Abstract
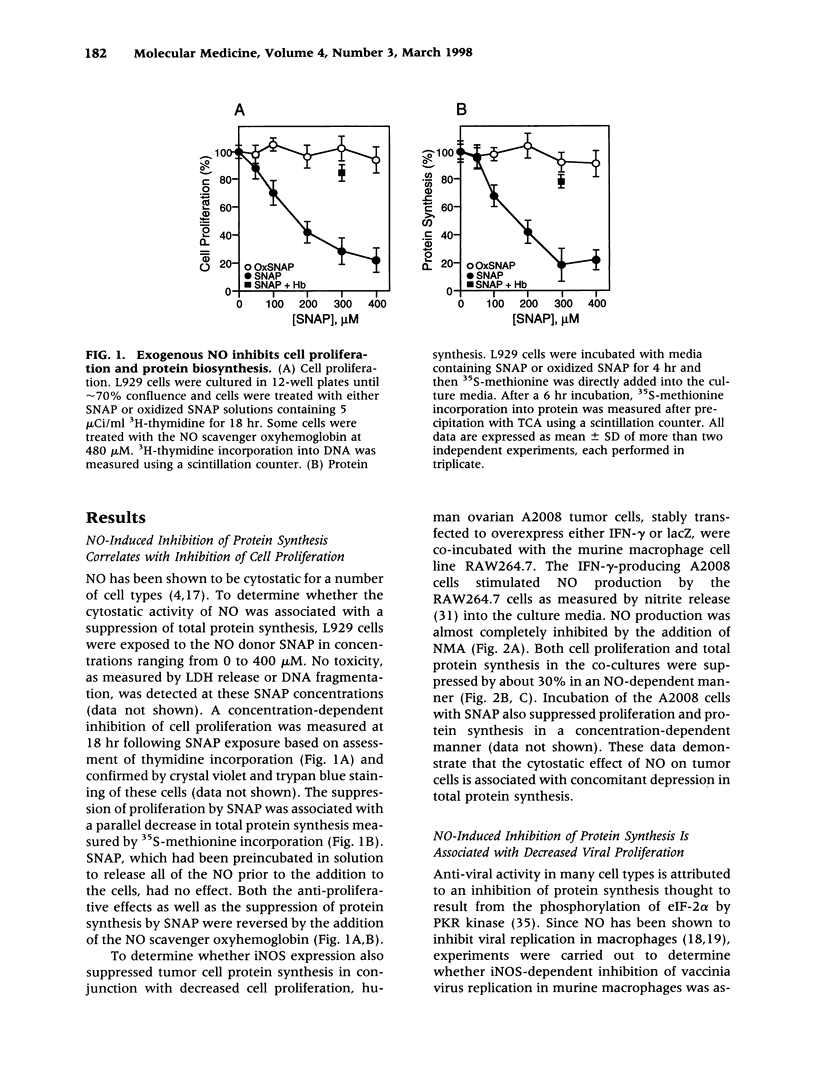
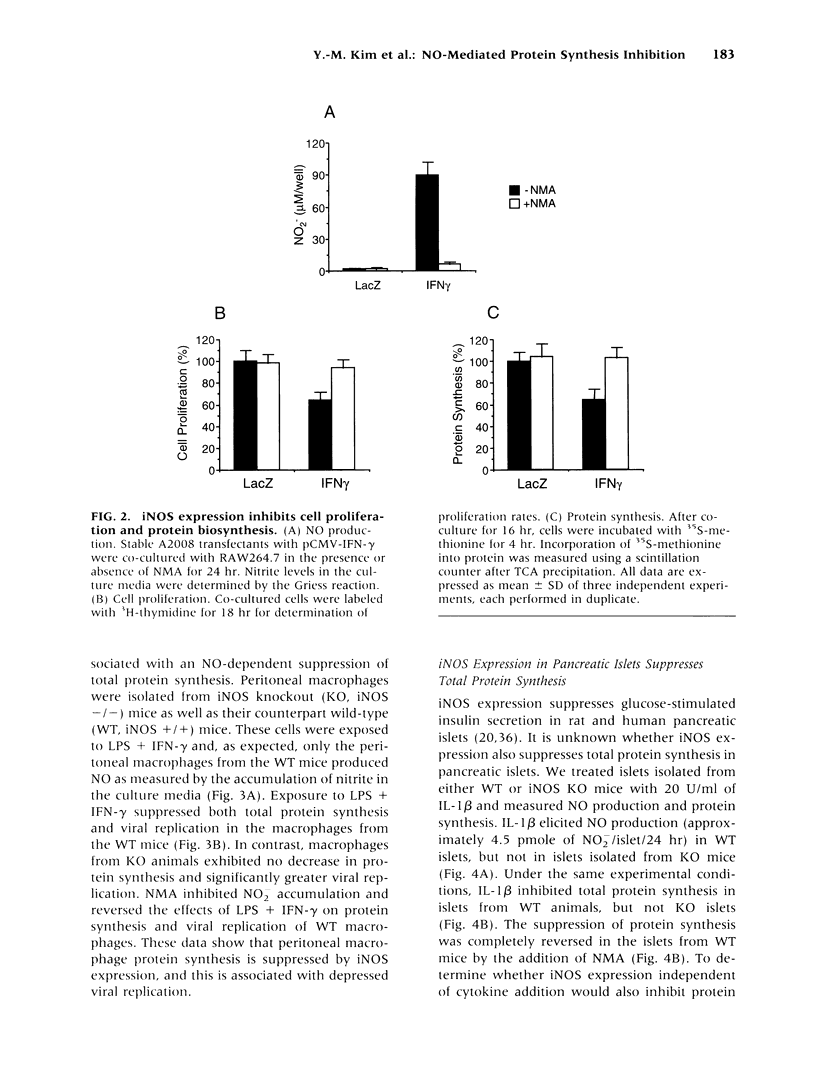
BACKGROUND: Nitric oxide (NO) is cytostatic for proliferating cells, inhibits microbial growth, and down-regulates the synthesis of specific proteins. Studies were undertaken to determine the mechanism by which NO inhibits total protein synthesis and whether the inhibition correlates with established cytostatic activities of NO. MATERIALS AND METHODS: In in vitro experiments, various cell types were exposed to NO using either donors or expression of inducible NO synthase (iNOS). The capacity of NO to suppress total protein synthesis, measured by incorporation of 35S-methionine into protein, was correlated with the capacity of NO to suppress cell proliferation, viral replication, or iNOS expression. Phosphorylation of eIF-2 alpha was examined as a possible mechanism for the suppressed protein synthesis by NO. RESULTS: Both NO donors and expression of the iNOS suppressed total protein synthesis in L929 cells and A2008 human ovarian tumor cells in parallel with decreased cell proliferation. Suppressed protein synthesis was also shown to correlate with decreased vaccinia virus proliferation in murine peritoneal macrophages in an iNOS-dependent manner. Furthermore, iNOS expression in pancreatic islets or RAW264.7 cells almost completely inhibited total protein synthesis, suggesting that nonspecific inhibition of protein synthesis may be the mechanism by which NO inhibited the synthesis of specific proteins such as insulin or iNOS itself. This possibility was confirmed in RAW264.7 cells where the inhibition of total protein synthesis correlated with the decreased iNOS protein. The decrease in protein levels occurred without changes in iNOS mRNA levels, implicating an inhibition of translation. Mechanistic studies revealed that iNOS expression in RAW264.7 cells resulted in the phosphorylation of eIF-2 alpha and inhibition of the 80S ribosomal complex formation. CONCLUSIONS: These results suggest that NO suppresses protein synthesis by stimulating the phosphorylation of eIF-2 alpha. Furthermore, our observations indicate that nonspecific inhibition of protein synthesis may be a generalized response of cells exposed to high levels of NO and that inhibition of protein synthesis may contribute to many of the described cytostatic actions of NO.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R. Nitric oxide. Novel biology with clinical relevance. Ann Surg. 1995 Apr;221(4):339–349. doi: 10.1097/00000658-199504000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Throop M. S., Gehrke L., Kuo I., Pal J. K., Brodsky M., London I. M. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7729–7733. doi: 10.1073/pnas.88.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Drillien R., Spehner D., Buller R. M. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 1992 Apr;187(2):433–442. doi: 10.1016/0042-6822(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Lancaster J. R., Jr, Sweetland M. A., McDaniel M. L. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem. 1991 Nov 15;266(32):21351–21354. [PubMed] [Google Scholar]
- Corbett J. A., Sweetland M. A., Wang J. L., Lancaster J. R., Jr, McDaniel M. L. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby J. S., Lee K., London I. M., Chen J. J. Erythroid expression of the heme-regulated eIF-2 alpha kinase. Mol Cell Biol. 1994 Jun;14(6):3906–3914. doi: 10.1128/mcb.14.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Ferrari F. K., Kispert P. H., Stadler J., Stuehr D. J., Simmons R. L., Billiar T. R. Nitric oxide and nitric oxide-generating compounds inhibit hepatocyte protein synthesis. FASEB J. 1991 Apr;5(7):2085–2092. doi: 10.1096/fasebj.5.7.1707021. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Phosphorylation of initiation factor eIF-2 alpha, binding of mRNA to 48 S complexes, and its reutilization in initiation of protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14556–14562. [PubMed] [Google Scholar]
- Der S. D., Lau A. S. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8841–8845. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias-Eisner R., Sherman M. P., Aeberhard E., Chaudhuri G. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9407–9411. doi: 10.1073/pnas.91.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. The eIF-2 alpha kinases: regulators of protein synthesis in starvation and stress. Semin Cell Biol. 1994 Dec;5(6):417–426. doi: 10.1006/scel.1994.1049. [DOI] [PubMed] [Google Scholar]
- Jenkins D. C., Charles I. G., Thomsen L. L., Moss D. W., Holmes L. S., Baylis S. A., Rhodes P., Westmore K., Emson P. C., Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Xie Q. W., Buller R. M., Nathan C., Duarte C., MacMicking J. D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993 Sep 10;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Bergonia H. A., Müller C., Pitt B. R., Watkins W. D., Lancaster J. R., Jr Loss and degradation of enzyme-bound heme induced by cellular nitric oxide synthesis. J Biol Chem. 1995 Mar 17;270(11):5710–5713. doi: 10.1074/jbc.270.11.5710. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Bergonia H. A., Müller C., Pitt B. R., Watkins W. D., Lancaster J. R., Jr Nitric oxide and intracellular heme. Adv Pharmacol. 1995;34:277–291. doi: 10.1016/s1054-3589(08)61092-3. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Hong S. J., Billiar T. R., Simmons R. L. Counterprotective effect of erythrocytes in experimental bacterial peritonitis is due to scavenging of nitric oxide and reactive oxygen intermediates. Infect Immun. 1996 Aug;64(8):3074–3080. doi: 10.1128/iai.64.8.3074-3080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Lancaster J. R., Jr Tetrahydrobiopterin-dependent nitrite oxidation to nitrate in isolated rat hepatocytes. FEBS Lett. 1993 Oct 18;332(3):255–259. doi: 10.1016/0014-5793(93)80644-a. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Son K. A nitric oxide production bioassay for interferon-gamma. J Immunol Methods. 1996 Nov 13;198(2):203–209. doi: 10.1016/s0022-1759(96)00162-7. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., de Vera M. E., Watkins S. C., Billiar T. R. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997 Jan 10;272(2):1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- Kwon G., Bohrer A., Han X., Corbett J. A., Ma Z., Gross R. W., McDaniel M. L., Turk J. Characterization of the sphingomyelin content of isolated pancreatic islets. Evaluation of the role of sphingomyelin hydrolysis in the action of interleukin-1 to induce islet overproduction of nitric oxide. Biochim Biophys Acta. 1996 Mar 29;1300(1):63–72. doi: 10.1016/0005-2760(95)00223-5. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Flaman J. M., Bobé P., Lemaire G., Henry Y. Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide. Relationship to cytostasis induced in tumor cells by cytotoxic macrophages. J Biol Chem. 1994 Aug 26;269(34):21891–21897. [PubMed] [Google Scholar]
- Lowenstein C. J., Hill S. L., Lafond-Walker A., Wu J., Allen G., Landavere M., Rose N. R., Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996 Apr 15;97(8):1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurides P. A., Akkaraju G. R., Jagus R. Evaluation of protein phosphorylation state by a combination of vertical slab gel isoelectric focusing and immunoblotting. Anal Biochem. 1989 Nov 15;183(1):144–151. doi: 10.1016/0003-2697(89)90182-6. [DOI] [PubMed] [Google Scholar]
- Meurs E. F., Watanabe Y., Kadereit S., Barber G. N., Katze M. G., Chong K., Williams B. R., Hovanessian A. G. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992 Oct;66(10):5805–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Petryshyn R., Trachsel H., London I. M. Regulation of protein synthesis in reticulocyte lysates: immune serum inhibits heme-regulated protein kinase activity and differentiates heme-regulated protein kinase from double-stranded RNA-induced protein kinase. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1575–1579. doi: 10.1073/pnas.76.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah K. V., Dhindsa R. S., Chen J. J., London I. M., Levin D. Recycling and phosphorylation of eukaryotic initiation factor 2 on 60S subunits of 80S initiation complexes and polysomes. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12063–12067. doi: 10.1073/pnas.89.24.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993 Apr 15;268(11):7603–7606. [PubMed] [Google Scholar]
- Sheffler L. A., Wink D. A., Melillo G., Cox G. W. Exogenous nitric oxide regulates IFN-gamma plus lipopolysaccharide-induced nitric oxide synthase expression in mouse macrophages. J Immunol. 1995 Jul 15;155(2):886–894. [PubMed] [Google Scholar]
- Son K., Kim Y. M. In vivo cisplatin-exposed macrophages increase immunostimulant-induced nitric oxide synthesis for tumor cell killing. Cancer Res. 1995 Dec 1;55(23):5524–5527. [PubMed] [Google Scholar]
- Stadler J., Bergonia H. A., Di Silvio M., Sweetland M. A., Billiar T. R., Simmons R. L., Lancaster J. R., Jr Nonheme iron-nitrosyl complex formation in rat hepatocytes: detection by electron paramagnetic resonance spectroscopy. Arch Biochem Biophys. 1993 Apr;302(1):4–11. doi: 10.1006/abbi.1993.1173. [DOI] [PubMed] [Google Scholar]
- Tzeng E., Kim Y. M., Pitt B. R., Lizonova A., Kovesdi I., Billiar T. R. Adenoviral transfer of the inducible nitric oxide synthase gene blocks endothelial cell apoptosis. Surgery. 1997 Aug;122(2):255–263. doi: 10.1016/s0039-6060(97)90016-7. [DOI] [PubMed] [Google Scholar]
- Wu S., Kaufman R. J. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997 Jan 10;272(2):1291–1296. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- Yang J. M., London I. M., Chen J. J. Effects of hemin and porphyrin compounds on intersubunit disulfide formation of heme-regulated eIF-2 alpha kinase and the regulation of protein synthesis in reticulocyte lysates. J Biol Chem. 1992 Oct 5;267(28):20519–20524. [PubMed] [Google Scholar]