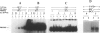Abstract
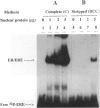
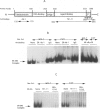
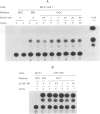
BACKGROUND: The receptor (ER) for estrogen (E2) is routinely assayed as a marker to determine the feasibility of anti-hormone therapy against breast cancer because ER-positive (ER+) tumors are much more likely to respond to anti-hormone therapy than are ER-negative (ER-). However 40% of ER+ breast cancer patients do not respond to anti-hormone therapy. We suggest that this unpredictability of therapeutic responses lies in the current ER assays, which measure only an initial component of the E2-responsive pathway, and that the difference depends upon altered downstream processes. We propose a functional criterion that subclassifies breast cancers on the basis of specific binding of ER to its cognate DNA sequence, the estrogen response element (ERE). MATERIALS AND METHODS: ER was identified in breast cancer cell lines by immunofluorescence assay, Western blot analysis, identification of ER-specific mRNA, and by interaction of the ER-ERE complex with three different ER-specific antibodies. ER-ERE complex formation was measured by electrophoretic mobility shift assay (EMSA). Transactivation of the E2-responsive gene was studied by transfection of cells with fusion gene construct with the promoter-containing ERE sequence and assay of reporter gene activity in the cell extracts. RESULTS: The growth of ER+ T47D cells was sensitive to tamoxifen, ICI-182,780, and ethynyl estradiol (EE2), whereas another ER+ breast cancer cell line, 21 PT, was resistant to these compounds. The estrogen receptor (ER) in the nuclear extracts of MCF-7 and T47D demonstrated hormone-dependent interaction with the response element (ERE) and also downstream transactivation of the E2-responsive PS2 promoter. But in the 21 PT cell line that was designated as ER- on the basis of ligand-binding assay and was found to be ER+ by all the other ER assays, ER-ERE interaction and PS2 promoter transactivation were independent of hormone. CONCLUSIONS: On the basis of the downstream functional assay of ER interaction with ERE, ER+ breast tumor cells can be subclassified into two categories. The first is E2-dependent (ERd+) and these cells should respond to anti-hormone therapy. The second type of ER interacts with ERE independent of E2 (ERi+) and constitutively transactivates responsive genes. It is predicted that the latter type of breast cancers will not respond to antihormone therapy.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan G. F., Leng X., Tsai S. Y., Weigel N. L., Edwards D. P., Tsai M. J., O'Malley B. W. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992 Sep 25;267(27):19513–19520. [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Berry M., Nunez A. M., Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D. K., Ahlers C. M., Dezube B. J., Pardee A. B. Cooperative inhibition of NF-kappa B and Tat-induced superactivation of human immunodeficiency virus type 1 long terminal repeat. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11044–11048. doi: 10.1073/pnas.90.23.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D. K., Salas T. R., Wang F., Ahlers C. M., Dezube B. J., Pardee A. B. A Tat-induced auto-up-regulatory loop for superactivation of the human immunodeficiency virus type 1 promoter. J Virol. 1995 Dec;69(12):7437–7444. doi: 10.1128/jvi.69.12.7437-7444.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. Estrogen receptor molecular biology. Hematol Oncol Clin North Am. 1994 Feb;8(1):101–112. [PubMed] [Google Scholar]
- Dauvois S., Danielian P. S., White R., Parker M. G. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua S. A., Chamness G. C., McGuire W. L. Estrogen receptor mutations in breast cancer. J Cell Biochem. 1993 Feb;51(2):135–139. doi: 10.1002/jcb.240510204. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., Chamness G. C., Tandon A. K., McDonnell D. P., Nawaz Z., O'Malley B. W., McGuire W. L. Variant human breast tumor estrogen receptor with constitutive transcriptional activity. Cancer Res. 1991 Jan 1;51(1):105–109. [PubMed] [Google Scholar]
- Furlow J. D., Murdoch F. E., Gorski J. High affinity binding of the estrogen receptor to a DNA response element does not require homodimer formation or estrogen. J Biol Chem. 1993 Jun 15;268(17):12519–12525. [PubMed] [Google Scholar]
- Gotteland M., Desauty G., Delarue J. C., Liu L., May E. Human estrogen receptor messenger RNA variants in both normal and tumor breast tissues. Mol Cell Endocrinol. 1995 Jul;112(1):1–13. doi: 10.1016/0303-7207(95)03576-s. [DOI] [PubMed] [Google Scholar]
- Halachmi S., Marden E., Martin G., MacKay H., Abbondanza C., Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994 Jun 3;264(5164):1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- Hanstein B., Eckner R., DiRenzo J., Halachmi S., Liu H., Searcy B., Kurokawa R., Brown M. p300 is a component of an estrogen receptor coactivator complex. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden A., Müller V., Jensen E. V. A new interpretation of antiestrogen action. Ann N Y Acad Sci. 1995 Jun 12;761:109–120. doi: 10.1111/j.1749-6632.1995.tb31373.x. [DOI] [PubMed] [Google Scholar]
- Jensen E. V. Hormone dependency of breast cancer. Cancer. 1981 May 15;47(10):2319–2326. doi: 10.1002/1097-0142(19810515)47:10<2319::aid-cncr2820471002>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Jensen E. V. Steroid hormones, receptors, and antagonists. Ann N Y Acad Sci. 1996 Apr 30;784:1–17. doi: 10.1111/j.1749-6632.1996.tb16223.x. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Greene G. L., Clark J. H., Tsai M. J., O'Malley B. W. Specific binding of estrogen receptor to the estrogen response element. Mol Cell Biol. 1989 Jan;9(1):43–49. doi: 10.1128/mcb.9.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W. L., Weis K. E., Katzenellenbogen B. S. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol Cell Biol. 1995 Apr;15(4):1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G. G., Gustafsson J. A. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997 Jun 23;410(1):87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Leygue E. R., Watson P. H., Murphy L. C. Estrogen receptor variants in normal human mammary tissue. J Natl Cancer Inst. 1996 Mar 6;88(5):284–290. doi: 10.1093/jnci/88.5.284. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Liu Z., Brattain M. G., Appert H. Differential display of reticulocalbin in the highly invasive cell line, MDA-MB-435, versus the poorly invasive cell line, MCF-7. Biochem Biophys Res Commun. 1997 Feb 13;231(2):283–289. doi: 10.1006/bbrc.1997.6083. [DOI] [PubMed] [Google Scholar]
- Martin K. J., Kwan C. P., O'Hare M. J., Pardee A. B., Sager R. Identification and verification of differential display cDNAs using gene-specific primers and hybridization arrays. Biotechniques. 1998 Jun;24(6):1018–1026. doi: 10.2144/98246cr01. [DOI] [PubMed] [Google Scholar]
- Martin K. J., Kwan C. P., Sager R. A direct-sequencing-based strategy for identifying and cloning cDNAs from differential display gels. Methods Mol Biol. 1997;85:77–85. doi: 10.1385/0-89603-489-5:77. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Dana S. L., Hoener P. A., Lieberman B. A., Imhof M. O., Stein R. B. Cellular mechanisms which distinguish between hormone- and antihormone-activated estrogen receptor. Ann N Y Acad Sci. 1995 Jun 12;761:121–137. doi: 10.1111/j.1749-6632.1995.tb31374.x. [DOI] [PubMed] [Google Scholar]
- Neuman E., Ladha M. H., Lin N., Upton T. M., Miller S. J., DiRenzo J., Pestell R. G., Hinds P. W., Dowdy S. F., Brown M. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997 Sep;17(9):5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. I., Gee J. M., Manning D. L., Wakeling A. E., Montano M. M., Katzenellenbogen B. S. Responses to pure antiestrogens (ICI 164384, ICI 182780) in estrogen-sensitive and -resistant experimental and clinical breast cancer. Ann N Y Acad Sci. 1995 Jun 12;761:148–163. doi: 10.1111/j.1749-6632.1995.tb31376.x. [DOI] [PubMed] [Google Scholar]
- Ozawa M. Structure of the gene encoding mouse reticulocalbin, a novel endoplasmic reticulum-resident Ca(2+)-binding protein with multiple EF-hand motifs. J Biochem. 1995 Jul;118(1):154–160. doi: 10.1093/oxfordjournals.jbchem.a124871. [DOI] [PubMed] [Google Scholar]
- Petersen O. W., Høyer P. E., van Deurs B. Frequency and distribution of estrogen receptor-positive cells in normal, nonlactating human breast tissue. Cancer Res. 1987 Nov 1;47(21):5748–5751. [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Examination of the DNA-binding ability of estrogen receptor in whole cells: implications for hormone-independent transactivation and the actions of antiestrogens. Mol Cell Biol. 1992 Oct;12(10):4531–4538. doi: 10.1128/mcb.12.10.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts D., Turnbull L., Ryall G., Bakhshi R., Rawson N. S., Gazet J. C., Nolan C., Coombes R. C. Estrogen and progesterone receptors in the normal female breast. Cancer Res. 1991 Apr 1;51(7):1817–1822. [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Sheng S., Anisowicz A., Sotiropoulou G., Zou Z., Stenman G., Swisshelm K., Chen Z., Hendrix M. J., Pemberton P. RNA genetics of breast cancer: maspin as paradigm. Cold Spring Harb Symp Quant Biol. 1994;59:537–546. doi: 10.1101/sqb.1994.059.01.060. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Lederkremer G. Z., Williams S., Fogliano M., Baldini G., Lodish H. F. Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J Cell Biol. 1996 Apr;133(2):257–268. doi: 10.1083/jcb.133.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schodin D. J., Zhuang Y., Shapiro D. J., Katzenellenbogen B. S. Analysis of mechanisms that determine dominant negative estrogen receptor effectiveness. J Biol Chem. 1995 Dec 29;270(52):31163–31171. doi: 10.1074/jbc.270.52.31163. [DOI] [PubMed] [Google Scholar]
- Shibata H., Spencer T. E., Oñate S. A., Jenster G., Tsai S. Y., Tsai M. J., O'Malley B. W. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–165. [PubMed] [Google Scholar]
- Smith C. L., Conneely O. M., O'Malley B. W. Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6120–6124. doi: 10.1073/pnas.90.13.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukovich D. A., Mukherjee R., Benfield P. A. A novel, cell-type-specific mechanism for estrogen receptor-mediated gene activation in the absence of an estrogen-responsive element. Mol Cell Biol. 1994 Nov;14(11):7134–7143. doi: 10.1128/mcb.14.11.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Vladusic E. A., Hornby A. E., Guerra-Vladusic F. K., Lupu R. Expression of estrogen receptor beta messenger RNA variant in breast cancer. Cancer Res. 1998 Jan 15;58(2):210–214. [PubMed] [Google Scholar]
- Yang N. N., Venugopalan M., Hardikar S., Glasebrook A. Identification of an estrogen response element activated by metabolites of 17beta-estradiol and raloxifene. Science. 1996 Aug 30;273(5279):1222–1225. doi: 10.1126/science.273.5279.1222. [DOI] [PubMed] [Google Scholar]
- Zajchowski D. A., Band V., Trask D. K., Kling D., Connolly J. L., Sager R. Suppression of tumor-forming ability and related traits in MCF-7 human breast cancer cells by fusion with immortal mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2314–2318. doi: 10.1073/pnas.87.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. X., Borg A., Wolf D. M., Oesterreich S., Fuqua S. A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997 Apr 1;57(7):1244–1249. [PubMed] [Google Scholar]
- Zwijsen R. M., Wientjens E., Klompmaker R., van der Sman J., Bernards R., Michalides R. J. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997 Feb 7;88(3):405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]