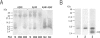Abstract
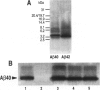
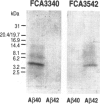
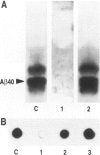
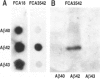
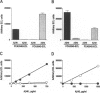
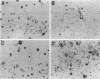
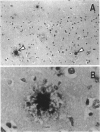
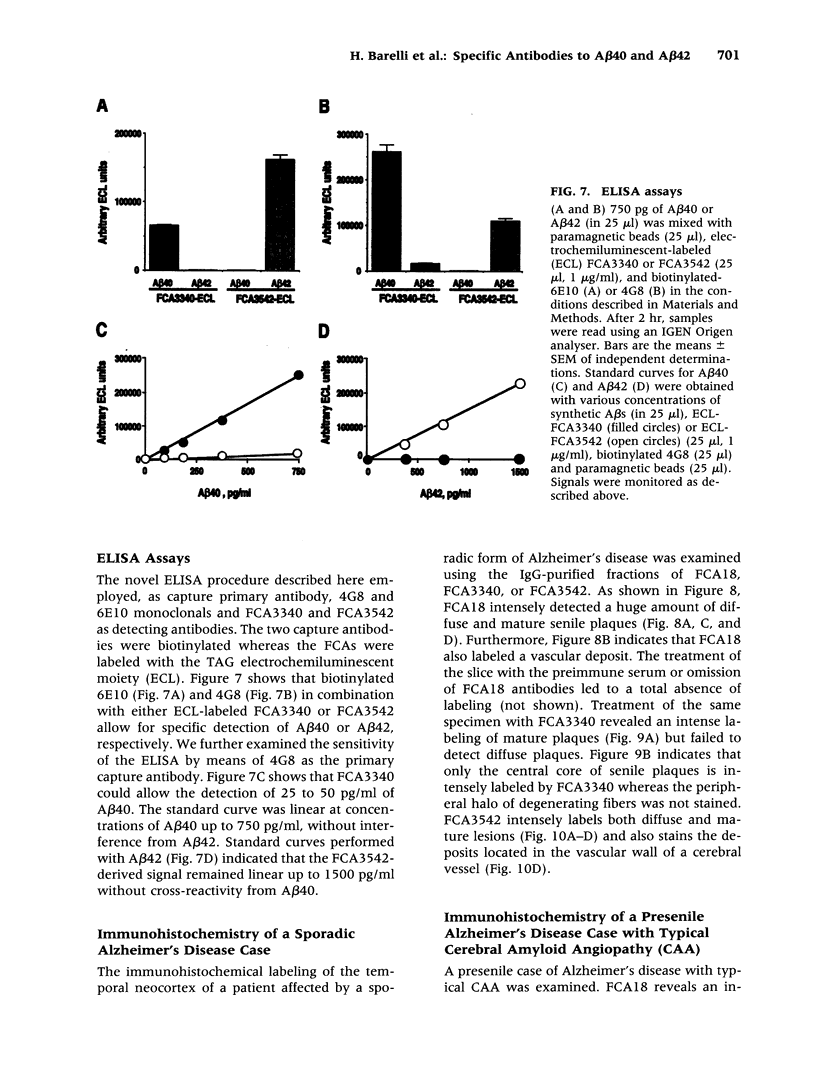
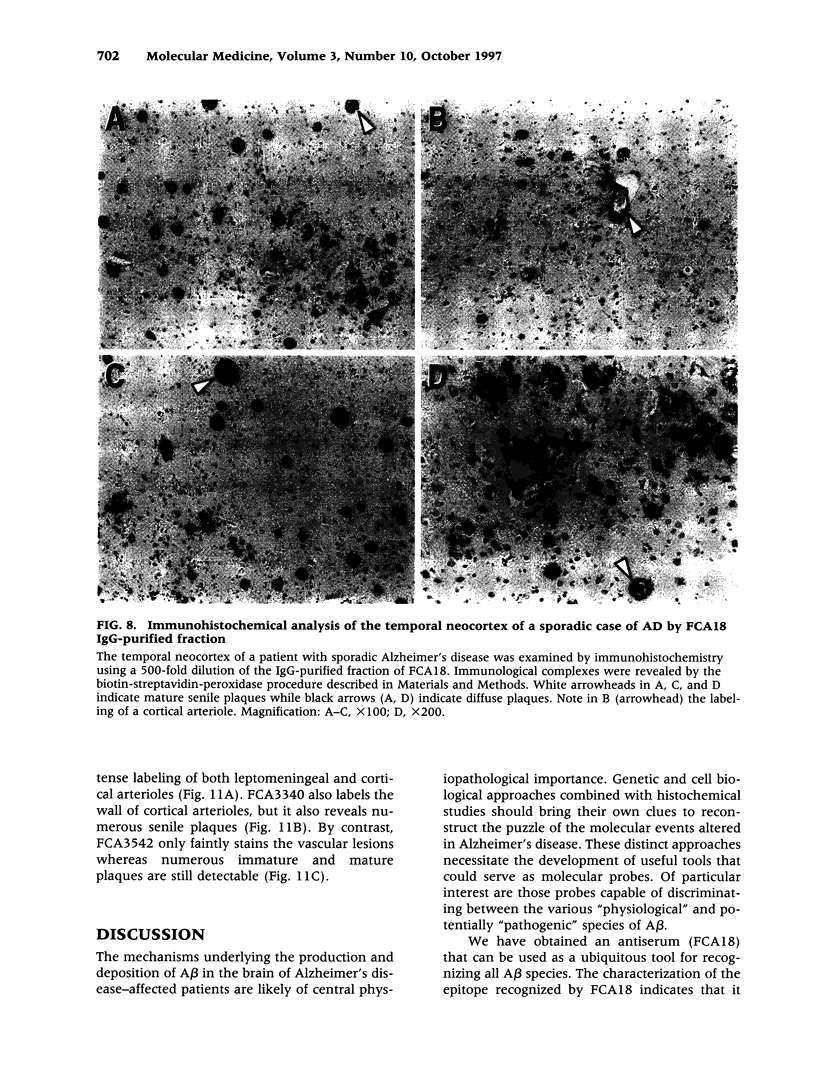
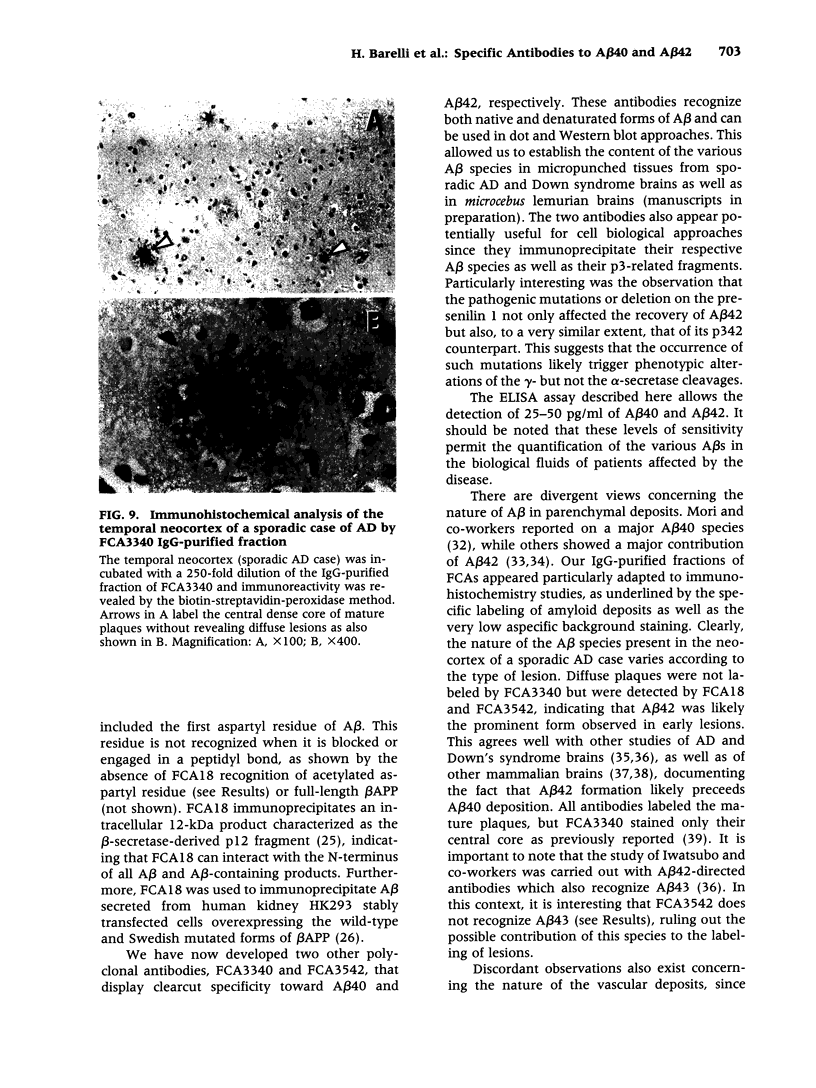
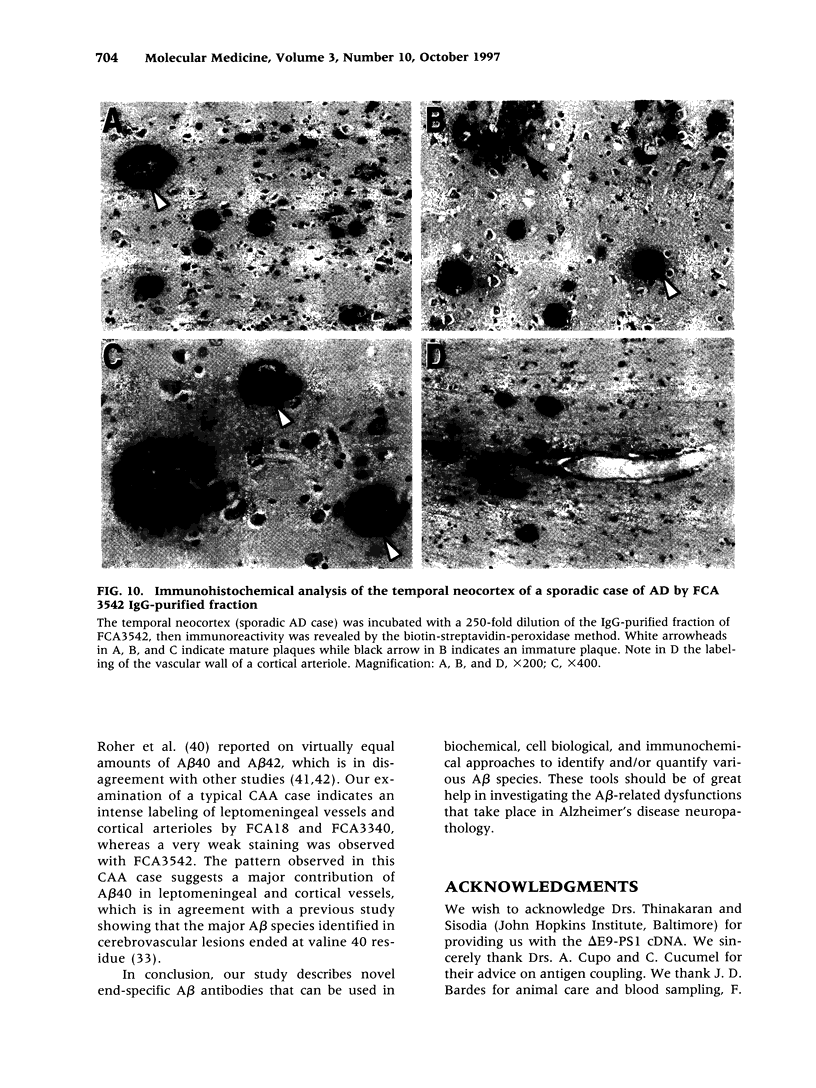
BACKGROUND: In Alzheimer's disease (AD), the main histological lesion is a proteinaceous deposit, the senile plaque, which is mainly composed of a peptide called A beta. The aggregation process is thought to occur through enhanced concentration of A beta 40 or increased production of the more readily aggregating 42 amino acid-long A beta 42 species. MATERIALS AND METHODS: Specificity of the antibodies was assessed by dot blot, Western blot, ELISA, and immunoprecipitation procedures on synthetic and endogenous A beta produced by secreted HK293 cells. A beta and p3 production by wild-type and mutated presenilin 1-expressing cells transiently transfected with beta APP751 was monitored after metabolic labeling and immunoprecipitation procedures. Immunohistochemical analysis was performed on brains of sporadic and typical cerebrovascular amyloid angiopathy (CAA) cases. RESULTS: Dot and Western blot analyses indicate that IgG-purified fractions of antisera recognize native and denaturated A beta s. FCA3340 and FCA 3542 display full specificity for A beta 40 and A beta 42, respectively. Antibodies immunoprecipitate their respective synthetic A beta species but also A beta s and their related p3 counterparts endogenously secreted by transfected human kidney 293 cells. This allowed us to show that mutations on presenilin 1 triggered similar increased ratios of A beta 42 and its p 342 counterpart over total A beta and p3. ELISA assays allow detection of about 25-50 pg/ml of A beta s and remain linear up to 750 to 1500 pg/ml without any cross-reactivity. FCA18 and FCA3542 label diffuse and mature plaques of a sporadic AD case whereas FCA3340 only reveals the mature lesions and particularly labels their central dense core. In a CAA case, FCA18 and FCA3340 reveal leptomeningeal and cortical arterioles whereas FCA3542 only faintly labels such structures. CONCLUSIONS: Polyclonal antibodies exclusively recognizing A beta 40 (FCA 3340) or A beta 42 (FCA3542) were obtained. These demonstrated that FAD-linked presenilins similarly affect both p342 and A beta 42, suggesting that these mutations misroute the beta APP to a compartment where gamma-secretase, but not alpha-secretase, cleavages are modified. Overall, these antibodies should prove useful for fundamental and diagnostic approaches, as suggested by their usefulness for biochemical, cell biological, and immunohistochemical techniques.
Full text
PDF



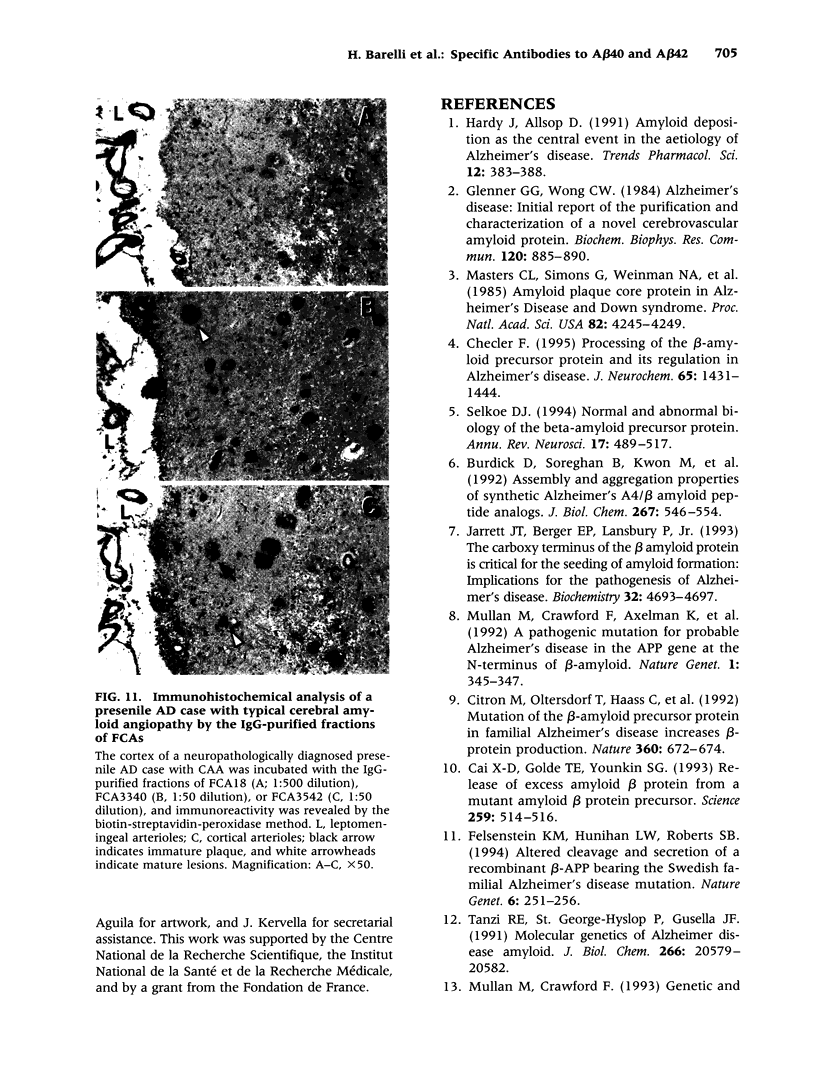


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996 Nov;17(5):1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Ramabhadran T. V., Greengard P. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F., Barelli H., Vincent J. P. Tissue distribution of a novel neurotensin-degrading metallopeptidase. An immunological approach using monospecific polyclonal antibodies. Biochem J. 1989 Jan 15;257(2):549–554. doi: 10.1042/bj2570549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer's disease. J Neurochem. 1995 Oct;65(4):1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- Chevallier N., Jiracek J., Vincent B., Baur C. P., Spillantini M. G., Goedert M., Dive V., Checler F. Examination of the role of endopeptidase 3.4.24.15 in A beta secretion by human transfected cells. Br J Pharmacol. 1997 Jun;121(3):556–562. doi: 10.1038/sj.bjp.0701151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., Johnson-Wood K., Lee M., Seubert P., Davis A. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997 Jan;3(1):67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Cucumel K., Garreau I., Mery J., Moinier D., Mansour A., Akil H., Cupo A. Production and characterization of site-directed antibodies against dermorphin and dermorphin-related peptides. Peptides. 1996;17(6):973–982. doi: 10.1016/0196-9781(96)00113-1. [DOI] [PubMed] [Google Scholar]
- Delaère P., He Y., Fayet G., Duyckaerts C., Hauw J. J. Beta A4 deposits are constant in the brain of the oldest old: an immunocytochemical study of 20 French centenarians. Neurobiol Aging. 1993 Mar-Apr;14(2):191–194. doi: 10.1016/0197-4580(93)90096-t. [DOI] [PubMed] [Google Scholar]
- Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Perez-tur J., Hutton M., Buee L., Harigaya Y., Yager D. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996 Oct 24;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Efthimiopoulos S., Felsenstein K. M., Sambamurti K., Robakis N. K., Refolo L. M. Study of the phorbol ester effect on Alzheimer amyloid precursor processing: sequence requirements and involvement of a cholera toxin sensitive protein. J Neurosci Res. 1994 May 1;38(1):81–90. doi: 10.1002/jnr.490380111. [DOI] [PubMed] [Google Scholar]
- Felsenstein K. M., Hunihan L. W., Roberts S. B. Altered cleavage and secretion of a recombinant beta-APP bearing the Swedish familial Alzheimer's disease mutation. Nat Genet. 1994 Mar;6(3):251–255. doi: 10.1038/ng0394-251. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Asami-Odaka A., Suzuki N., Shimada H., Ihara Y., Iwatsubo T. Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer's disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques. Am J Pathol. 1996 Jan;148(1):259–265. [PMC free article] [PubMed] [Google Scholar]
- Gillespie S. L., Golde T. E., Younkin S. G. Secretory processing of the Alzheimer amyloid beta/A4 protein precursor is increased by protein phosphorylation. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1285–1290. doi: 10.1016/0006-291x(92)90442-n. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991 Oct;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Mann D. M., Odaka A., Suzuki N., Ihara Y. Amyloid beta protein (A beta) deposition: A beta 42(43) precedes A beta 40 in Down syndrome. Ann Neurol. 1995 Mar;37(3):294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994 Jul;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Berger E. P., Lansbury P. T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993 May 11;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Duffy L. K., Morris J. H., Selkoe D. J. Protein chemical and immunocytochemical studies of meningovascular beta-amyloid protein in Alzheimer's disease and normal aging. Brain Res. 1988 Nov 22;474(1):100–111. doi: 10.1016/0006-8993(88)90673-7. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., Pettingell W. H., Yu C. E., Jondro P. D., Schmidt S. D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995 Aug 18;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsueda G. R., Stewart J. M. A p-methylbenzhydrylamine resin for improved solid-phase synthesis of peptide amides. Peptides. 1981 Spring;2(1):45–50. doi: 10.1016/s0196-9781(81)80010-1. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Papayannopoulos I. A., Styles J., Bobin S. A., Lin Y. Y., Biemann K., Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993 Feb 15;301(1):41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Mori H., Takio K., Ogawara M., Selkoe D. J. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J Biol Chem. 1992 Aug 25;267(24):17082–17086. [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Tamaoka A., Sawamura N., Shoji S., Nakayama H., Ono F., Sakakibara I., Yoshikawa Y., Mori H., Goto N. Carboxyl end-specific monoclonal antibodies to amyloid beta protein (A beta) subtypes (A beta 40 and A beta 42(43)) differentiate A beta in senile plaques and amyloid angiopathy in brains of aged cynomolgus monkeys. Neurosci Lett. 1995 Dec 8;201(2):151–154. doi: 10.1016/0304-3940(95)12160-9. [DOI] [PubMed] [Google Scholar]
- Prelli F., Castaño E., Glenner G. G., Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem. 1988 Aug;51(2):648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995 Aug 31;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Wolkow C., Wang R., Cotter R. J., Reardon I. M., Zürcher-Neely H. A., Heinrikson R. L., Ball M. J. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J Biol Chem. 1993 Feb 15;268(5):3072–3083. [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Woods A. S., Cotter R. J., Gowing E., Ball M. J. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995 Jun 29;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Slack B. E., Nitsch R. M., Livneh E., Kunz G. M., Jr, Breu J., Eldar H., Wurtman R. J. Regulation by phorbol esters of amyloid precursor protein release from Swiss 3T3 fibroblasts overexpressing protein kinase C alpha. J Biol Chem. 1993 Oct 5;268(28):21097–21101. [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., George-Hyslop P. S., Gusella J. F. Molecular genetics of Alzheimer disease amyloid. J Biol Chem. 1991 Nov 5;266(31):20579–20582. [PubMed] [Google Scholar]