Abstract
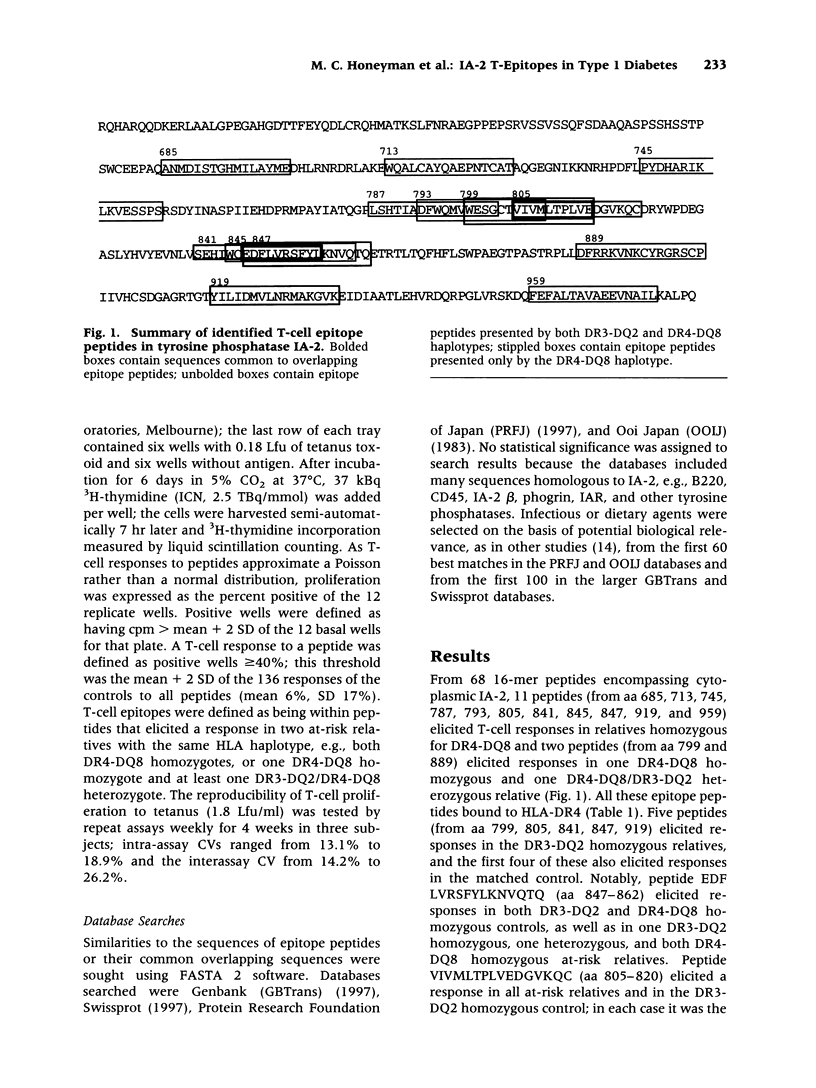
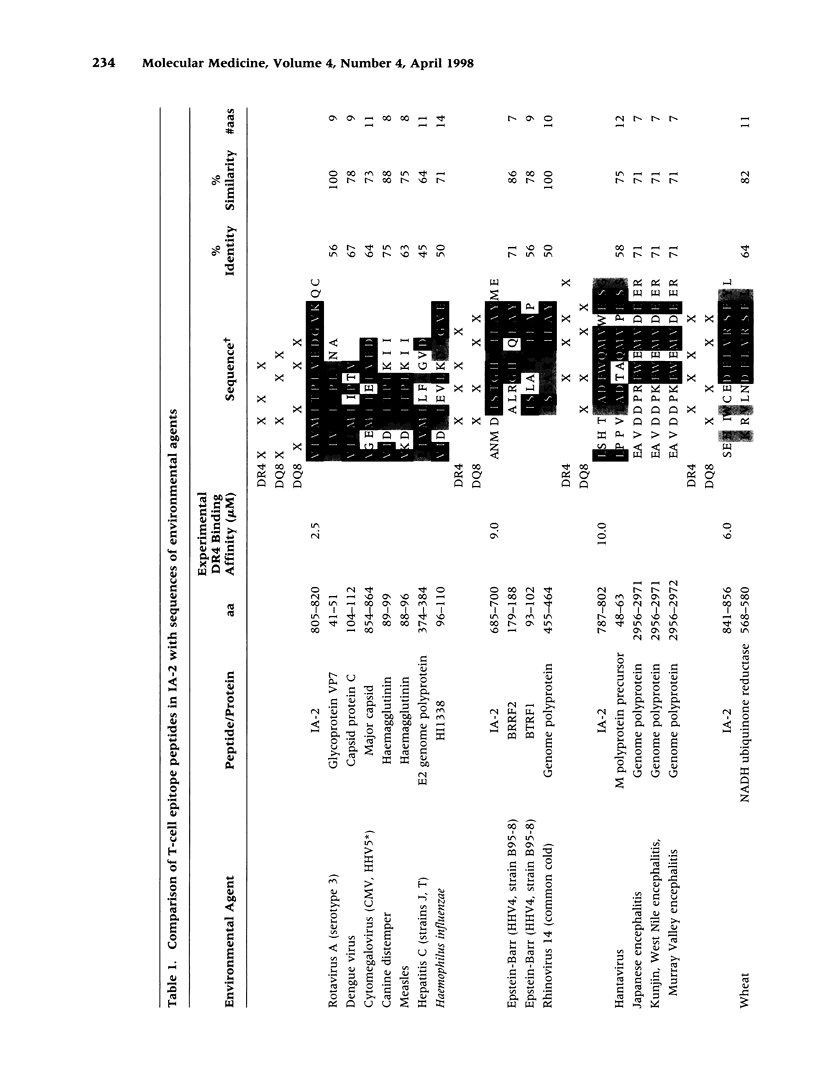
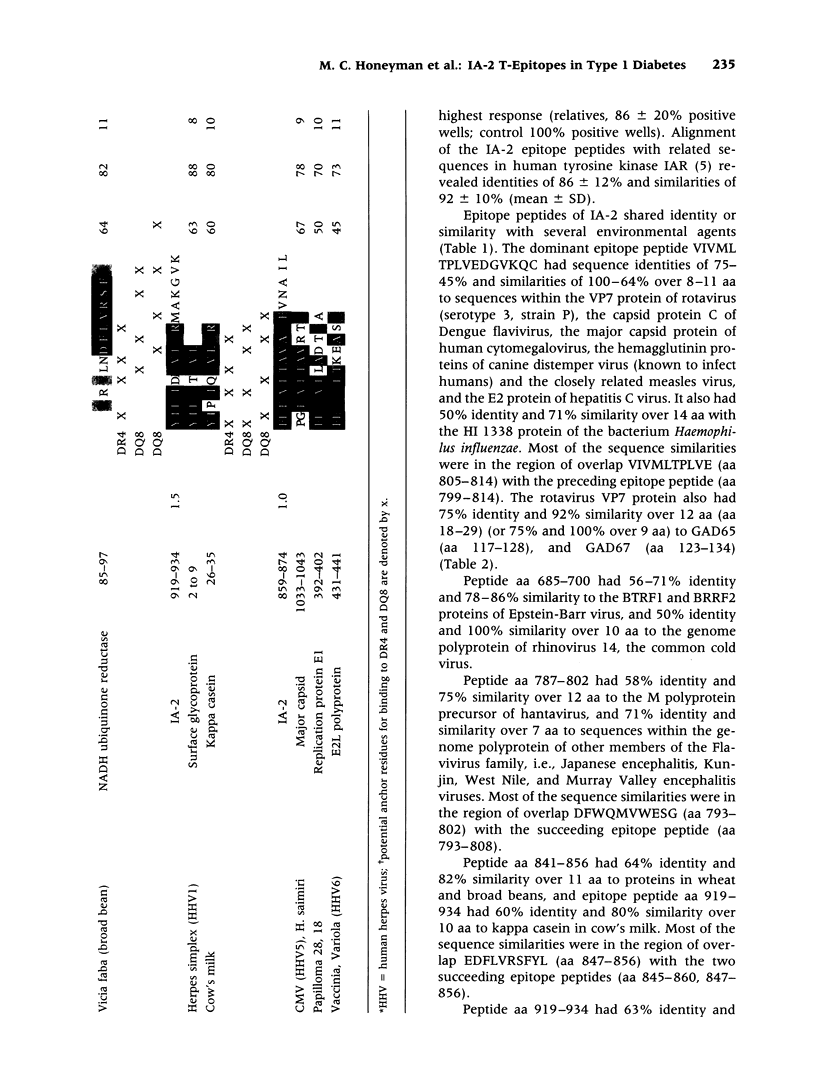
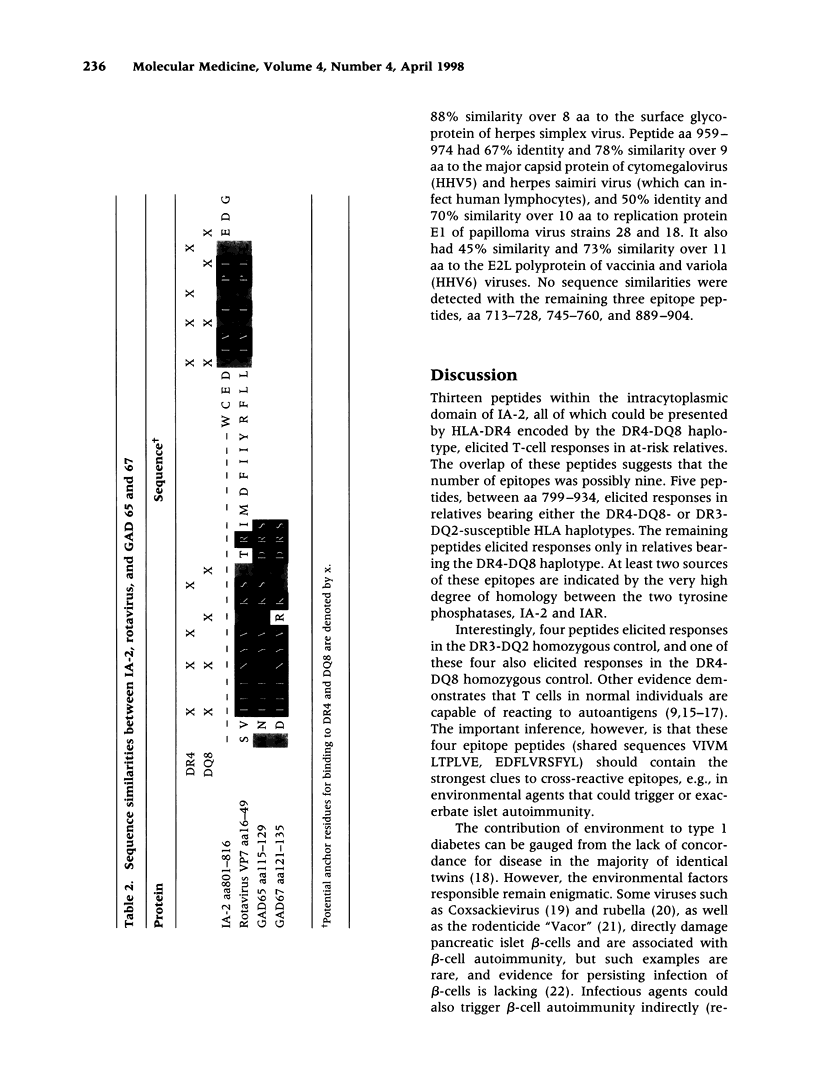
The tyrosine phosphatase IA-2 is a molecular target of pancreatic islet autoimmunity in type 1 diabetes. T-cell epitope peptides in autoantigens have potential diagnostic and therapeutic applications, and they may hold clues to environmental agents with similar sequences that could trigger or exacerbate autoimmune disease. We identified 13 epitope peptides in IA-2 by measuring peripheral blood T-cell proliferation to 68 overlapping, synthetic peptides encompassing the intracytoplasmic domain of IA-2 in six at-risk type 1 diabetes relatives selected for HLA susceptibility haplotypes. The dominant epitope, VIVMLTPLVEDGVKQC (aa 805-820), which elicited the highest T-cell responses in all at-risk relatives, has 56% identity and 100% similarity over 9 amino acids (aa) with a sequence in VP7, a major immunogenic protein of human rotavirus. Both peptides bind to HLA-DR4(*0401) and are deduced to present identical aa to the T-cell receptor. The contiguous sequence of VP7 has 75% identity and 92% similarity over 12 aa with a known T-cell epitope in glutamic acid decarboxylase (GAD), another autoantigen in type 1 diabetes. This dominant IA-2 epitope peptide also has 75-45% identity and 88-64% similarity over 8-14 aa to sequences in Dengue, cytomegalovirus, measles, hepatitis C, and canine distemper viruses, and the bacterium Haemophilus influenzae. Three other IA-2 epitope peptides are 71-100% similar over 7-12 aa to herpes, rhino-, hanta- and flaviviruses. Two others are 80-82% similar over 10-11 aa to sequences in milk, wheat, and bean proteins. Further studies should now be carried out to directly test the hypothesis that T-cell activation by rotavirus and possibly other viruses, and dietary proteins, could trigger or exacerbate beta-cell autoimmunity through molecular mimicry with IA-2 and (for rotavirus) GAD.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Bowman M. A., Campbell L., Darrow B. L., Kaufman D. L., Maclaren N. K. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994 Nov;94(5):2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Unicomb L. E., Barnes G. L. Epidemiology of rotavirus serotypes in Melbourne, Australia, from 1973 to 1989. J Clin Microbiol. 1991 May;29(5):862–868. doi: 10.1128/jcm.29.5.862-868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacio E., Lampasona V., Genovese S., Ferrari M., Bosi E. Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol. 1995 Dec 1;155(11):5419–5426. [PubMed] [Google Scholar]
- Brusic V., Rudy G., Kyne A. P., Harrison L. C. MHCPEP--a database of MHC-binding peptides: update 1995. Nucleic Acids Res. 1996 Jan 1;24(1):242–244. doi: 10.1093/nar/24.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo M. G., Fava D., Monetini L., Barone F., Pozzilli P. Cell-mediated immune response to beta casein in recent-onset insulin-dependent diabetes: implications for disease pathogenesis. Lancet. 1996 Oct 5;348(9032):926–928. doi: 10.1016/S0140-6736(95)12065-3. [DOI] [PubMed] [Google Scholar]
- Cui L., Yu W. P., DeAizpurua H. J., Schmidli R. S., Pallen C. J. Cloning and characterization of islet cell antigen-related protein-tyrosine phosphatase (PTP), a novel receptor-like PTP and autoantigen in insulin-dependent diabetes. J Biol Chem. 1996 Oct 4;271(40):24817–24823. [PubMed] [Google Scholar]
- Durinovic-Bellò I., Hummel M., Ziegler A. G. Cellular immune response to diverse islet cell antigens in IDDM. Diabetes. 1996 Jun;45(6):795–800. doi: 10.2337/diab.45.6.795. [DOI] [PubMed] [Google Scholar]
- Forrest J. M., Menser M. A., Burgess J. A. High frequency of diabetes mellitus in young adults with congenital rubella. Lancet. 1971 Aug 14;2(7720):332–334. doi: 10.1016/s0140-6736(71)90057-2. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., McGill M., Farquharson M. A., Hilton D. A. A search for evidence of viral infection in pancreases of newly diagnosed patients with IDDM. Diabetologia. 1997 Jan;40(1):53–61. doi: 10.1007/s001250050642. [DOI] [PubMed] [Google Scholar]
- Hammer J., Bono E., Gallazzi F., Belunis C., Nagy Z., Sinigaglia F. Precise prediction of major histocompatibility complex class II-peptide interaction based on peptide side chain scanning. J Exp Med. 1994 Dec 1;180(6):2353–2358. doi: 10.1084/jem.180.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. C. Cow's milk and IDDM. Lancet. 1996 Oct 5;348(9032):905–906. doi: 10.1016/S0140-6736(05)65333-0. [DOI] [PubMed] [Google Scholar]
- Hawkes C. J., Wasmeier C., Christie M. R., Hutton J. C. Identification of the 37-kDa antigen in IDDM as a tyrosine phosphatase-like protein (phogrin) related to IA-2. Diabetes. 1996 Sep;45(9):1187–1192. doi: 10.2337/diab.45.9.1187. [DOI] [PubMed] [Google Scholar]
- Heath R. R., Stagg S., Xu F., McCrae M. A. Mapping of the target antigens of the rotavirus-specific cytotoxic T cell response. J Gen Virol. 1997 May;78(Pt 5):1065–1075. doi: 10.1099/0022-1317-78-5-1065. [DOI] [PubMed] [Google Scholar]
- Hoorfar J., Buschard K., Dagnaes-Hansen F. Prophylactic nutritional modification of the incidence of diabetes in autoimmune non-obese diabetic (NOD) mice. Br J Nutr. 1993 Mar;69(2):597–607. doi: 10.1079/bjn19930059. [DOI] [PubMed] [Google Scholar]
- Hyöty H., Hiltunen M., Knip M., Laakkonen M., Vähäsalo P., Karjalainen J., Koskela P., Roivainen M., Leinikki P., Hovi T. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995 Jun;44(6):652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- Hänninen A., Salmi M., Simell O., Jalkanen S. Mucosa-associated (beta 7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes. 1996 Sep;45(9):1173–1180. doi: 10.2337/diab.45.9.1173. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Crosby I. Proliferative lymphocyte responses to virus antigens homologous to GAD65 in IDDM. Diabetologia. 1996 Nov;39(11):1318–1324. doi: 10.1007/s001250050576. [DOI] [PubMed] [Google Scholar]
- Karam J. H., Lewitt P. A., Young C. W., Nowlain R. E., Frankel B. J., Fujiya H., Freedman Z. R., Grodsky G. M. Insulinopenic diabetes after rodenticide (Vacor) ingestion: a unique model of acquired diabetes in man. Diabetes. 1980 Dec;29(12):971–978. doi: 10.2337/diab.29.12.971. [DOI] [PubMed] [Google Scholar]
- Kumar D., Gemayel N. S., Deapen D., Kapadia D., Yamashita P. H., Lee M., Dwyer J. H., Roy-Burman P., Bray G. A., Mack T. M. North-American twins with IDDM. Genetic, etiological, and clinical significance of disease concordance according to age, zygosity, and the interval after diagnosis in first twin. Diabetes. 1993 Sep;42(9):1351–1363. doi: 10.2337/diab.42.9.1351. [DOI] [PubMed] [Google Scholar]
- Lan M. S., Lu J., Goto Y., Notkins A. L. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994 May;13(5):505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Lu J., Li Q., VanderVegt F. P., Wasserfall C., Maclaren N. K., Lan M. S. IA-2 and IA-2 beta are major autoantigens in IDDM and the precursors of the 40 kDa and 37 kDa tryptic fragments. J Autoimmun. 1996 Oct;9(5):677–682. doi: 10.1006/jaut.1996.0088. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Paronen J., Klemetti P., Kantele J. M., Savilahti E., Perheentupa J., Akerblom H. K., Vaarala O. Glutamate decarboxylase-reactive peripheral blood lymphocytes from patients with IDDM express gut-specific homing receptor alpha4beta7-integrin. Diabetes. 1997 Apr;46(4):583–588. doi: 10.2337/diab.46.4.583. [DOI] [PubMed] [Google Scholar]
- Patel S. D., Cope A. P., Congia M., Chen T. T., Kim E., Fugger L., Wherrett D., Sonderstrup-McDevitt G. Identification of immunodominant T cell epitopes of human glutamic acid decarboxylase 65 by using HLA-DR(alpha1*0101,beta1*0401) transgenic mice. Proc Natl Acad Sci U S A. 1997 Jul 22;94(15):8082–8087. doi: 10.1073/pnas.94.15.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin D. U., Pleasic S. M., Shapiro J. A., Yoo-Warren H., Oles J., Hicks J. M., Goldstein D. E., Rae P. M. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994 Mar 15;152(6):3183–3188. [PubMed] [Google Scholar]
- Rott L. S., Rosé J. R., Bass D., Williams M. B., Greenberg H. B., Butcher E. C. Expression of mucosal homing receptor alpha4beta7 by circulating CD4+ cells with memory for intestinal rotavirus. J Clin Invest. 1997 Sep 1;100(5):1204–1208. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy G., Stone N., Harrison L. C., Colman P. G., McNair P., Brusic V., French M. B., Honeyman M. C., Tait B., Lew A. M. Similar peptides from two beta cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol Med. 1995 Sep;1(6):625–633. [PMC free article] [PubMed] [Google Scholar]
- Schloot N. C., Roep B. O., Wegmann D. R., Yu L., Wang T. B., Eisenbarth G. S. T-cell reactivity to GAD65 peptide sequences shared with coxsackie virus protein in recent-onset IDDM, post-onset IDDM patients and control subjects. Diabetologia. 1997 Mar;40(3):332–338. doi: 10.1007/s001250050683. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Romagnoli P., Guttinger M., Takacs B., Pink J. R. Selection of T cell epitopes and vaccine engineering. Methods Enzymol. 1991;203:370–386. doi: 10.1016/0076-6879(91)03021-8. [DOI] [PubMed] [Google Scholar]
- Solimena M., Dirkx R., Jr, Hermel J. M., Pleasic-Williams S., Shapiro J. A., Caron L., Rabin D. U. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 1996 May 1;15(9):2102–2114. [PMC free article] [PubMed] [Google Scholar]
- Tian J., Lehmann P. V., Kaufman D. L. T cell cross-reactivity between coxsackievirus and glutamate decarboxylase is associated with a murine diabetes susceptibility allele. J Exp Med. 1994 Nov 1;180(5):1979–1984. doi: 10.1084/jem.180.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden W., Anderton S. M., Van Der Zee R., Prakken B. J., Broeren C. P., Wauben M. H. (Altered) self peptides and the regulation of self reactivity in the peripheral T cell pool. Immunol Rev. 1996 Feb;149:55–73. doi: 10.1111/j.1600-065x.1996.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Verge C. F., Howard N. J., Irwig L., Simpson J. M., Mackerras D., Silink M. Environmental factors in childhood IDDM. A population-based, case-control study. Diabetes Care. 1994 Dec;17(12):1381–1389. doi: 10.2337/diacare.17.12.1381. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., Austin M., Onodera T., Notkins A. L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979 May 24;300(21):1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]


