Abstract
BACKGROUND: Advanced glycation end products (AGEs) are the reactive derivatives of nonenzymatic glucose-macromolecule condensation products. Aging human tissues accumulate AGEs in an age-dependent manner and contribute to age-related functional changes in vital organs. We have shown previously that AGE scavenger receptors are present on monocyte/macrophages, lymphocytes, and other cells. However, it remains unclear whether the human brain can efficiently eliminate AGE-modified proteins and whether excessive AGEs can contribute to inflammatory changes leading to brain injury in aging. MATERIALS AND METHODS: To explore the expression and characteristics of AGE-binding proteins on CNS glia components and their putative function, such as degradation of AGE-modified proteins, primary human astrocytes and human monocytes (as a microglial cell surrogate) and murine microglia (N9) cells and cell membrane extracts were used. Immunohistochemistry was used to examine the distribution of AGE-binding proteins in the human hippocampus; RT-PCR techniques were used to examine the biologic effects of AGEs and a model AGE compound, FFI, on AGE-binding protein modulation and cytokine responses of human astrocytes and monocytes. RESULTS: Our results showed that AGE-binding proteins AGE-R1, -R2, and -R3 are present in glial cells. Western blot analyses and radiolabeled ligand binding studies show that AGE-R1 and -R3 from human astrocytes bind AGE-modified proteins; binding could be blocked by anti-AGE-R1 and anti-AGE-R3 antibodies, respectively. Immunohistochemistry showed that AGE-R1 and -R2 are expressed mainly in neurons; only some glial cells express these AGE-binding proteins. In contrast, AGE-R3 was found only on those astrocytes whose positively stained foot processes extend and surround the sheath of microcapillaries. RT-PCR results showed that mRNAs of the three AGE-binding proteins are expressed constitutively in human astrocytes and monocytes, and receptor transcripts are not regulated by exogenous AGEs, the model AGE compound FFI, or phorbol ester. At the concentrations used, GM-CSF appears to be the only cytokine whose transcript and protein levels are regulated in human astrocytes by exogenous AGEs. CONCLUSIONS: The selective presence of AGE-binding proteins in pyramidal neurons and glial cells and their roles in degrading AGE-modified protein in glial cells suggest that the human brain has a mechanism(s) to clear AGE-modified proteins. Without this capacity, accumulation of AGEs extracellularly could stimulate glial cells to produce the major inflammatory cytokine GM-CSF, which has been shown to be capable of up-regulating AGE-R3. It remains to be determined whether AGE-binding proteins could be aberrant or down-regulated under certain pathological conditions, resulting in an insidious inflammatory state of the CNS in some aging humans.
Full text
PDF








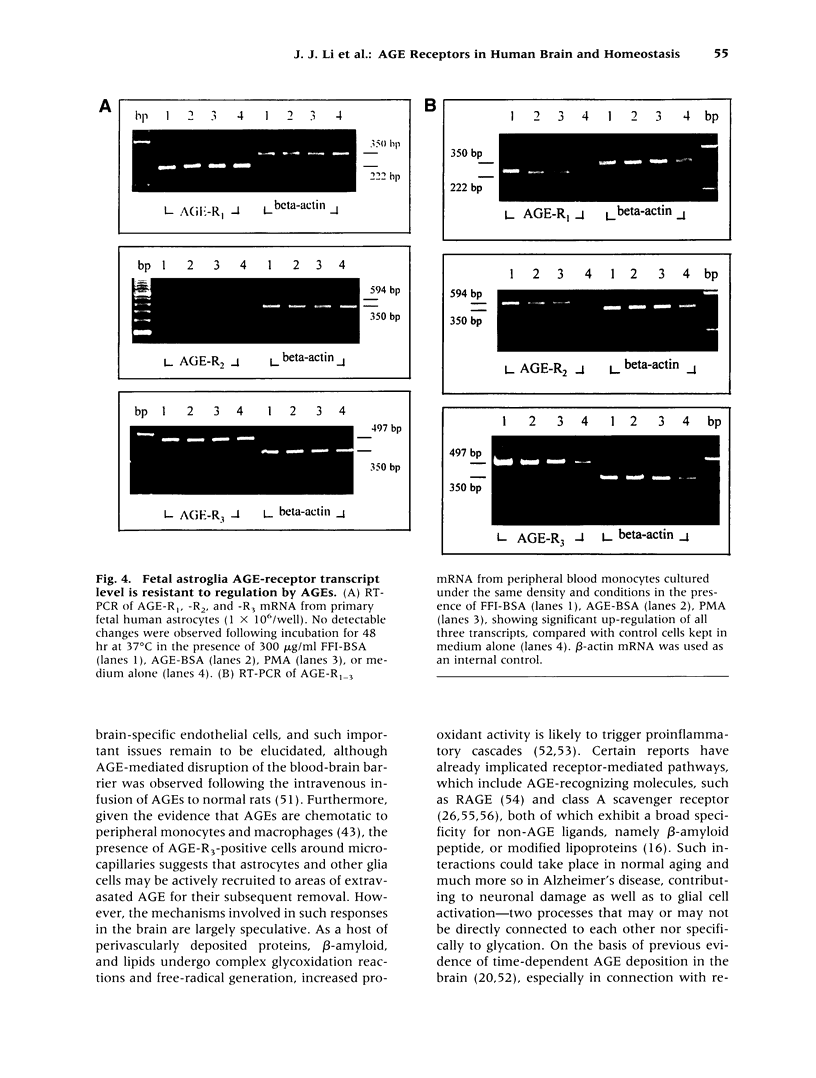
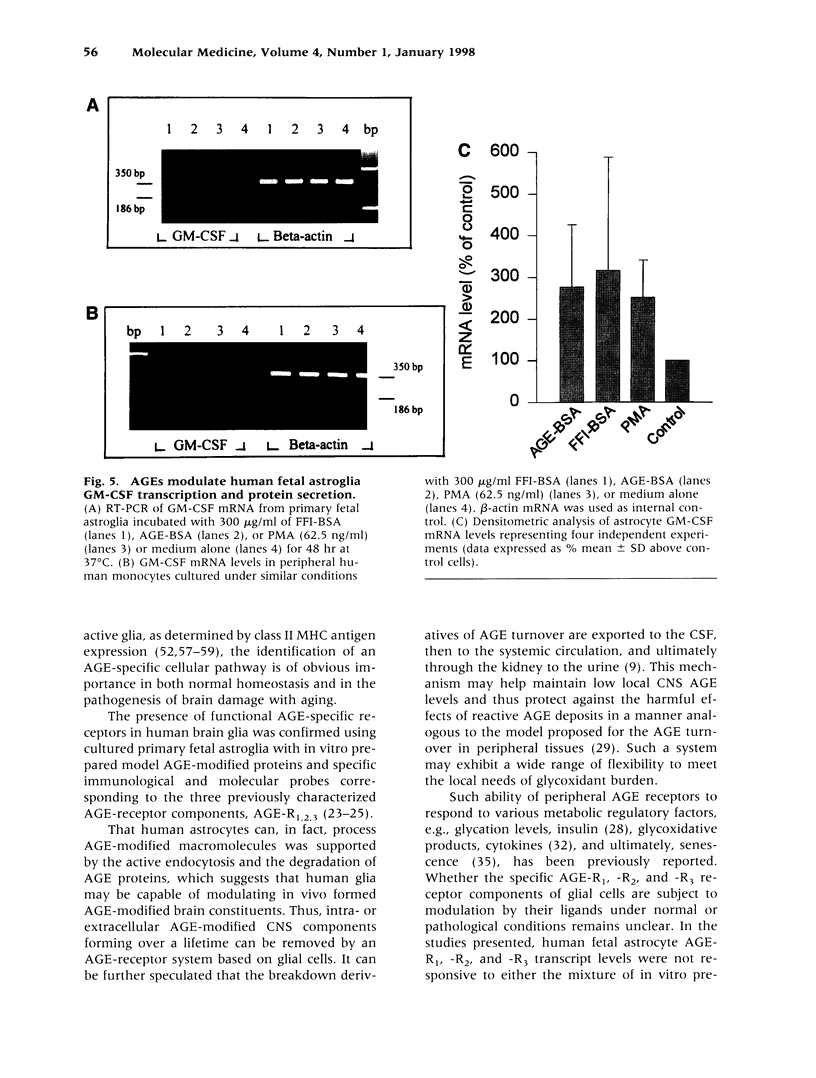
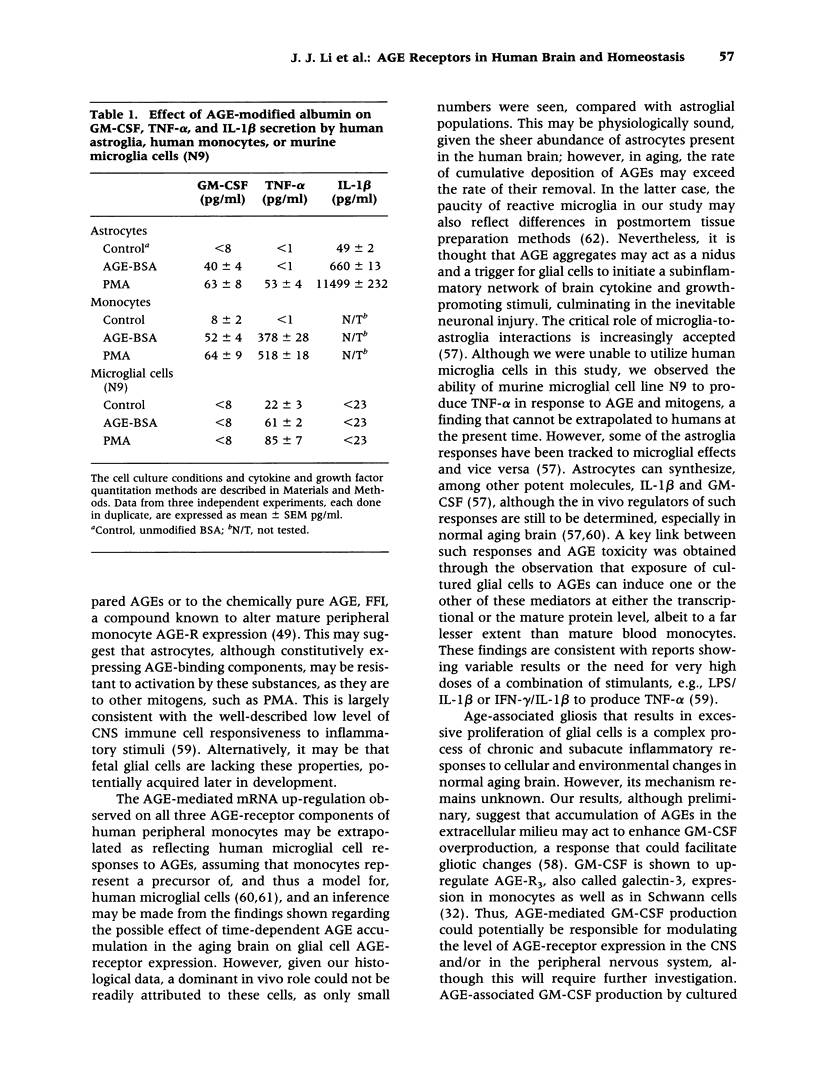



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel M., Ritthaler U., Zhang Y., Deng Y., Schmidt A. M., Greten J., Sernau T., Wahl P., Andrassy K., Ritz E. Expression of receptors for advanced glycosylated end-products in renal disease. Nephrol Dial Transplant. 1995;10(9):1662–1667. [PubMed] [Google Scholar]
- Abraham E. C., Cherian M., Smith J. B. Site selectivity in the glycation of alpha A- and alpha B-crystallins by glucose. Biochem Biophys Res Commun. 1994 Jun 30;201(3):1451–1456. doi: 10.1006/bbrc.1994.1866. [DOI] [PubMed] [Google Scholar]
- Aoki Y., Yanagisawa Y., Yazaki K., Oguchi H., Kiyosawa K., Furuta S. Protective effect of vitamin E supplementation on increased thermal stability of collagen in diabetic rats. Diabetologia. 1992 Oct;35(10):913–916. doi: 10.1007/BF00401418. [DOI] [PubMed] [Google Scholar]
- Araki N., Higashi T., Mori T., Shibayama R., Kawabe Y., Kodama T., Takahashi K., Shichiri M., Horiuchi S. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur J Biochem. 1995 Jun 1;230(2):408–415. doi: 10.1111/j.1432-1033.1995.0408h.x. [DOI] [PubMed] [Google Scholar]
- Araki N., Ueno N., Chakrabarti B., Morino Y., Horiuchi S. Immunochemical evidence for the presence of advanced glycation end products in human lens proteins and its positive correlation with aging. J Biol Chem. 1992 May 25;267(15):10211–10214. [PubMed] [Google Scholar]
- Ateshkadi A., Johnson C. A., Founds H. W., Zimmerman S. W. Serum advanced glycosylation end-products in patients on hemodialysis and CAPD. Perit Dial Int. 1995;15(2):129–133. [PubMed] [Google Scholar]
- Boyd-White J., Williams J. C., Jr Effect of cross-linking on matrix permeability. A model for AGE-modified basement membranes. Diabetes. 1996 Mar;45(3):348–353. doi: 10.2337/diab.45.3.348. [DOI] [PubMed] [Google Scholar]
- Brain M. C. The haemolytic-uraemic syndrome. Semin Hematol. 1969 Apr;6(2):162–180. [PubMed] [Google Scholar]
- Brett J., Schmidt A. M., Yan S. D., Zou Y. S., Weidman E., Pinsky D., Nowygrod R., Neeper M., Przysiecki C., Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993 Dec;143(6):1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bucala R., Mitchell R., Arnold K., Innerarity T., Vlassara H., Cerami A. Identification of the major site of apolipoprotein B modification by advanced glycosylation end products blocking uptake by the low density lipoprotein receptor. J Biol Chem. 1995 May 5;270(18):10828–10832. doi: 10.1074/jbc.270.18.10828. [DOI] [PubMed] [Google Scholar]
- Bucala R., Vlassara H. Advanced glycosylation end products in diabetic renal and vascular disease. Am J Kidney Dis. 1995 Dec;26(6):875–888. doi: 10.1016/0272-6386(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Cacabelos R., Alvarez X. A., Fernández-Novoa L., Franco A., Mangues R., Pellicer A., Nishimura T. Brain interleukin-1 beta in Alzheimer's disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994 Mar;16(2):141–151. [PubMed] [Google Scholar]
- Cerami A., Vlassara H., Brownlee M. Role of nonenzymatic glycosylation in atherogenesis. J Cell Biochem. 1986;30(2):111–120. doi: 10.1002/jcb.240300203. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Sinicropi S., Yen S. H., Ko L. W., Mattiace L. A., Bucala R., Vlassara H. Glycation and microglial reaction in lesions of Alzheimer's disease. Neurobiol Aging. 1996 Sep-Oct;17(5):733–743. doi: 10.1016/0197-4580(96)00116-9. [DOI] [PubMed] [Google Scholar]
- El Khoury J., Hickman S. E., Thomas C. A., Cao L., Silverstein S. C., Loike J. D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996 Aug 22;382(6593):716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989 Oct 1;170(4):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. W. Macrophage scavenger receptors. Curr Opin Lipidol. 1994 Apr;5(2):143–148. doi: 10.1097/00041433-199404000-00011. [DOI] [PubMed] [Google Scholar]
- Giaume C., McCarthy K. D. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996 Aug;19(8):319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Kirstein M., Brett J., Radoff S., Ogawa S., Stern D., Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg G. W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996 Aug;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Li J. J., Surini M., Catsicas S., Kawashima E., Bouras C. Age-dependent accumulation of advanced glycosylation end products in human neurons. Neurobiol Aging. 1995 Jan-Feb;16(1):69–76. doi: 10.1016/0197-4580(95)80009-g. [DOI] [PubMed] [Google Scholar]
- Li J. J., Voisin D., Quiquerez A. L., Bouras C. Differential expression of advanced glycosylation end-products in neurons of different species. Brain Res. 1994 Apr 4;641(2):285–288. doi: 10.1016/0006-8993(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Li Y. M., Mitsuhashi T., Wojciechowicz D., Shimizu N., Li J., Stitt A., He C., Banerjee D., Vlassara H. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M., Frank A., Hernanz A. Relationship of interleukin-1 beta and beta 2-microglobulin with neuropeptides in cerebrospinal fluid of patients with dementia of the Alzheimer type. J Neuroimmunol. 1993 Nov-Dec;48(2):235–240. doi: 10.1016/0165-5728(93)90197-7. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Rydel R. E. Alzheimer's disease. Amyloid ox-tox transducers. Nature. 1996 Aug 22;382(6593):674–675. doi: 10.1038/382674a0. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Benveniste E. N. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996 Aug;19(8):331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- Merrill J. E. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Dev Neurosci. 1992;14(1):1–10. doi: 10.1159/000111642. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Li Y. M., Fishbane S., Vlassara H. Depletion of reactive advanced glycation endproducts from diabetic uremic sera using a lysozyme-linked matrix. J Clin Invest. 1997 Aug 15;100(4):847–854. doi: 10.1172/JCI119600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi T., Vlassara H., Founds H. W., Li Y. M. Standardizing the immunological measurement of advanced glycation endproducts using normal human serum. J Immunol Methods. 1997 Aug 22;207(1):79–88. doi: 10.1016/s0022-1759(97)00110-5. [DOI] [PubMed] [Google Scholar]
- Mrak R. E., Sheng J. G., Griffin W. S. Glial cytokines in Alzheimer's disease: review and pathogenic implications. Hum Pathol. 1995 Aug;26(8):816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Ebato M., Sato K., Okumura K. Expression of tumour necrosis factor-alpha, -beta and interferon-gamma genes within human neuroglial tumour cells and brain specimens. Cytokine. 1994 Mar;6(2):171–180. doi: 10.1016/1043-4666(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Ransom B. R. Vertebrate glial classification, lineage, and heterogeneity. Ann N Y Acad Sci. 1991;633:19–26. doi: 10.1111/j.1749-6632.1991.tb15591.x. [DOI] [PubMed] [Google Scholar]
- Rozemuller J. M., Eikelenboom P., Stam F. C., Beyreuther K., Masters C. L. A4 protein in Alzheimer's disease: primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol. 1989 Nov;48(6):674–691. doi: 10.1097/00005072-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Saada A., Reichert F., Rotshenker S. Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the up-regulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J Cell Biol. 1996 Apr;133(1):159–167. doi: 10.1083/jcb.133.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saishoji T., Higashi T., Ikeda K., Sano H., Jinnouchi Y., Ogawa M., Horiuchi S. Advanced glycation end products stimulate plasminogen activator activity via GM-CSF in RAW 264.7 cells. Biochem Biophys Res Commun. 1995 Dec 5;217(1):278–285. doi: 10.1006/bbrc.1995.2775. [DOI] [PubMed] [Google Scholar]
- Salazar R., Brandt R., Krantz S. Expression of fructosyllysine receptors on human monocytes and monocyte-like cell lines. Biochim Biophys Acta. 1995 Apr 6;1266(1):57–63. doi: 10.1016/0167-4889(94)00221-y. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Mora R., Cao R., Yan S. D., Brett J., Ramakrishnan R., Tsang T. C., Simionescu M., Stern D. The endothelial cell binding site for advanced glycation end products consists of a complex: an integral membrane protein and a lactoferrin-like polypeptide. J Biol Chem. 1994 Apr 1;269(13):9882–9888. [PubMed] [Google Scholar]
- Sell D. R., Lane M. A., Johnson W. A., Masoro E. J., Mock O. B., Reiser K. M., Fogarty J. F., Cutler R. G., Ingram D. K., Roth G. S. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):485–490. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Yang Z., Makita Z., Radoff S., Kirstein M., Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991 Oct 1;174(4):931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Richey P. L., Taneda S., Kutty R. K., Sayre L. M., Monnier V. M., Perry G. Advanced Maillard reaction end products, free radicals, and protein oxidation in Alzheimer's disease. Ann N Y Acad Sci. 1994 Nov 17;738:447–454. doi: 10.1111/j.1749-6632.1994.tb21836.x. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Sayre L. M., Monnier V. M., Perry G. Radical AGEing in Alzheimer's disease. Trends Neurosci. 1995 Apr;18(4):172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- Vitek M. P., Bhattacharya K., Glendening J. M., Stopa E., Vlassara H., Bucala R., Manogue K., Cerami A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H. Advanced nonenzymatic tissue glycosylation: cell-mediated interactions implicated in the complications associated with diabetes and aging. Blood Purif. 1990;8(4):223–232. doi: 10.1159/000169970. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Macrophage receptor-mediated processing and regulation of advanced glycosylation endproduct (AGE)-modified proteins: role in diabetes and aging. Prog Clin Biol Res. 1989;304:205–218. [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med. 1986 Oct 1;164(4):1301–1309. doi: 10.1084/jem.164.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Bucala R. Recent progress in advanced glycation and diabetic vascular disease: role of advanced glycation end product receptors. Diabetes. 1996 Jul;45 (Suppl 3):S65–S66. doi: 10.2337/diab.45.3.s65. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Li Y. M., Imani F., Wojciechowicz D., Yang Z., Liu F. T., Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995 Sep;1(6):634–646. [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Moldawer L., Chan B. Macrophage/monocyte receptor for nonenzymatically glycosylated protein is upregulated by cachectin/tumor necrosis factor. J Clin Invest. 1989 Dec;84(6):1813–1820. doi: 10.1172/JCI114366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H. Serum advanced glycosylation end products: a new class of uremic toxins? Blood Purif. 1994;12(1):54–59. doi: 10.1159/000170145. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Striker L. J., Teichberg S., Fuh H., Li Y. M., Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996 Aug 22;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Schmidt A. M., Brett J., Godman G., Zou Y. S., Scott C. W., Caputo C., Frappier T., Smith M. A. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]







