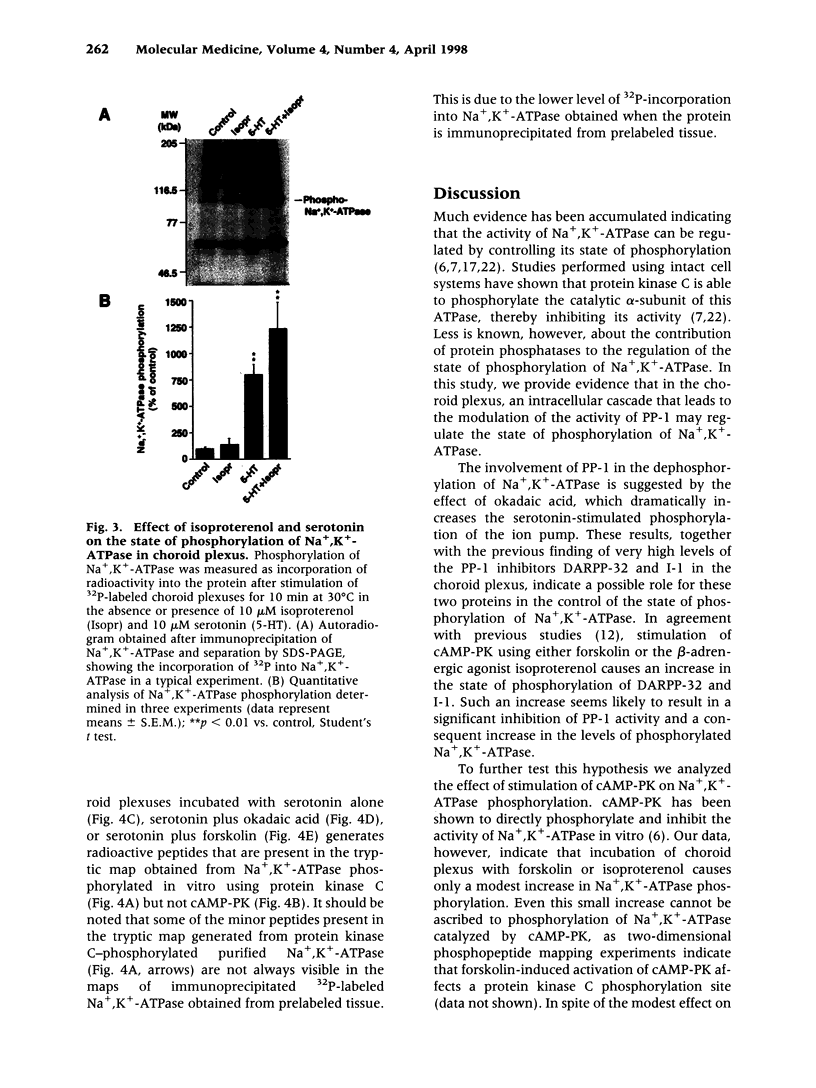
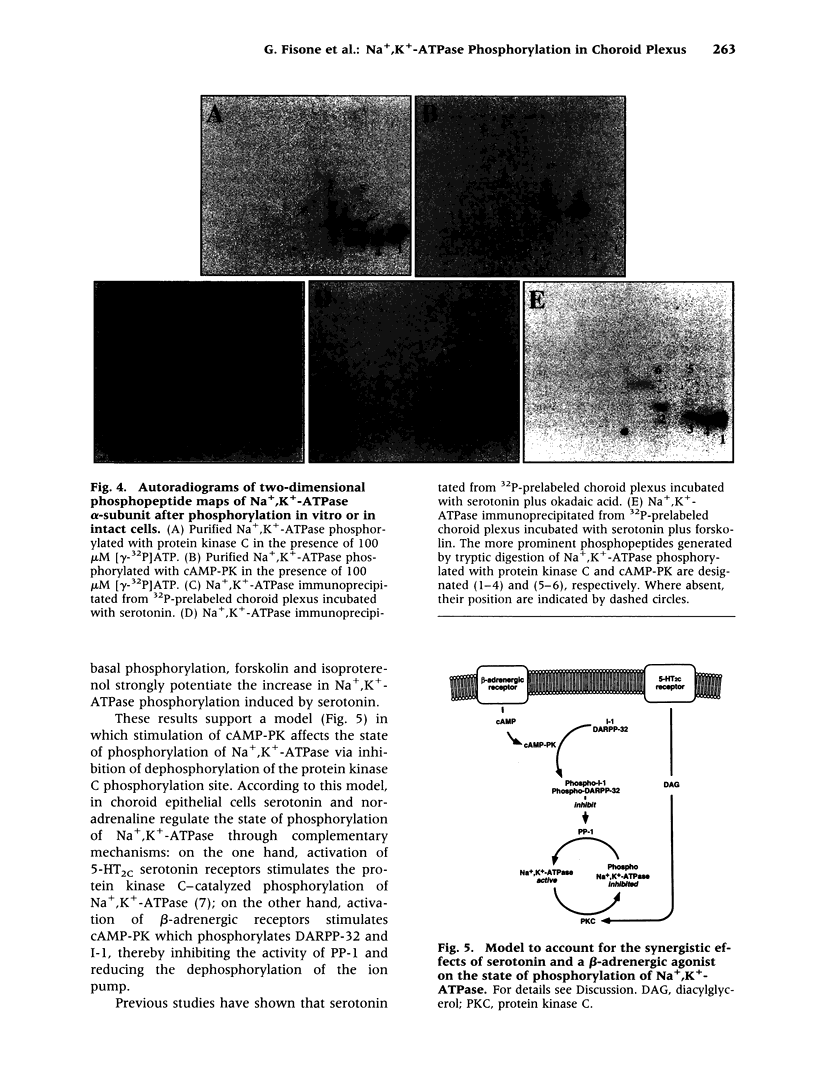
Abstract
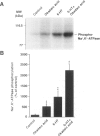
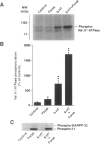
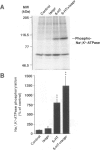
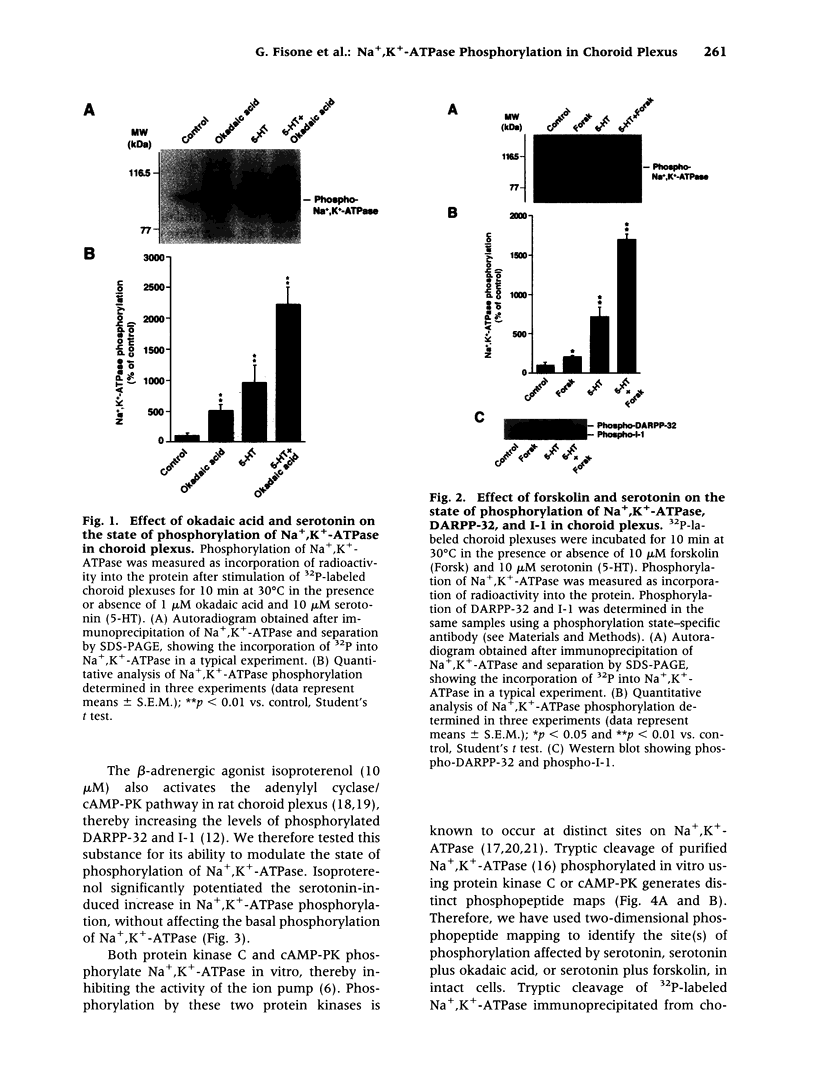
BACKGROUND: The ion pump Na+,K(+)-ATPase is responsible for the secretion of cerebrospinal fluid from the choroid plexus. In this tissue, the activity of Na+,K(+)-ATPase is inhibited by serotonin via stimulation of protein kinase C-catalyzed phosphorylation. The choroid plexus is highly enriched in two phosphoproteins which act as regulators of protein phosphatase-1 activity, DARPP-32 and inhibitor-1. Phosphorylation catalyzed by cAMP-dependent protein kinase on a single threonyl residue converts DARPP-32 and inhibitor-1 into potent inhibitors of protein phosphatase-1. Previous work has shown that in the choroid plexus, phosphorylation of DARPP-32 and I-1 is enhanced by isoproterenol and other agents that activate cAMP-PK. We have now examined the possible involvement of the cAMP-PK/protein phosphatase-1 pathway in the regulation of Na+,K(+)-ATPase. MATERIALS AND METHODS: The state of phosphorylation of Na+,K(+)-ATPase was measured by determining the amount of radioactivity incorporated into the ion pump following immunoprecipitation from 32P-prelabeled choroid plexuses incubated with various drugs (see below). Two-dimensional phosphopeptide mapping was employed to identify the protein kinase involved in the phosphorylation of Na+,K(+)-ATPase. RESULTS: The serotonin-mediated increase in Na+,K(+)-ATPase phosphorylation is potentiated by okadaic acid, an inhibitor of protein phosphatases-1 and -2A, as well as by forskolin or the beta-adrenergic agonist, isoproterenol, activators of cAMP-dependent protein kinase. Two-dimensional phosphopeptide maps suggest that this potentiating action occurs at the level of a protein kinase C phosphorylation site. Forskolin and isoproterenol also stimulate the phosphorylation of DARPP-32 and protein phosphatase inhibitor-1, which in their phosphorylated form are potent inhibitors of protein phosphatase-1. CONCLUSIONS: The results presented here support a model in which okadaic acid, forskolin, and isoproterenol achieve their synergistic effects with serotonin through phosphorylation of DARPP-32 and inhibitor-1, inhibition of protein phosphatase-1, and a reduction of dephosphorylation of Na+,K(+)-ATPase at a protein kinase C phosphorylation site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguin P., Beggah A. T., Chibalin A. V., Burgener-Kairuz P., Jaisser F., Mathews P. M., Rossier B. C., Cotecchia S., Geering K. Phosphorylation of the Na,K-ATPase alpha-subunit by protein kinase A and C in vitro and in intact cells. Identification of a novel motif for PKC-mediated phosphorylation. J Biol Chem. 1994 Sep 30;269(39):24437–24445. [PubMed] [Google Scholar]
- Bertorello A. M., Aperia A., Walaas S. I., Nairn A. C., Greengard P. Phosphorylation of the catalytic subunit of Na+,K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson S. J., Alexander A. Net production of cerebrospinal fluid is decreased by SCH-23390. Ann Neurol. 1990 Jun;27(6):631–635. doi: 10.1002/ana.410270608. [DOI] [PubMed] [Google Scholar]
- Feschenko M. S., Sweadner K. J. Structural basis for species-specific differences in the phosphorylation of Na,K-ATPase by protein kinase C. J Biol Chem. 1995 Jun 9;270(23):14072–14077. doi: 10.1074/jbc.270.23.14072. [DOI] [PubMed] [Google Scholar]
- Fisone G., Cheng S. X., Nairn A. C., Czernik A. J., Hemmings H. C., Jr, Hög J. O., Bertorello A. M., Kaiser R., Bergman T., Jörnvall H. Identification of the phosphorylation site for cAMP-dependent protein kinase on Na+,K(+)-ATPase and effects of site-directed mutagenesis. J Biol Chem. 1994 Mar 25;269(12):9368–9373. [PubMed] [Google Scholar]
- Fisone G., Snyder G. L., Fryckstedt J., Caplan M. J., Aperia A., Greengard P. Na+,K(+)-ATPase in the choroid plexus. Regulation by serotonin/protein kinase C pathway. J Biol Chem. 1995 Feb 10;270(6):2427–2430. doi: 10.1074/jbc.270.6.2427. [DOI] [PubMed] [Google Scholar]
- Haywood J. R., Vogh B. P. Some measurements of autonomic nervous system influence on production of cerebrospinal fluid in the cat. J Pharmacol Exp Ther. 1979 Feb;208(2):341–346. [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Girault J. A., Nairn A. C., Bertuzzi G., Greengard P. Distribution of protein phosphatase inhibitor-1 in brain and peripheral tissues of various species: comparison with DARPP-32. J Neurochem. 1992 Sep;59(3):1053–1061. doi: 10.1111/j.1471-4159.1992.tb08347.x. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P., Tung H. Y., Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984 Aug 9;310(5977):503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li D., Aperia A., Celsi G., da Cruz e Silva E. F., Greengard P., Meister B. Protein phosphatase-1 in the kidney: evidence for a role in the regulation of medullary Na(+)-K(+)-ATPase. Am J Physiol. 1995 Nov;269(5 Pt 2):F673–F680. doi: 10.1152/ajprenal.1995.269.5.F673. [DOI] [PubMed] [Google Scholar]
- Lindvall-Axelsson M., Mathew C., Nilsson C., Owman C. Effect of 5-hydroxytryptamine on the rate of cerebrospinal fluid production in rabbit. Exp Neurol. 1988 Feb;99(2):362–368. doi: 10.1016/0014-4886(88)90154-9. [DOI] [PubMed] [Google Scholar]
- Lindvall M., Edvinsson L., Owman C. Effect of sympathomimetic drugs and corresponding receptor antagonists on the rate of cerebrospinal fluid production. Exp Neurol. 1979 Apr;64(1):132–145. doi: 10.1016/0014-4886(79)90010-4. [DOI] [PubMed] [Google Scholar]
- Lindvall M., Edvinsson L., Owman C. Sympathetic nervous control of cerebrospinal fluid production from the choroid plexus. Science. 1978 Jul 14;201(4351):176–178. doi: 10.1126/science.663649. [DOI] [PubMed] [Google Scholar]
- Lindvall M., Gustafson A., Hedner P., Owman C. Stimulation of cyclic adenosine 3',5'-monophosphate formation in rabbit choroid plexus by beta-receptor agonists and vasoactive intestinal polypeptide. Neurosci Lett. 1985 Mar 15;54(2-3):153–157. doi: 10.1016/s0304-3940(85)80071-9. [DOI] [PubMed] [Google Scholar]
- Middleton J. P., Khan W. A., Collinsworth G., Hannun Y. A., Medford R. M. Heterogeneity of protein kinase C-mediated rapid regulation of Na/K-ATPase in kidney epithelial cells. J Biol Chem. 1993 Jul 25;268(21):15958–15964. [PubMed] [Google Scholar]
- Nathanson J. A. Beta-adrenergic-sensitive adenylate cyclase in secretory cells of choroid plexus. Science. 1979 May 25;204(4395):843–844. doi: 10.1126/science.220707. [DOI] [PubMed] [Google Scholar]
- Pietrini G., Matteoli M., Banker G., Caplan M. J. Isoforms of the Na,K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8414–8418. doi: 10.1073/pnas.89.18.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollay M., Hisey B., Reynolds E., Tomkins P., Stevens F. A., Smith R. Choroid plexus Na+/K+-activated adenosine triphosphatase and cerebrospinal fluid formation. Neurosurgery. 1985 Nov;17(5):768–772. doi: 10.1227/00006123-198511000-00007. [DOI] [PubMed] [Google Scholar]
- Snyder G. L., Girault J. A., Chen J. Y., Czernik A. J., Kebabian J. W., Nathanson J. A., Greengard P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J Neurosci. 1992 Aug;12(8):3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steardo L., Nathanson J. A. Brain barrier tissues: end organs for atriopeptins. Science. 1987 Jan 23;235(4787):470–473. doi: 10.1126/science.2879355. [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V., Mackic J. B., Wang L., McComb J. G., McDonough A. Differential expression of Na,K-ATPase alpha and beta subunit isoforms at the blood-brain barrier and the choroid plexus. J Biol Chem. 1993 Apr 15;268(11):8019–8025. [PubMed] [Google Scholar]