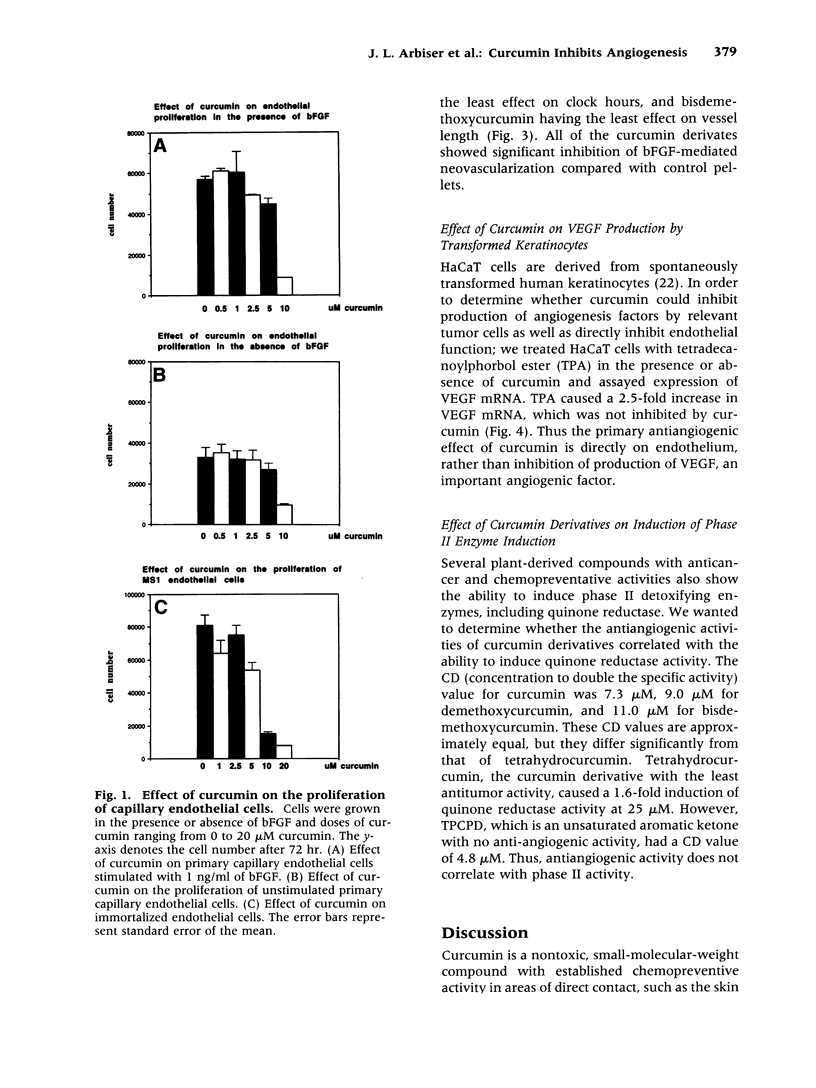
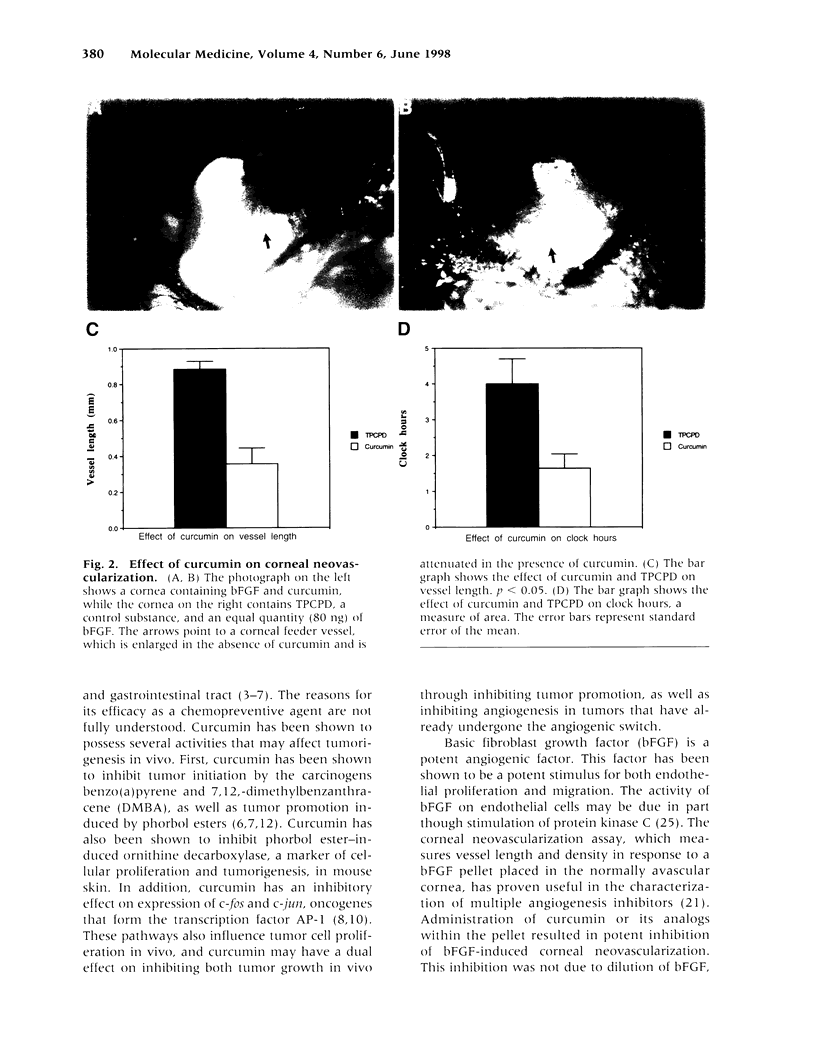
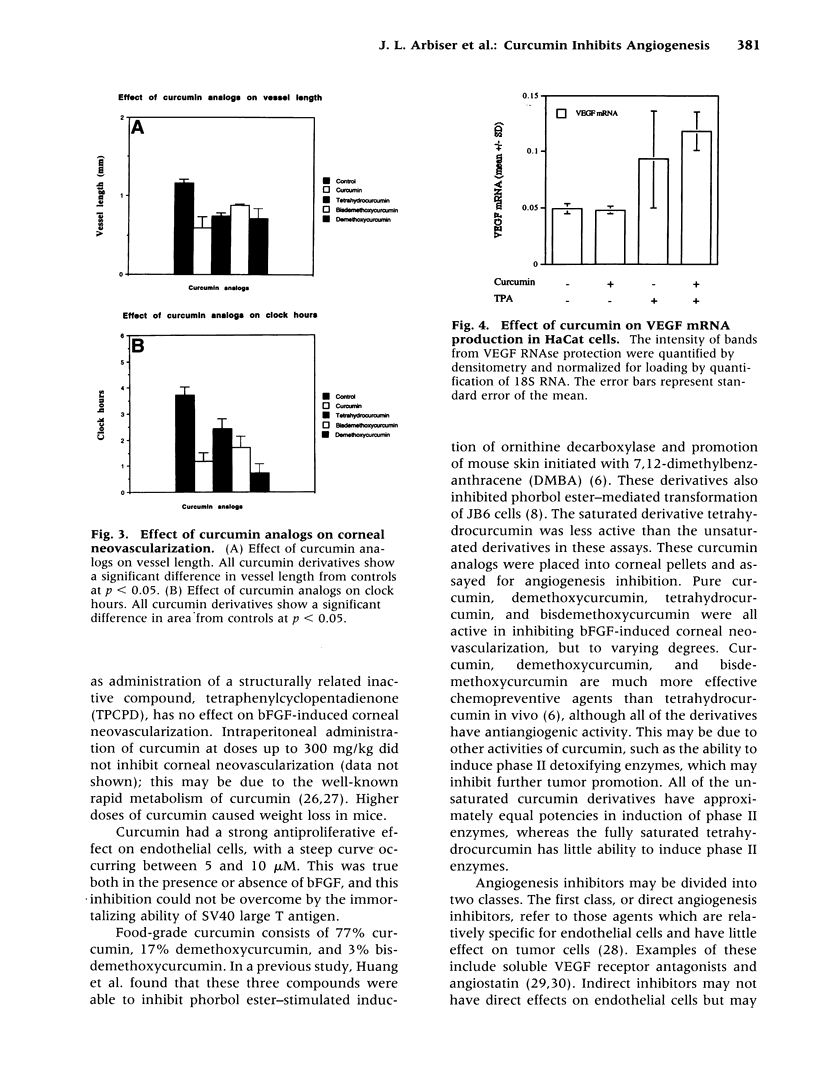
Abstract
BACKGROUND: Curcumin is a small-molecular-weight compound that is isolated from the commonly used spice turmeric. In animal models, curcumin and its derivatives have been shown to inhibit the progression of chemically induced colon and skin cancers. The genetic changes in carcinogenesis in these organs involve different genes, but curcumin is effective in preventing carcinogenesis in both organs. A possible explanation for this finding is that curcumin may inhibit angiogenesis. MATERIALS AND METHODS: Curcumin was tested for its ability to inhibit the proliferation of primary endothelial cells in the presence and absence of basic fibroblast growth factor (bFGF), as well as its ability to inhibit proliferation of an immortalized endothelial cell line. Curcumin and its derivatives were subsequently tested for their ability to inhibit bFGF-induced corneal neovascularization in the mouse cornea. Finally, curcumin was tested for its ability to inhibit phorbol ester-stimulated vascular endothelial growth factor (VEGF) mRNA production. RESULTS: Curcumin effectively inhibited endothelial cell proliferation in a dose-dependent manner. Curcumin and its derivatives demonstrated significant inhibition of bFGF-mediated corneal neovascularization in the mouse. Curcumin had no effect on phorbol ester-stimulated VEGF production. CONCLUSIONS: These results indicate that curcumin has direct antiangiogenic activity in vitro and in vivo. The activity of curcumin in inhibiting carcinogenesis in diverse organs such as the skin and colon may be mediated in part through angiogenesis inhibition.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbiser J. L., Moses M. A., Fernandez C. A., Ghiso N., Cao Y., Klauber N., Frank D., Brownlee M., Flynn E., Parangi S. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conney A. H., Lysz T., Ferraro T., Abidi T. F., Manchand P. S., Laskin J. D., Huang M. T. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Adv Enzyme Regul. 1991;31:385–396. doi: 10.1016/0065-2571(91)90025-h. [DOI] [PubMed] [Google Scholar]
- Dietrich W. F., Lander E. S., Smith J. S., Moser A. R., Gould K. A., Luongo C., Borenstein N., Dove W. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993 Nov 19;75(4):631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- Fahey J. W., Zhang Y., Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotsis T., Pepper M., Adlercreutz H., Fleischmann G., Hase T., Montesano R., Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gess B., Sandner P., Kurtz A. Differential effects of kinase inhibitors on erythropoietin and vascular endothelial growth factor gene expression in rat hepatocytes. Pflugers Arch. 1996 Jul;432(3):426–432. doi: 10.1007/s004240050154. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Hod Y. A simplified ribonuclease protection assay. Biotechniques. 1992 Dec;13(6):852–854. [PubMed] [Google Scholar]
- Huang M. T., Deschner E. E., Newmark H. L., Wang Z. Y., Ferraro T. A., Conney A. H. Effect of dietary curcumin and ascorbyl palmitate on azoxymethanol-induced colonic epithelial cell proliferation and focal areas of dysplasia. Cancer Lett. 1992 Jun 15;64(2):117–121. doi: 10.1016/0304-3835(92)90071-3. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Lou Y. R., Ma W., Newmark H. L., Reuhl K. R., Conney A. H. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994 Nov 15;54(22):5841–5847. [PubMed] [Google Scholar]
- Huang M. T., Ma W., Lu Y. P., Chang R. L., Fisher C., Manchand P. S., Newmark H. L., Conney A. H. Effects of curcumin, demethoxycurcumin, bisdemethoxycurcumin and tetrahydrocurcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion. Carcinogenesis. 1995 Oct;16(10):2493–2497. doi: 10.1093/carcin/16.10.2493. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Lee S. C., Lin J. K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. C., Mii S., Harrington E. O., Chang J. D., Mallette S., Ware J. A. Requirement for protein kinase C activation in basic fibroblast growth factor-induced human endothelial cell proliferation. Circ Res. 1995 Aug;77(2):231–238. doi: 10.1161/01.res.77.2.231. [DOI] [PubMed] [Google Scholar]
- Kenyon B. M., Voest E. E., Chen C. C., Flynn E., Folkman J., D'Amato R. J. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996 Jul;37(8):1625–1632. [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Omer C. A., Conner M. W., Anthony N. J., Davide J. P., deSolms S. J., Giuliani E. A., Gomez R. P., Graham S. L., Hamilton K. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med. 1995 Aug;1(8):792–797. doi: 10.1038/nm0895-792. [DOI] [PubMed] [Google Scholar]
- Korutla L., Cheung J. Y., Mendelsohn J., Kumar R. Inhibition of ligand-induced activation of epidermal growth factor receptor tyrosine phosphorylation by curcumin. Carcinogenesis. 1995 Aug;16(8):1741–1745. doi: 10.1093/carcin/16.8.1741. [DOI] [PubMed] [Google Scholar]
- Larcher F., Robles A. I., Duran H., Murillas R., Quintanilla M., Cano A., Conti C. J., Jorcano J. L. Up-regulation of vascular endothelial growth factor/vascular permeability factor in mouse skin carcinogenesis correlates with malignant progression state and activated H-ras expression levels. Cancer Res. 1996 Dec 1;56(23):5391–5396. [PubMed] [Google Scholar]
- Lu Y. P., Chang R. L., Lou Y. R., Huang M. T., Newmark H. L., Reuhl K. R., Conney A. H. Effect of curcumin on 12-O-tetradecanoylphorbol-13-acetate- and ultraviolet B light-induced expression of c-Jun and c-Fos in JB6 cells and in mouse epidermis. Carcinogenesis. 1994 Oct;15(10):2363–2370. doi: 10.1093/carcin/15.10.2363. [DOI] [PubMed] [Google Scholar]
- MacPhee M., Chepenik K. P., Liddell R. A., Nelson K. K., Siracusa L. D., Buchberg A. M. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 1995 Jun 16;81(6):957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- O'Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Moses M., Lane W. S., Cao Y., Sage E. H., Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994 Oct 21;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Oshima M., Dinchuk J. E., Kargman S. L., Oshima H., Hancock B., Kwong E., Trzaskos J. M., Evans J. F., Taketo M. M. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996 Nov 29;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B., Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C. V., Rivenson A., Simi B., Reddy B. S. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995 Jan 15;55(2):259–266. [PubMed] [Google Scholar]
- Rao C. V., Simi B., Reddy B. S. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993 Nov;14(11):2219–2225. doi: 10.1093/carcin/14.11.2219. [DOI] [PubMed] [Google Scholar]
- Ravindranath V., Chandrasekhara N. In vitro studies on the intestinal absorption of curcumin in rats. Toxicology. 1981;20(2-3):251–257. doi: 10.1016/0300-483x(81)90056-1. [DOI] [PubMed] [Google Scholar]
- Redston M. S., Papadopoulos N., Caldas C., Kinzler K. W., Kern S. E. Common occurrence of APC and K-ras gene mutations in the spectrum of colitis-associated neoplasias. Gastroenterology. 1995 Feb;108(2):383–392. doi: 10.1016/0016-5085(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Singh S., Aggarwal B. B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995 Oct 20;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Stoner G. D., Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:169–180. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- Su L. K., Kinzler K. W., Vogelstein B., Preisinger A. C., Moser A. R., Luongo C., Gould K. A., Dove W. F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992 May 1;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Wahlström B., Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978 Aug;43(2):86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]




