Abstract
BACKGROUND: There are two fundamental forms of cell death: apoptosis and necrosis. Molecular studies of cell death thus far favor a model in which apoptosis and necrosis share very few molecular regulators. It appears that apoptotic processes triggered by a variety of stimuli converge on the activation of a member of the caspase family, such as caspase 3, which leads to the execution of apoptosis. It has been suggested that blocking of caspase activation in an apoptotic process may divert cell death to a necrotic demise, suggesting that apoptosis and necrosis may share some upstream events. Activation of caspase is preceded by the release of mitochondrial cytochrome C. MATERIALS AND METHODS: We first studied cell death induced by beta-lapachone by MTT and colony-formation assay. To determine whether the cell death induced by beta-lapachone occurs through necrosis or apoptosis, we used the PI staining procedure to determine the sub-G1 fraction and the Annexin-V staining for externalization of phophatidylserine. We next compared the release of mitochondrial cytochrome C in apoptosis and necrosis. Mitochondrial cytochrome C was determined by Western blot analysis. To investigate changes in mitochondria that resulted in cytochrome C release, the mitochondrial membrane potential (delta psi) was analyzed by the accumulation of rhodamine 123, a membrane-permeant cationic fluorescent dye. The activation of caspase in apoptosis and necrosis were measured by using a profluorescent substrate for caspase-like proteases, PhiPhiLuxG6D2. RESULTS: beta-lapachone induced cell death in a spectrum of human carcinoma cells, including nonproliferating cells. It induced apoptosis in human ovary, colon, and lung cancer cells, and necrotic cell death in four human breast cancer cell lines. Mitochondrial cytochrome C release was found in both apoptosis and necrosis. This cytochrome C release occurred shortly after beta-lapachone treatment when cells were fully viable by trypan blue exclusion and MTT assay, suggesting that cytochrome C release is an early event in beta-lapachone induced apoptosis as well as necrosis. The mitochondrial cytochrome C release induced by beta-lapachone is associated with a decrease in mitochondrial transmembrane potential (delta psi). There was activation of caspase 3 in apoptotic cell death, but not in necrotic cell death. This lack of activation of CPP 32 in human breast cancer cells is consistent with the necrotic cell death induced by beta-lapachone as determined by absence of sub-G1 fraction, externalization of phosphatidylserine. CONCLUSIONS: beta-lapachone induces either apoptotic or necrotic cell death in a variety of human carcinoma cells including ovary, colon, lung, prostate, and breast, suggesting a wide spectrum of anti-cancer activity in vitro. Both apoptotic and necrotic cell death induced by beta-lapachone are preceded by a rapid release of cytochrome C, followed by the activation of caspase 3 in apoptotic cell death but not in necrotic cell death. Our results suggest that beta-lapachone is a potential anti-cancer drug acting on the mitochondrial cytochrome C-caspase pathway, and that cytochrome C is involved in the early phase of necrosis.
Full text
PDF


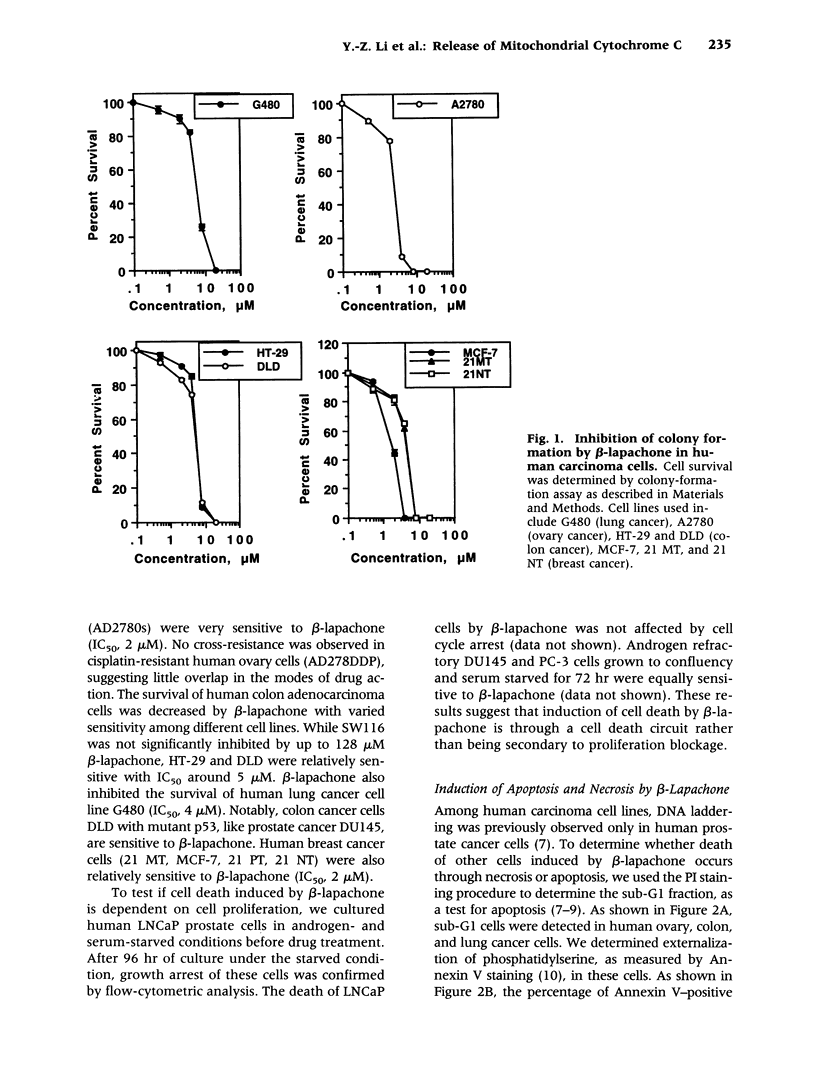
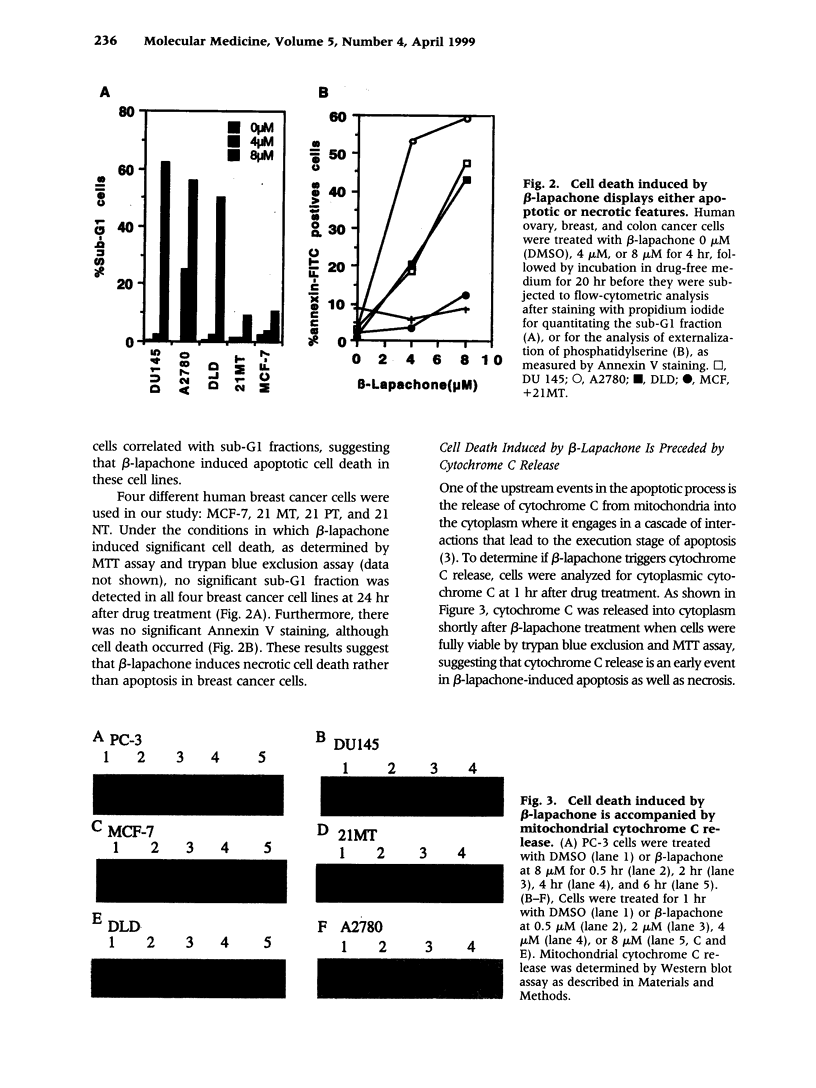
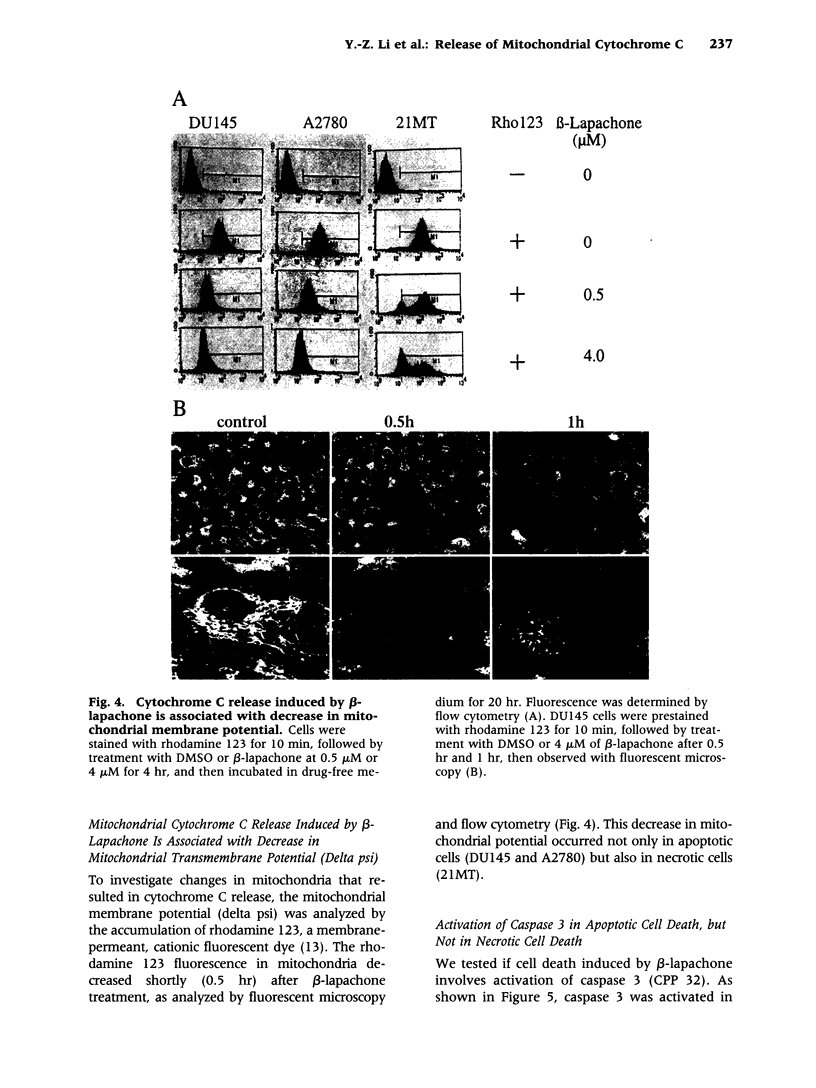
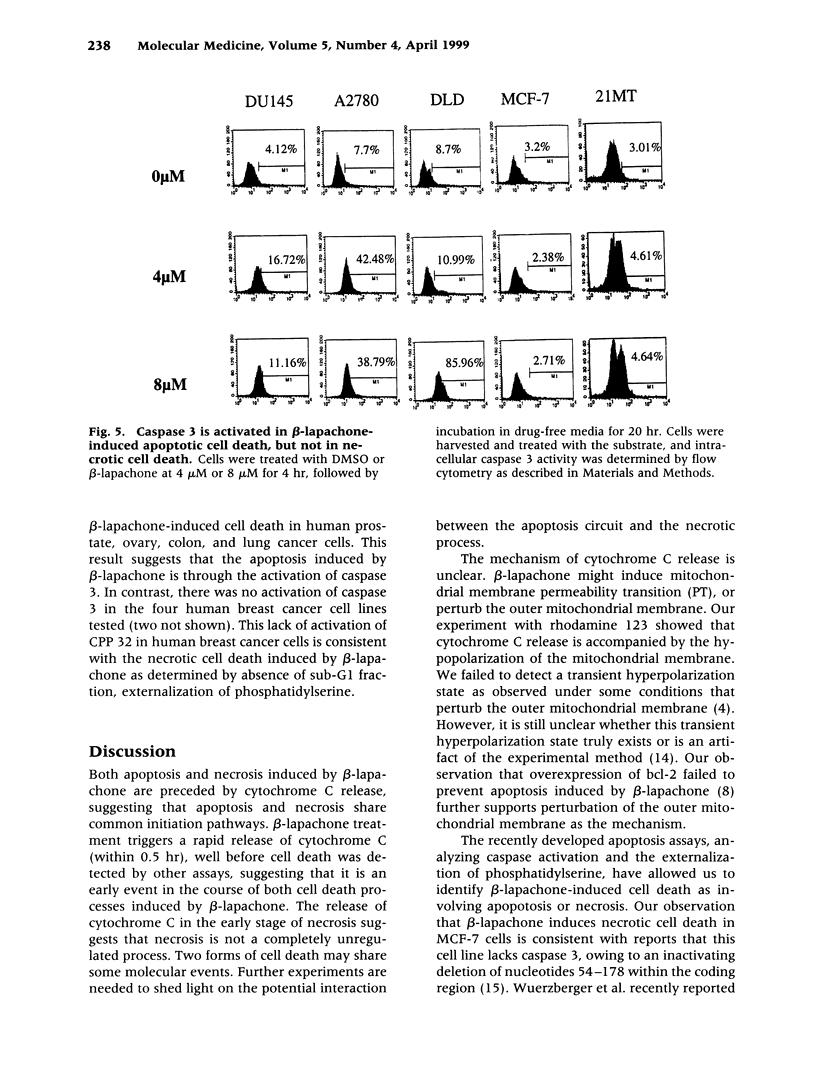

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998 Aug 28;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Jänicke R. U., Ng P., Sprengart M. L., Porter A. G. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998 Jun 19;273(25):15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- Li C. J., Friedman D. J., Wang C., Metelev V., Pardee A. B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995 Apr 21;268(5209):429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- Li C. J., Wang C., Pardee A. B. Induction of apoptosis by beta-lapachone in human prostate cancer cells. Cancer Res. 1995 Sep 1;55(17):3712–3715. [PubMed] [Google Scholar]
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996 Jul 12;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Métivier D., Dallaporta B., Zamzami N., Larochette N., Susin S. A., Marzo I., Kroemer G. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol Lett. 1998 Apr;61(2-3):157–163. doi: 10.1016/s0165-2478(98)00013-3. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Packard B. Z., Toptygin D. D., Komoriya A., Brand L. Profluorescent protease substrates: intramolecular dimers described by the exciton model. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11640–11645. doi: 10.1073/pnas.93.21.11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchon S. M., Wuerzberger S., Frydman B., Witiak D. T., Hutson P., Church D. R., Wilding G., Boothman D. A. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53-independent response. Cancer Res. 1995 Sep 1;55(17):3706–3711. [PMC free article] [PubMed] [Google Scholar]
- Raffray M., Cohen G. M. Apoptosis and necrosis in toxicology: a continuum or distinct modes of cell death? Pharmacol Ther. 1997 Sep;75(3):153–177. doi: 10.1016/s0163-7258(97)00037-5. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G., Chandel N. S., Williamson E. K., Schumacker P. T., Thompson C. B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997 Nov 28;91(5):627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Wuerzberger S. M., Pink J. J., Planchon S. M., Byers K. L., Bornmann W. G., Boothman D. A. Induction of apoptosis in MCF-7:WS8 breast cancer cells by beta-lapachone. Cancer Res. 1998 May 1;58(9):1876–1885. [PubMed] [Google Scholar]
- Zhang G., Gurtu V., Kain S. R., Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques. 1997 Sep;23(3):525–531. doi: 10.2144/97233pf01. [DOI] [PubMed] [Google Scholar]
- Zou H., Henzel W. J., Liu X., Lutschg A., Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997 Aug 8;90(3):405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]