., Darche S., Casrouge A., Zöller M., Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N., Sinha R., Kabak S., Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994 Feb 1;179(2):609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D., Goronzy J. J., Weyand C. M. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996 May 1;97(9):2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E., David-Ameline J., Peyrat M. A., Moreau-Aubry A., Pinczon D., Lim A., Even J., Semana G., Berthelot J. M., Breathnach R. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996 Nov 1;184(5):1791–1800. doi: 10.1084/jem.184.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyk L., Hawes G. E., Chatila M. K., Breedveld F. C., Kurnick J. T., van den Elsen P. J. T cell receptors in rheumatoid arthritis. Arthritis Rheum. 1995 May;38(5):577–589. doi: 10.1002/art.1780380502. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Waase I., Kayser C., Carlson P. J., Goronzy J. J., Weyand C. M. Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum. 1996 Jun;39(6):904–913. doi: 10.1002/art.1780390606. [DOI] [PubMed] [Google Scholar]
- Walser-Kuntz D. R., Weyand C. M., Weaver A. J., O'Fallon W. M., Goronzy J. J. Mechanisms underlying the formation of the T cell receptor repertoire in rheumatoid arthritis. Immunity. 1995 Jun;2(6):597–605. doi: 10.1016/1074-7613(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Schönberger J., Oppitz U., Hunder N. N., Hicok K. C., Goronzy J. J. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994 Mar 1;179(3):951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Xie C., Goronzy J. J. Homozygosity for the HLA-DRB1 allele selects for extraarticular manifestations in rheumatoid arthritis. J Clin Invest. 1992 Jun;89(6):2033–2039. doi: 10.1172/JCI115814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., Fang Q., Demarco D., VonFeldt J., Zurier R. B., Weiner D. B. Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest. 1992 Aug;90(2):326–333. doi: 10.1172/JCI115866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. The molecular basis of susceptibility to rheumatoid arthritis. Adv Immunol. 1994;56:389–466. doi: 10.1016/s0065-2776(08)60456-3. [DOI] [PubMed] [Google Scholar]