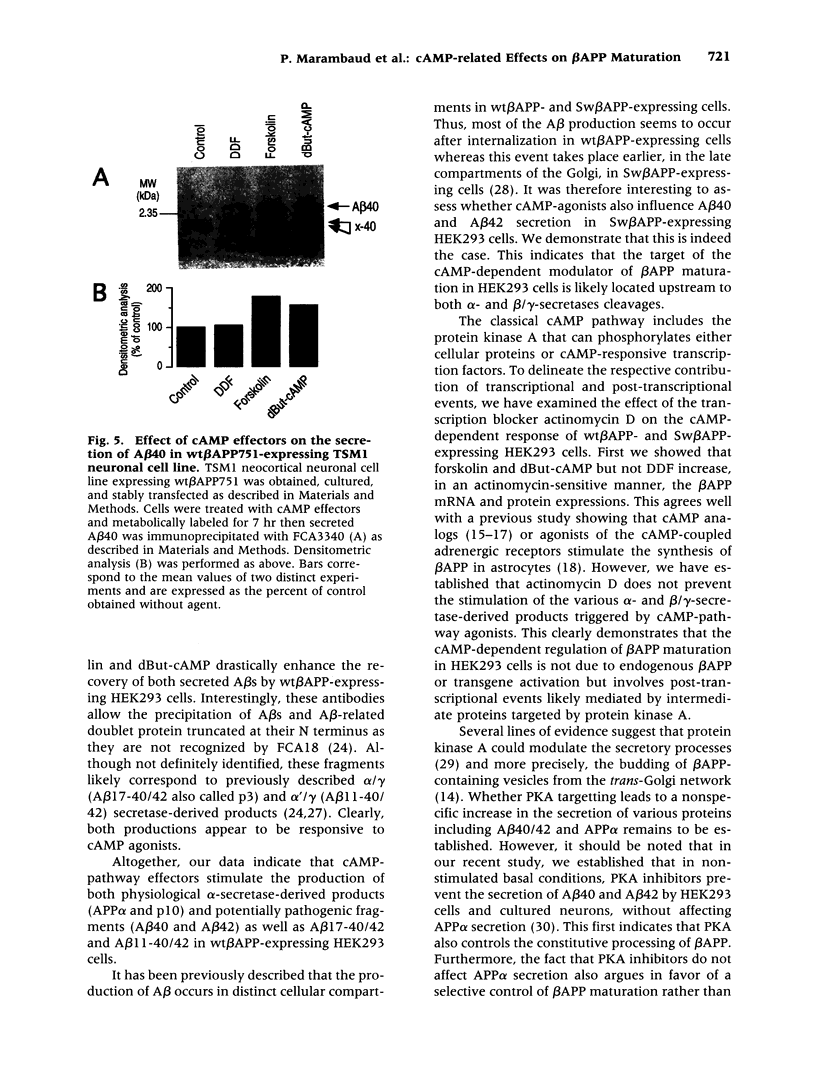
Abstract
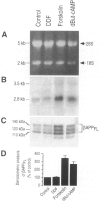
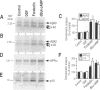
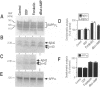
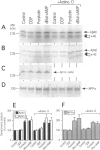
BACKGROUND: The physiopathological maturation of the beta-amyloid precursor protein can be modulated by effectors targeting a protein kinase C-dependent pathway. These agents increase the recovery of APP alpha, the physiological alpha-secretase-derived product of beta APP processing, and concomittantly lower the production of the pathogenic beta/gamma-secretase-derived A beta fragment. METHODS: We set up stably transfected HEK293 cells expressing wild-type or Swedish mutated beta APP. By combined metabolic labeling and/or immunoprecipitation procedures, we assessed the effect of various cAMP effectors on the production of the beta APP maturation products A beta 40, A beta 42, APP alpha, and its C-terminal counterpart. RESULTS: We show here that the cAMP-dependent protein kinase (PKA) effectors, dibutyryl-cAMP (dBut-cAMP) and forskolin, but not the inactive analog dideoxyforskolin, enhance the secretion of APP alpha and the intracellular production of its C-terminal counterpart (p10) in stably transfected HEK293 cells. The above agonists also drastically increase both A beta 40 and A beta 42 secretions and intracellular A beta recovery. The same influence was observed with HEK293 cells overexpressing the Swedish mutated beta APP. We attempted to delineate the relative contribution of transcriptional and post-transcriptional events in the cAMP-mediated response. We show here that the dBut-cAMP and forskolin-induced increase of APP alpha and A beta s secretions is not prevented by the transcription inhibitor actinomycin D. CONCLUSION: Our data suggest a major contribution of post-transcriptional events in the cAMP-dependent effect on beta APP maturation. It appears likely that cAMP triggers the PKA-dependent phosphorylation of a protein involved in beta APP maturation and occurring upstream to alpha- and beta/gamma-secretase cleavages.
Full text
PDF


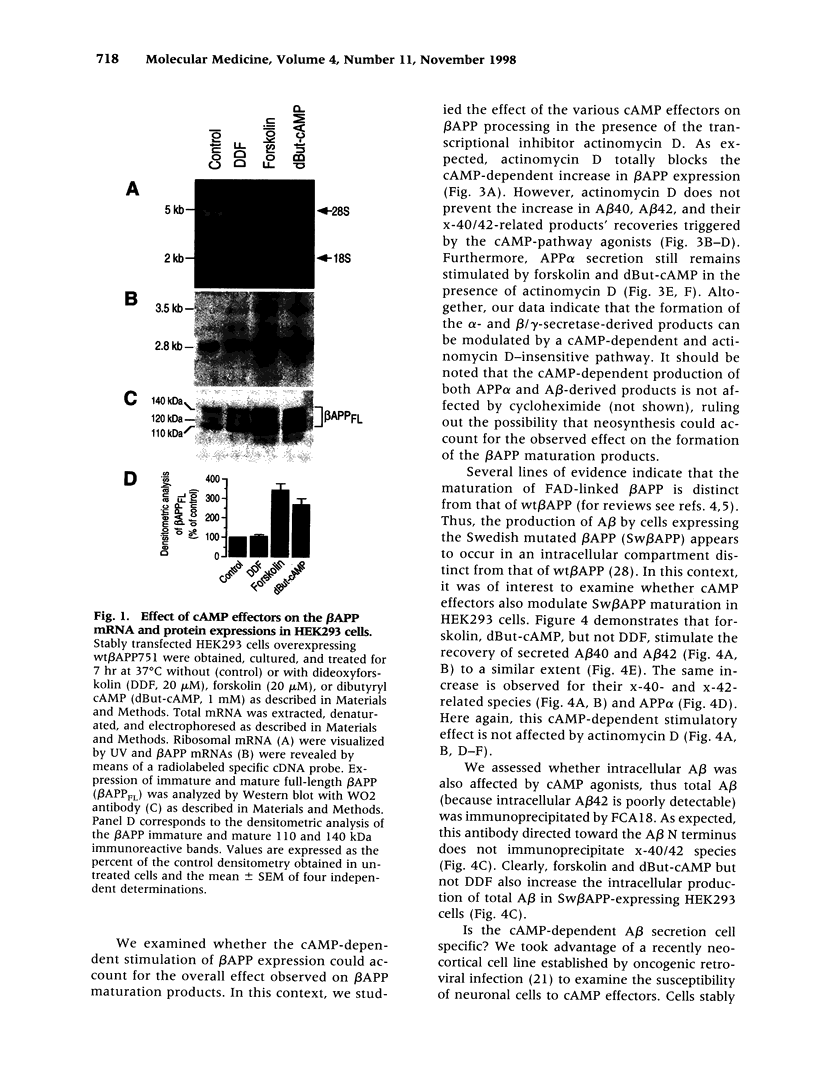
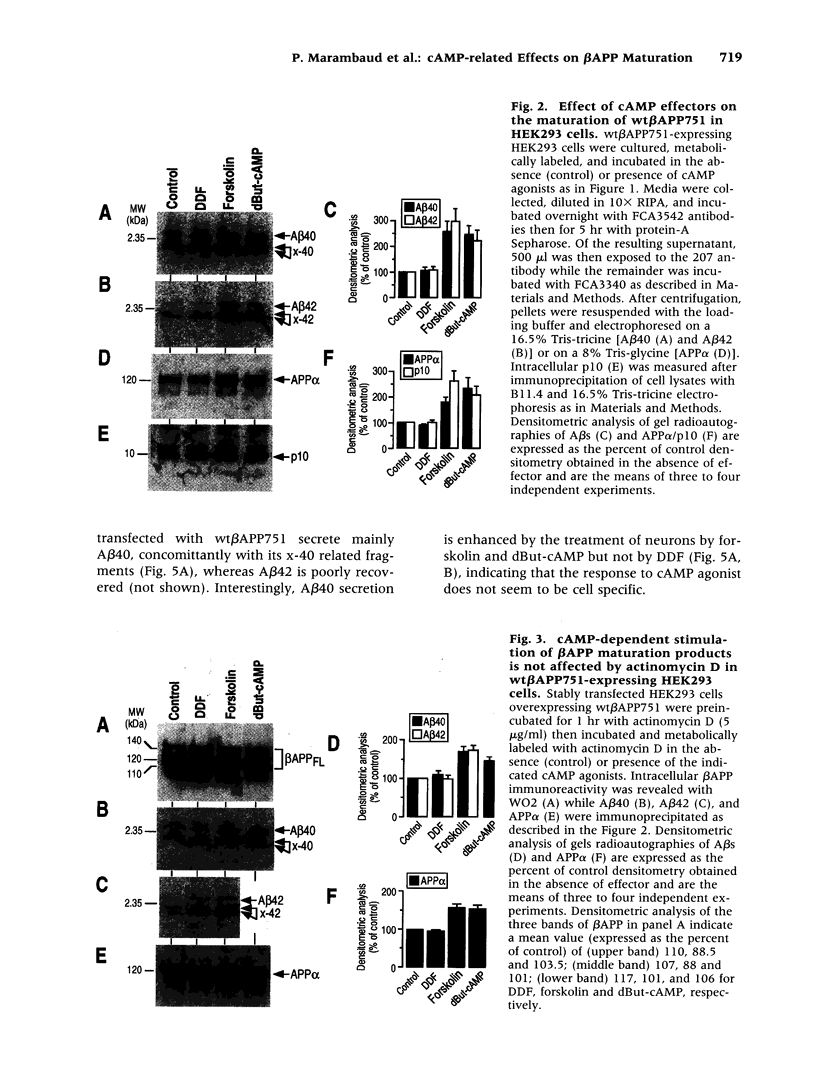
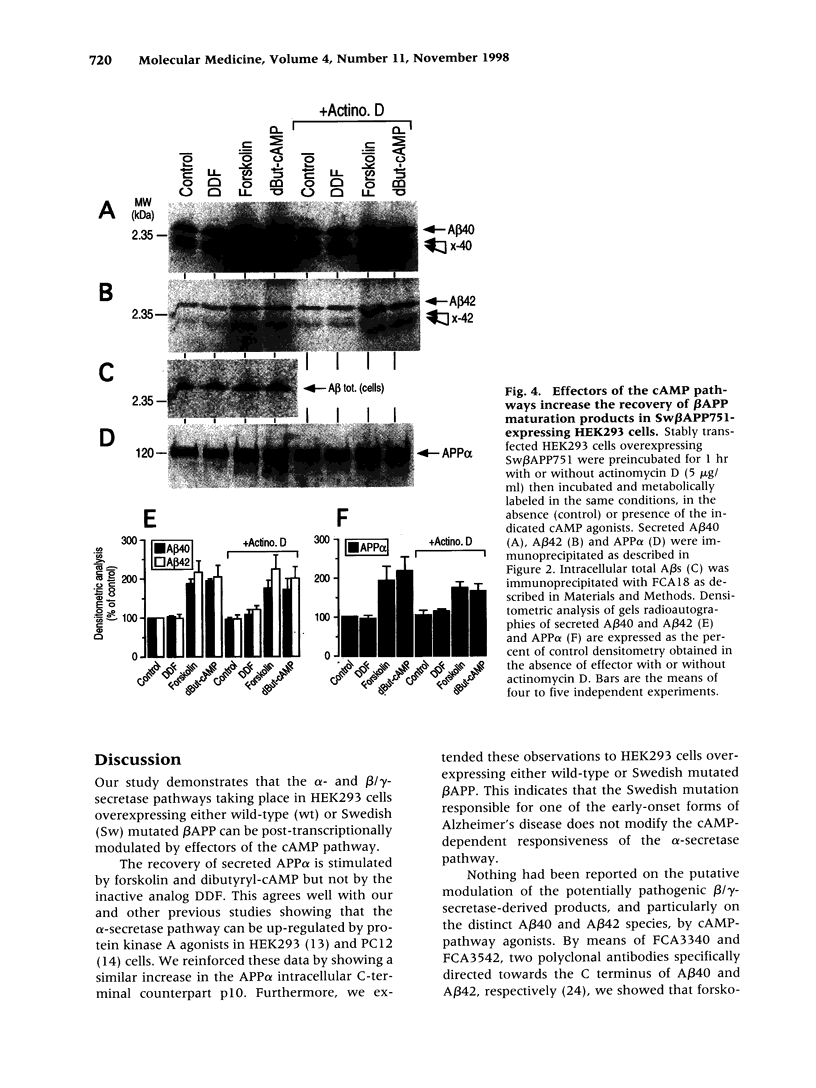


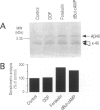
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barelli H., Lebeau A., Vizzavona J., Delaere P., Chevallier N., Drouot C., Marambaud P., Ancolio K., Buxbaum J. D., Khorkova O. Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol Med. 1997 Oct;3(10):695–707. [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Koo E. H., Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Ramabhadran T. V., Greengard P. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer's disease. J Neurochem. 1995 Oct;65(4):1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- Chevallier N., Jiracek J., Vincent B., Baur C. P., Spillantini M. G., Goedert M., Dive V., Checler F. Examination of the role of endopeptidase 3.4.24.15 in A beta secretion by human transfected cells. Br J Pharmacol. 1997 Jun;121(3):556–562. doi: 10.1038/sj.bjp.0701151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Jaenisch R. Clonal cell lines produced by infection of neocortical neuroblasts using multiple oncogenes transduced by retroviruses. Mol Cell Neurosci. 1996 Apr;7(4):304–321. doi: 10.1006/mcne.1996.0023. [DOI] [PubMed] [Google Scholar]
- Cook D. G., Forman M. S., Sung J. C., Leight S., Kolson D. L., Iwatsubo T., Lee V. M., Doms R. W. Alzheimer's A beta(1-42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med. 1997 Sep;3(9):1021–1023. doi: 10.1038/nm0997-1021. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Simons M., Multhaup G., Van Leuven F., Beyreuther K., Dotti C. G. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J. 1995 Oct 16;14(20):4932–4938. doi: 10.1002/j.1460-2075.1995.tb00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G., Bock E., Schousboe A., Linnemann D. Two types of amyloid precursor protein (APP) mRNA in rat glioma cell lines: upregulation via a cyclic AMP-dependent pathway. Brain Res Mol Brain Res. 1996 Apr;37(1-2):151–156. doi: 10.1016/0169-328x(95)00302-9. [DOI] [PubMed] [Google Scholar]
- Gillespie S. L., Golde T. E., Younkin S. G. Secretory processing of the Alzheimer amyloid beta/A4 protein precursor is increased by protein phosphorylation. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1285–1290. doi: 10.1016/0006-291x(92)90442-n. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Haass C., Lemere C. A., Capell A., Citron M., Seubert P., Schenk D., Lannfelt L., Selkoe D. J. The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995 Dec;1(12):1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991 Oct;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hartmann T., Bieger S. C., Brühl B., Tienari P. J., Ida N., Allsop D., Roberts G. W., Masters C. L., Dotti C. G., Unsicker K. Distinct sites of intracellular production for Alzheimer's disease A beta40/42 amyloid peptides. Nat Med. 1997 Sep;3(9):1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- Hung A. Y., Haass C., Nitsch R. M., Qiu W. Q., Citron M., Wurtman R. J., Growdon J. H., Selkoe D. J. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem. 1993 Nov 5;268(31):22959–22962. [PubMed] [Google Scholar]
- Ida N., Hartmann T., Pantel J., Schröder J., Zerfass R., Förstl H., Sandbrink R., Masters C. L., Beyreuther K. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996 Sep 13;271(37):22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- Lee R. K., Araki W., Wurtman R. J. Stimulation of amyloid precursor protein synthesis by adrenergic receptors coupled to cAMP formation. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5422–5426. doi: 10.1073/pnas.94.10.5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P., Chevallier N., Barelli H., Wilk S., Checler F. Proteasome contributes to the alpha-secretase pathway of amyloid precursor protein in human cells. J Neurochem. 1997 Feb;68(2):698–703. doi: 10.1046/j.1471-4159.1997.68020698.x. [DOI] [PubMed] [Google Scholar]
- Marambaud P., Lopez-Perez E., Wilk S., Checler F. Constitutive and protein kinase C-regulated secretory cleavage of Alzheimer's beta-amyloid precursor protein: different control of early and late events by the proteasome. J Neurochem. 1997 Dec;69(6):2500–2505. doi: 10.1046/j.1471-4159.1997.69062500.x. [DOI] [PubMed] [Google Scholar]
- Marambaud P., Wilk S., Checler F. Protein kinase A phosphorylation of the proteasome: a contribution to the alpha-secretase pathway in human cells. J Neurochem. 1996 Dec;67(6):2616–2619. doi: 10.1046/j.1471-4159.1996.67062616.x. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury C. P. Molecular pathogenesis of beta-amyloidosis in Alzheimer's disease and other cerebral amyloidoses. Lab Invest. 1995 Jan;72(1):4–16. [PubMed] [Google Scholar]
- Savage M. J., Trusko S. P., Howland D. S., Pinsker L. R., Mistretta S., Reaume A. G., Greenberg B. D., Siman R., Scott R. W. Turnover of amyloid beta-protein in mouse brain and acute reduction of its level by phorbol ester. J Neurosci. 1998 Mar 1;18(5):1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- Shekarabi M., Bourbonnière M., Dagenais A., Nalbantoglu J. Transcriptional regulation of amyloid precursor protein during dibutyryl cyclic AMP-induced differentiation of NG108-15 cells. J Neurochem. 1997 Mar;68(3):970–978. doi: 10.1046/j.1471-4159.1997.68030970.x. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau L. E., Emery D. G., Haydon P. G. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron. 1996 Oct;17(4):789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- Xu H., Gouras G. K., Greenfield J. P., Vincent B., Naslund J., Mazzarelli L., Fried G., Jovanovic J. N., Seeger M., Relkin N. R. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med. 1998 Apr;4(4):447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- Xu H., Sweeney D., Greengard P., Gandy S. Metabolism of Alzheimer beta-amyloid precursor protein: regulation by protein kinase A in intact cells and in a cell-free system. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4081–4084. doi: 10.1073/pnas.93.9.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]