Abstract
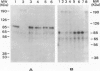
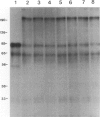
BACKGROUND: Acquired C1-inhibitor (C1-inh) deficiency is usually associated with the presence of circulating C1-inh autoantibodies. These autoantibodies have been shown previously to bind to two synthetic peptides corresponding to C1-inh amino acid residues 438-449 (peptide 2) and 448-459 (peptide 3) but not to peptide 1 (residues 428-440). MATERIALS AND METHODS: Affinity-purified C1-inh autoantibodies from two patients with acquired C1-inh deficiency were studied for their effects on the inhibition of C1s activity by C1-inh using SDS-PAGE and hydrolysis of a synthetic ester. RESULTS: Functional studies confirmed that the anti-C1-inh autoantibodies abrogated C1-inh activity, and their maximum effect was produced when the concentrations of C1-inh and autoantibody were approximately equimolar. The autoantibodies prevent the formation of the C1s-C1-inh complex, but they do not dissociate the preformed complex, suggesting that the autoantibodies act prior to the formation of the enzyme-inhibitor complex. In the presence of autoantibodies, C1s cleaves C1-inh, and a stable covalent bond between C1s and C1-inh does not form. Peptides 2 and 3, but not peptide 1 inhibited autoantibody activity, thus C1-inh inhibitory activity for C1s was expressed fully. CONCLUSIONS: Our data indicate that the anti-C1-inh autoantibodies convert C1-inh to a substrate by preventing the formation of the stable covalent protease-serpin complex. The data also suggest a possible therapeutic use for peptides 2 and 3 or their derivatives in the management of patients with type II acquired angioedema (AAE).
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsenz J., Bork K., Loos M. Autoantibody-mediated acquired deficiency of C1 inhibitor. N Engl J Med. 1987 May 28;316(22):1360–1366. doi: 10.1056/NEJM198705283162202. [DOI] [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Silverton E. W., Davies D. R. Refined crystal structure of gamma-chymotrypsin at 1.9 A resolution. Comparison with other pancreatic serine proteases. J Mol Biol. 1981 Jun 5;148(4):449–479. doi: 10.1016/0022-2836(81)90186-8. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Aulak K., Parad R. B., Stecklein H. P., Eldering E., Hack C. E., Kramer J., Strunk R. C., Bissler J., Rosen F. S. C1 inhibitor hinge region mutations produce dysfunction by different mechanisms. Nat Genet. 1992 Aug;1(5):354–358. doi: 10.1038/ng0892-354. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol. 1988;6:595–628. doi: 10.1146/annurev.iy.06.040188.003115. [DOI] [PubMed] [Google Scholar]
- Fothergill J., Kemp G., Paton N., Carter P., Gray P. The structures of human C1r and C1s and their relationship to other serine proteases. Behring Inst Mitt. 1989 Jul;(84):72–79. [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A. Human C1 inhibitor: improved isolation and preliminary structural characterization. Biochemistry. 1983 Oct 11;22(21):5001–5007. doi: 10.1021/bi00290a019. [DOI] [PubMed] [Google Scholar]
- He S., Sim R. B., Whaley K. A secondary C1s interaction site on C1-inhibitor is essential for formation of a stable enzyme-inhibitor complex. FEBS Lett. 1997 Mar 17;405(1):42–46. doi: 10.1016/s0014-5793(96)01529-3. [DOI] [PubMed] [Google Scholar]
- He S., Tsang S., North J., Chohan N., Sim R. B., Whaley K. Epitope mapping of C1 inhibitor autoantibodies from patients with acquired C1 inhibitor deficiency. J Immunol. 1996 Mar 1;156(5):2009–2013. [PubMed] [Google Scholar]
- Ishizaki E., Yoshioka Y., Mori Y., Koyama J. Isolation of two forms of activated C1s, a subcomponent of the first component of rabbit complement. J Biochem. 1976 Dec;80(6):1423–1427. doi: 10.1093/oxfordjournals.jbchem.a131415. [DOI] [PubMed] [Google Scholar]
- Jackson J., Sim R. B., Whaley K., Feighery C. Autoantibody facilitated cleavage of C1-inhibitor in autoimmune angioedema. J Clin Invest. 1989 Feb;83(2):698–707. doi: 10.1172/JCI113934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J., Sim R. B., Whelan A., Feighery C. An IgG autoantibody which inactivates C1-inhibitor. Nature. 1986 Oct 23;323(6090):722–724. doi: 10.1038/323722a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandle R., Baron C., Roux E., Sundel R., Gelfand J., Aulak K., Davis A. E., 3rd, Rosen F. S., Bing D. H. Acquired C1 inhibitor deficiency as a result of an autoantibody to the reactive center region of C1 inhibitor. J Immunol. 1994 May 1;152(9):4680–4685. [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mori Y., Ueda E., Takeuchi T., Taniuchi S., Koyama J. Proteolytic cleavage of an activated subcomponent of the first component of rabbit complement, C1s. J Biochem. 1980 Jun;87(6):1757–1763. doi: 10.1093/oxfordjournals.jbchem.a132920. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Smith K. F., Amatayakul S., Ashford D., Rademacher T. W., Dwek R. A., Lachmann P. J., Harrison R. A. Two-domain structure of the native and reactive centre cleaved forms of C1 inhibitor of human complement by neutron scattering. J Mol Biol. 1990 Aug 5;214(3):751–763. doi: 10.1016/0022-2836(90)90290-3. [DOI] [PubMed] [Google Scholar]
- Pilatte Y., Hammer C. H., Frank M. M., Fries L. F. A new simplified procedure for C1 inhibitor purification. A novel use for jacalin-agarose. J Immunol Methods. 1989 Jun 2;120(1):37–43. doi: 10.1016/0022-1759(89)90286-x. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. C1 inhibitor-dependent dissociation of human complement component C1 bound to immune complexes. Biochem J. 1979 Jun 1;179(3):449–457. doi: 10.1042/bj1790449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B. The human complement system serine proteases C1r and C1s and their proenzymes. Methods Enzymol. 1981;80(Pt 100):26–42. doi: 10.1016/s0076-6879(81)80006-7. [DOI] [PubMed] [Google Scholar]
- Skriver K., Wikoff W. R., Patston P. A., Tausk F., Schapira M., Kaplan A. P., Bock S. C. Substrate properties of C1 inhibitor Ma (alanine 434----glutamic acid). Genetic and structural evidence suggesting that the P12-region contains critical determinants of serine protease inhibitor/substrate status. J Biol Chem. 1991 May 15;266(14):9216–9221. [PubMed] [Google Scholar]
- Stein P. E., Carrell R. W. What do dysfunctional serpins tell us about molecular mobility and disease? Nat Struct Biol. 1995 Feb;2(2):96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace E. M., Perkins S. J., Sim R. B., Willis A. C., Feighery C., Jackson J. Degradation of C1-inhibitor by plasmin: implications for the control of inflammatory processes. Mol Med. 1997 Jun;3(6):385–396. [PMC free article] [PubMed] [Google Scholar]
- Ziccardi R. J. Demonstration of the interaction of native C1 with monomeric immunoglobulins and C1 inhibitor. J Immunol. 1985 Apr;134(4):2559–2563. [PubMed] [Google Scholar]