Abstract
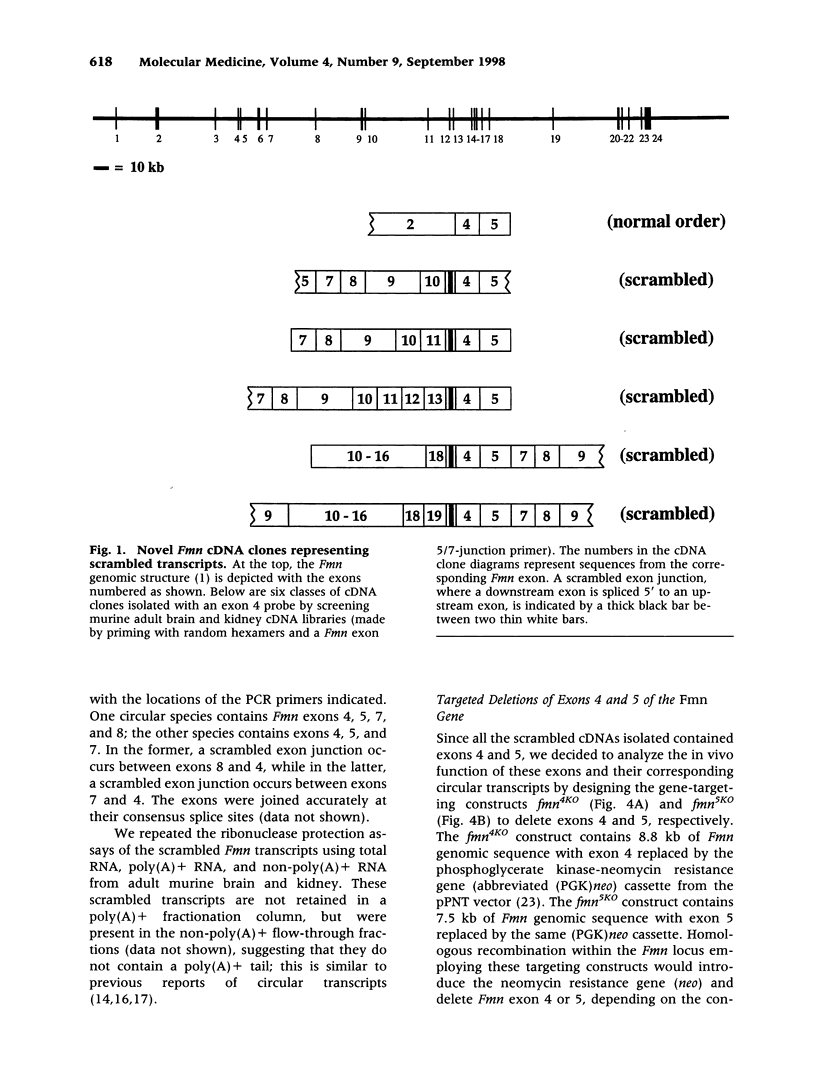
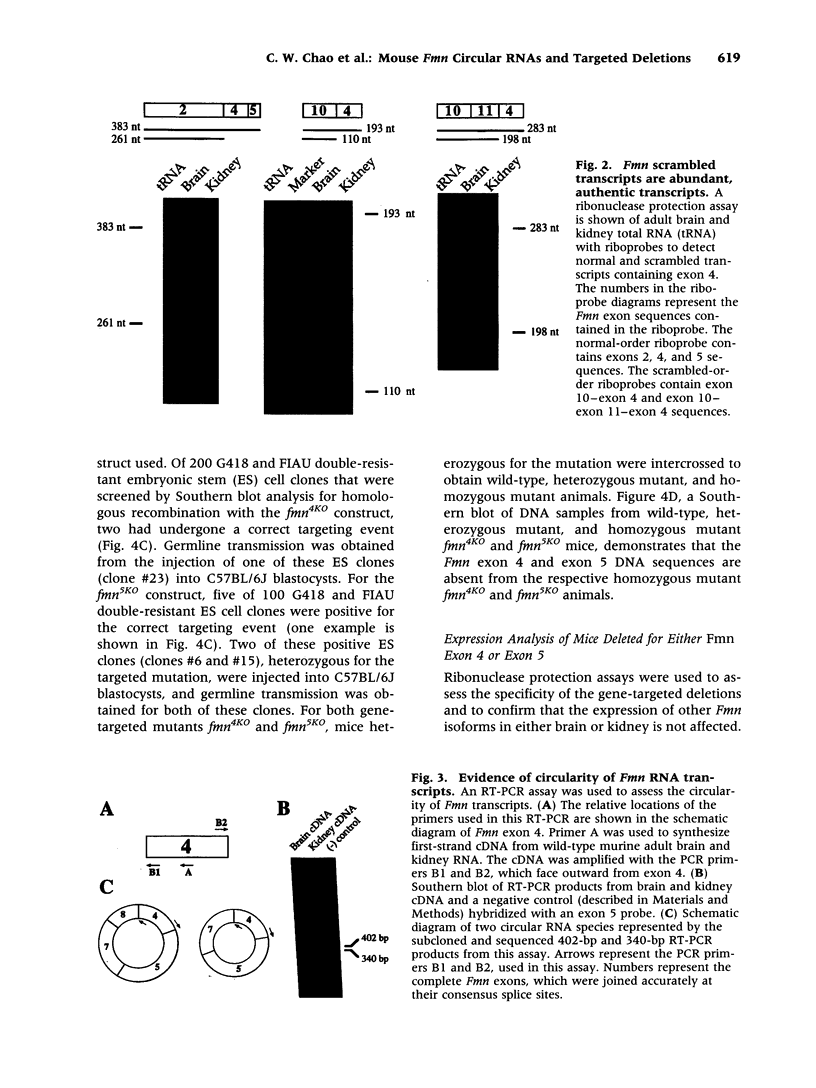
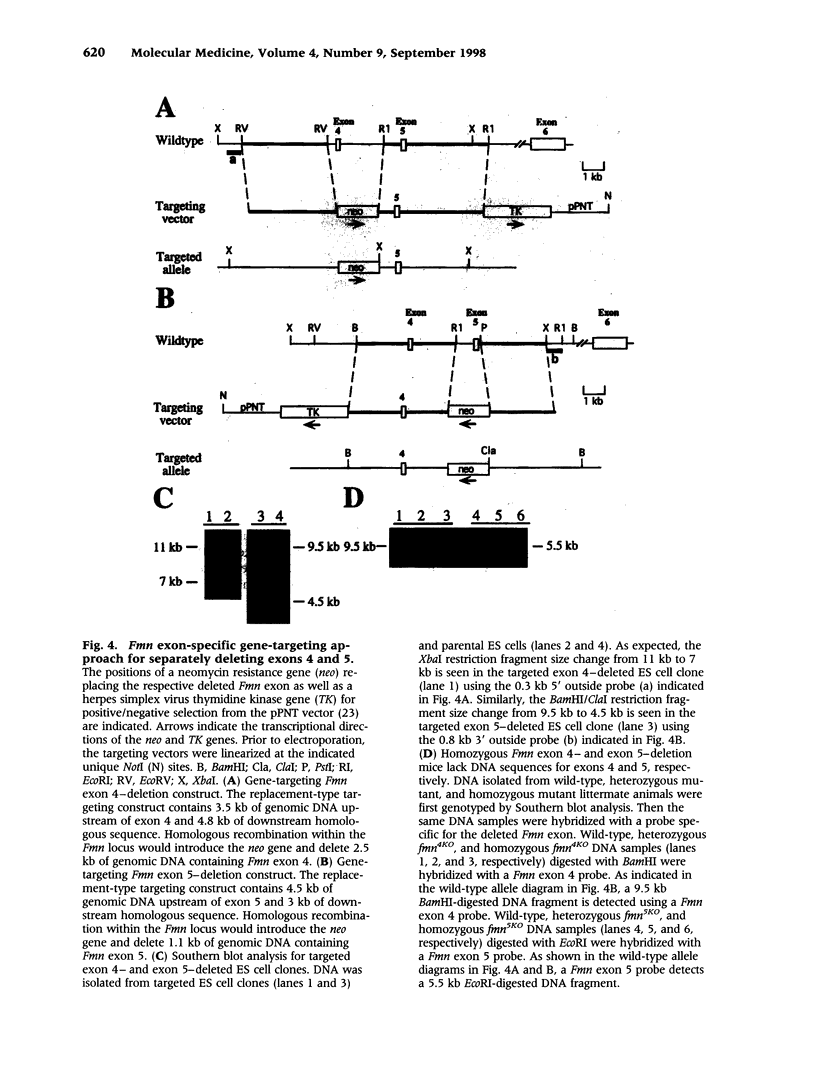
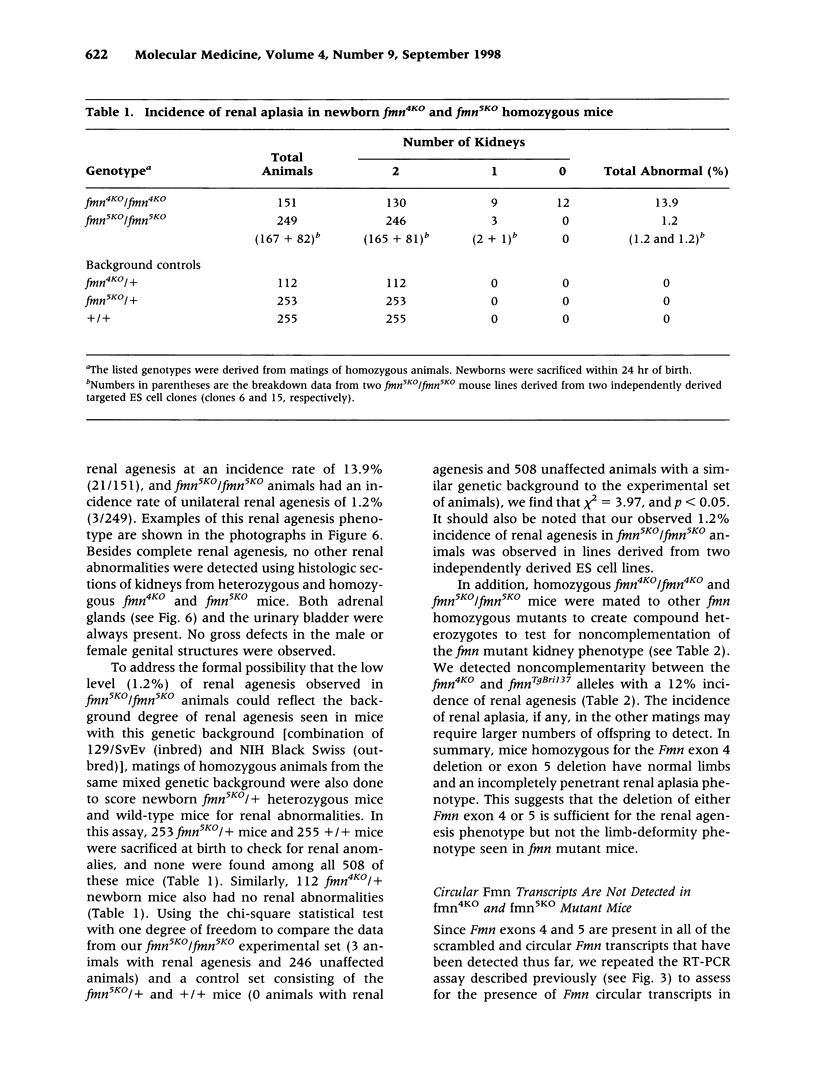
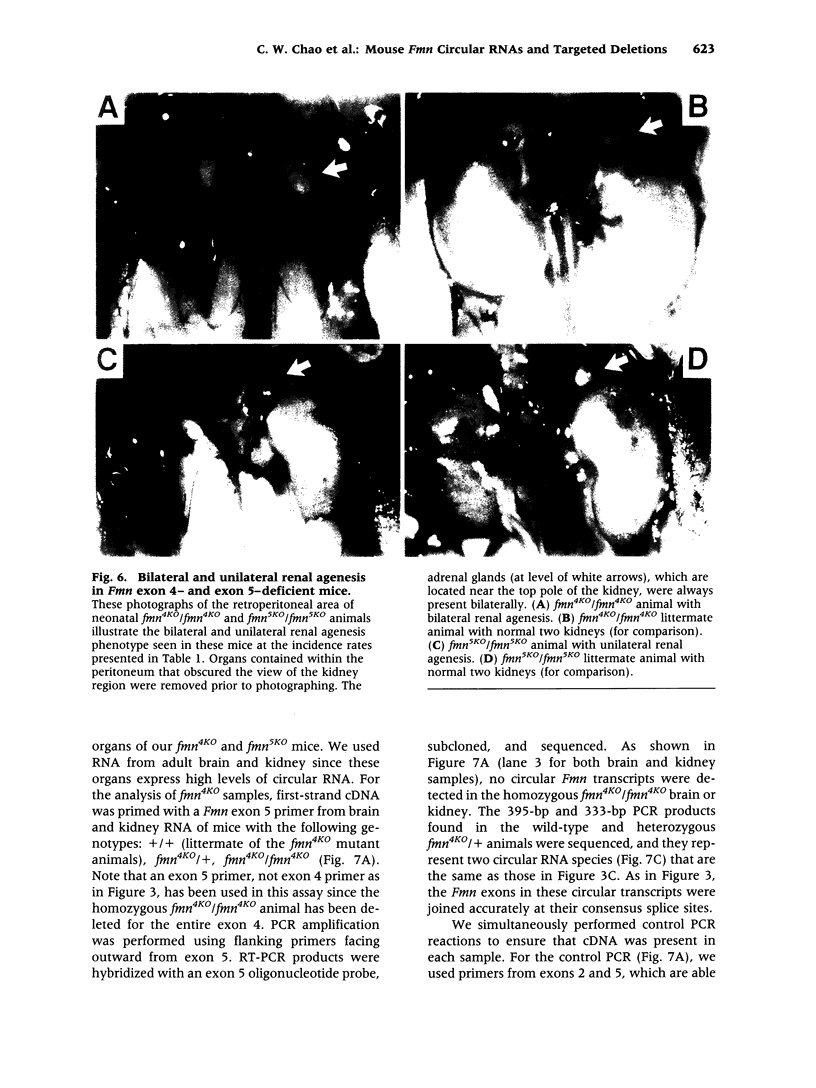
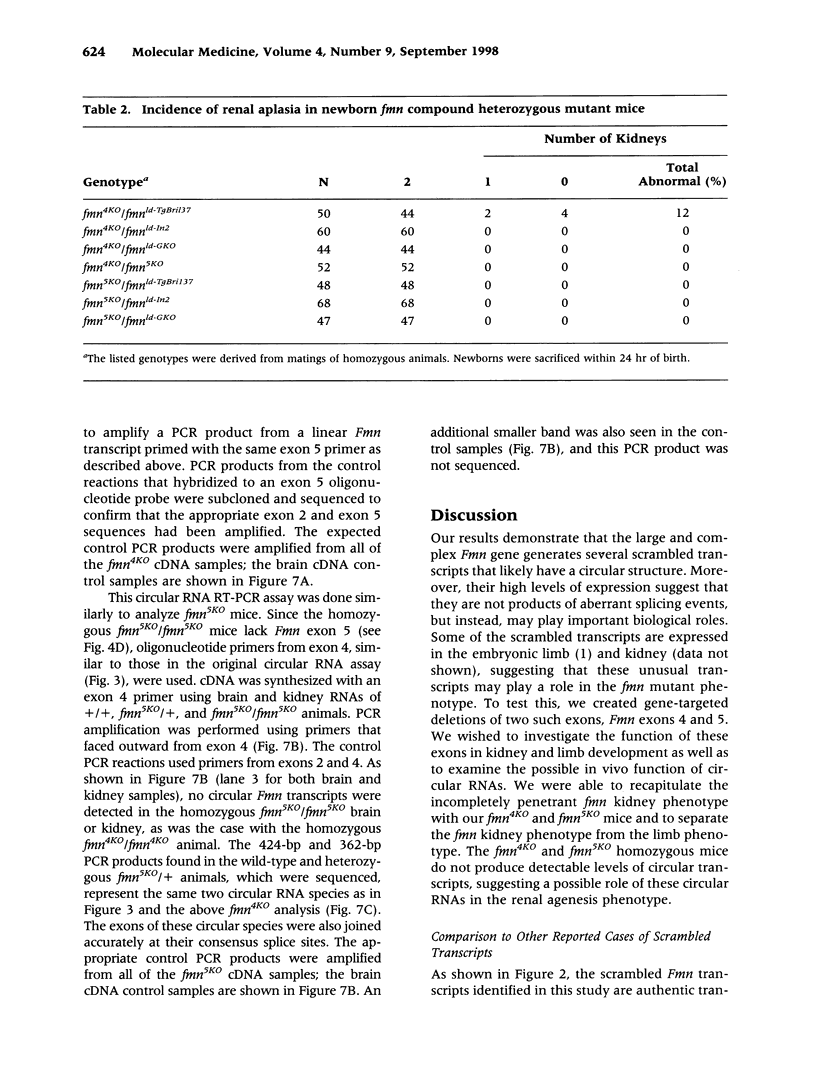
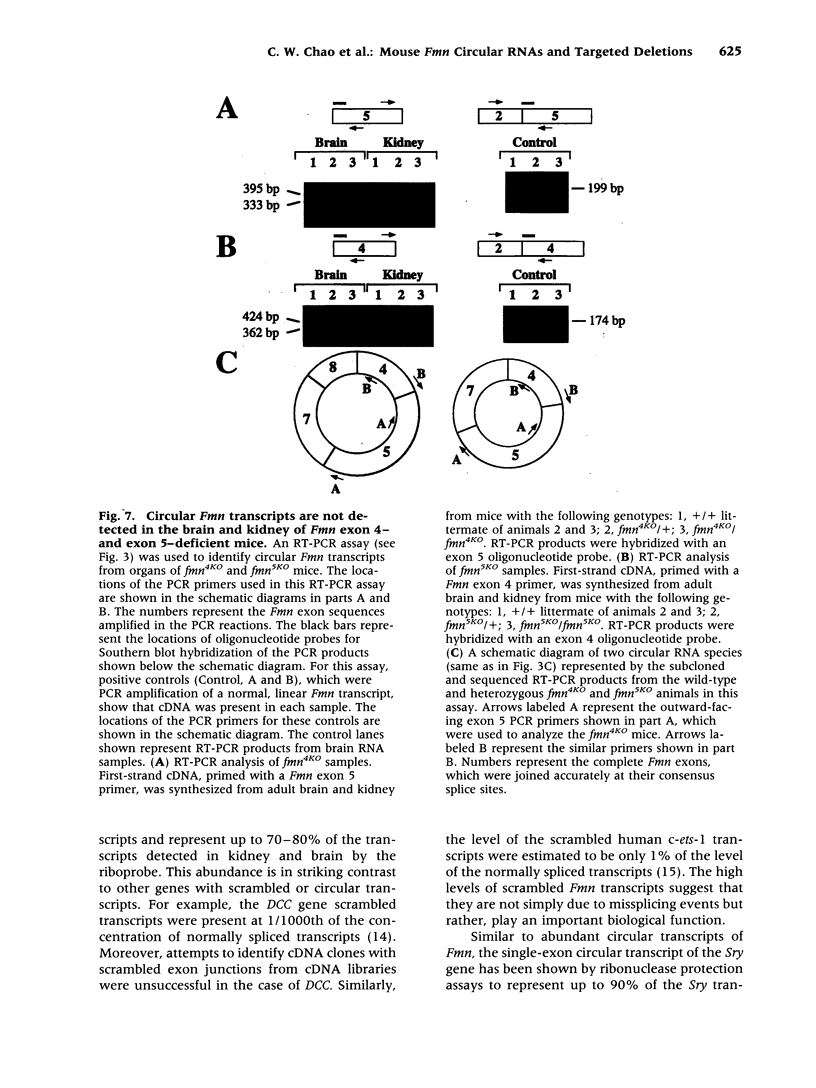
BACKGROUND: Mutations in the mouse formin (Fmn) gene result in limb deformities and incompletely penetrant renal aplasia. A molecular genetic approach was taken to characterize novel circular RNAs from the Fmn gene and to understand the developmental effects of gene-targeted mutations. MATERIALS AND METHODS: RT-PCR and ribonuclease protection analyses were done to characterize the circular RNA transcripts arising from the Fmn gene. Two lines of mice with gene-targeted deletions of specific Fmn exons, namely exon 4 or exon 5, were generated and analyzed. RESULTS: In our analysis of formin cDNAs, we discovered a class of transcripts in which the exon order is reversed such that downstream exons are joined to the acceptor end of a specific exon that lies 5' to them in the genome. RT-PCR and ribonuclease protection analyses indicate that these transcripts are circular and are the major transcripts arising from this locus in adult brain and kidney. To gain insight into the biological function of these transcripts, we have systematically deleted the relevant exons using gene-targeted homologous recombination. The resulting mice fail to produce circular transcripts, but appear to produce normal amounts of the linear RNA isoforms from this locus. While these deficient mice have normal limbs, they display variably penetrant renal aplasia characteristic of other mutant formin alleles. CONCLUSIONS: Our results demonstrate novel circular transcripts arising from the Fmn gene. Moreover, their high levels of expression suggest that they are not products of aberrant splicing events, but instead, may play important biological roles. Mice with gene-targeted deletions of Fmn exons 4 or 5 lack these circular transcripts and have an incompletely penetrant renal agenesis phenotype. While the biologic function of circular Fmn RNA transcripts is not entirely known, our work suggests their possible involvement in kidney development.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailleul B. During in vivo maturation of eukaryotic nuclear mRNA, splicing yields excised exon circles. Nucleic Acids Res. 1996 Mar 15;24(6):1015–1019. doi: 10.1093/nar/24.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B., Swain A., Nicolis S., Hacker A., Walter M., Koopman P., Goodfellow P., Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993 Jun 4;73(5):1019–1030. doi: 10.1016/0092-8674(93)90279-y. [DOI] [PubMed] [Google Scholar]
- Chan D. C., Leder P. Genetic evidence that formins function within the nucleus. J Biol Chem. 1996 Sep 20;271(38):23472–23477. doi: 10.1074/jbc.271.38.23472. [DOI] [PubMed] [Google Scholar]
- Chan D. C., Wynshaw-Boris A., Leder P. Formin isoforms are differentially expressed in the mouse embryo and are required for normal expression of fgf-4 and shh in the limb bud. Development. 1995 Oct;121(10):3151–3162. doi: 10.1242/dev.121.10.3151. [DOI] [PubMed] [Google Scholar]
- Cocquerelle C., Mascrez B., Hétuin D., Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993 Jan;7(1):155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- Deng C. X., Wynshaw-Boris A., Shen M. M., Daugherty C., Ornitz D. M., Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994 Dec 15;8(24):3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- Deng C., Wynshaw-Boris A., Zhou F., Kuo A., Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996 Mar 22;84(6):911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Haramis A. G., Brown J. M., Zeller R. The limb deformity mutation disrupts the SHH/FGF-4 feedback loop and regulation of 5' HoxD genes during limb pattern formation. Development. 1995 Dec;121(12):4237–4245. doi: 10.1242/dev.121.12.4237. [DOI] [PubMed] [Google Scholar]
- Jackson-Grusby L., Kuo A., Leder P. A variant limb deformity transcript expressed in the embryonic mouse limb defines a novel formin. Genes Dev. 1992 Jan;6(1):29–37. doi: 10.1101/gad.6.1.29. [DOI] [PubMed] [Google Scholar]
- Maas R., Elfering S., Glaser T., Jepeal L. Deficient outgrowth of the ureteric bud underlies the renal agenesis phenotype in mice manifesting the limb deformity (ld) mutation. Dev Dyn. 1994 Mar;199(3):214–228. doi: 10.1002/aja.1001990306. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Cho K. R., Fearon E. R., Kern S. E., Ruppert J. M., Oliner J. D., Kinzler K. W., Vogelstein B. Scrambled exons. Cell. 1991 Feb 8;64(3):607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Chan D. C., Leder P. The mouse formin (Fmn) gene: genomic structure, novel exons, and genetic mapping. Genomics. 1997 Feb 1;39(3):303–311. doi: 10.1006/geno.1996.4519. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Generoso W. M., Russell L. B., Cain K. T., Cacheiro N. L., Bultman S. J., Selby P. B., Dickinson M. E., Hogan B. L., Rutledge J. C. Molecular and genetic characterization of a radiation-induced structural rearrangement in mouse chromosome 2 causing mutations at the limb deformity and agouti loci. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2588–2592. doi: 10.1073/pnas.87.7.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Maas R. L., Zeller R., Vogt T. F., Leder P. 'Formins': proteins deduced from the alternative transcripts of the limb deformity gene. Nature. 1990 Aug 30;346(6287):850–853. doi: 10.1038/346850a0. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Stewart T. A., Davis L. G., D'Eustachio P., Leder P. An inherited limb deformity created by insertional mutagenesis in a transgenic mouse. Nature. 1985 Nov 7;318(6041):36–40. doi: 10.1038/318036a0. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Ryan G., Deng C. X., Chan D. C., Jackson-Grusby L., Larson D., Dunmore J. H., Leder P. The role of a single formin isoform in the limb and renal phenotypes of limb deformity. Mol Med. 1997 Jun;3(6):372–384. [PMC free article] [PubMed] [Google Scholar]
- Zaphiropoulos P. G. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6536–6541. doi: 10.1073/pnas.93.13.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R., Jackson-Grusby L., Leder P. The limb deformity gene is required for apical ectodermal ridge differentiation and anteroposterior limb pattern formation. Genes Dev. 1989 Oct;3(10):1481–1492. doi: 10.1101/gad.3.10.1481. [DOI] [PubMed] [Google Scholar]