Abstract
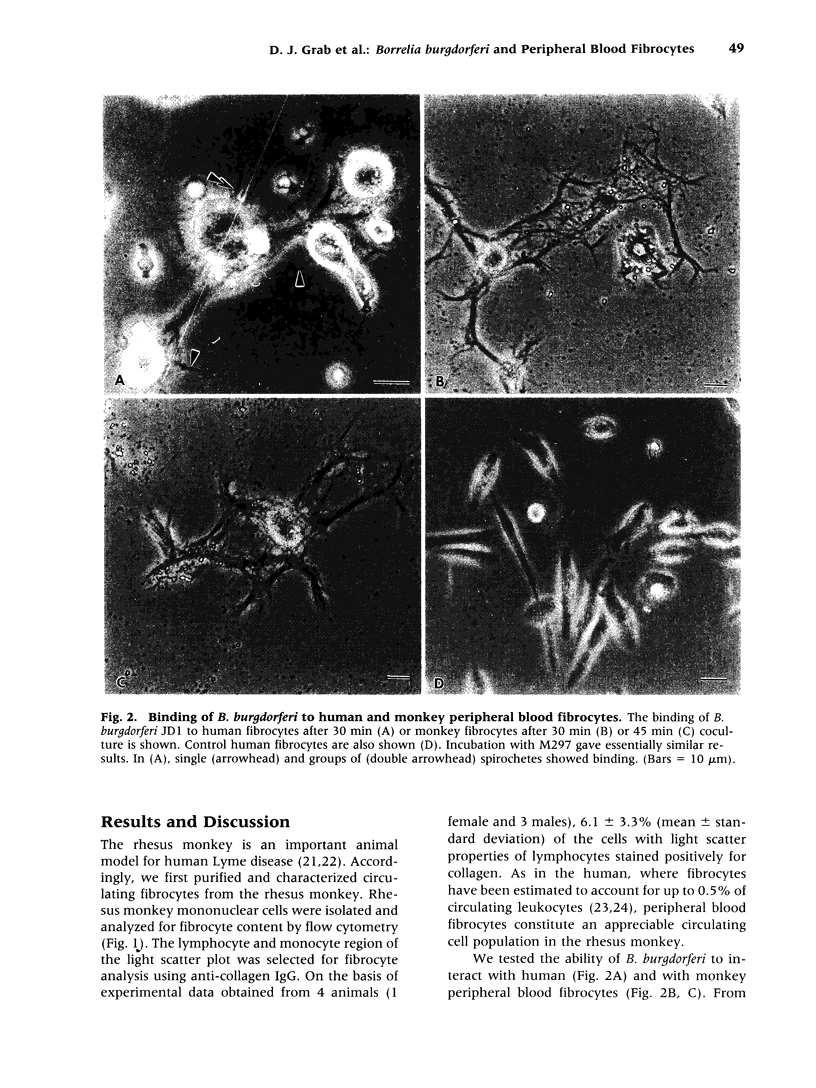
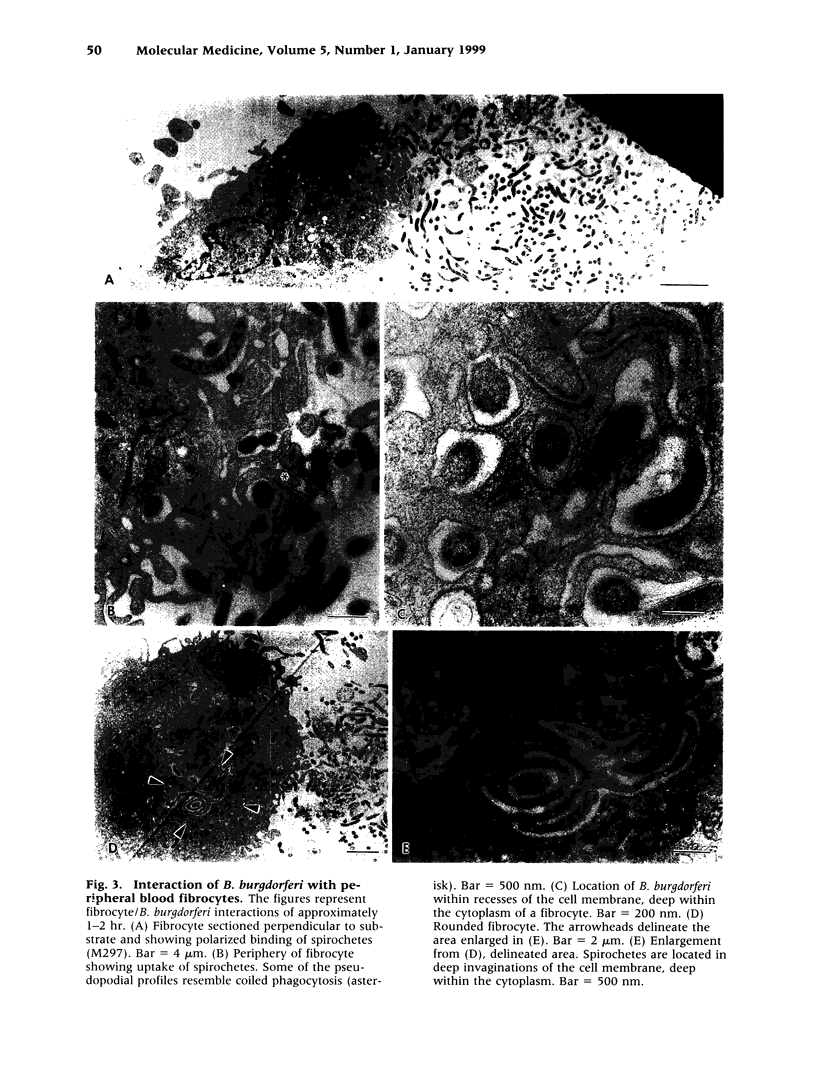
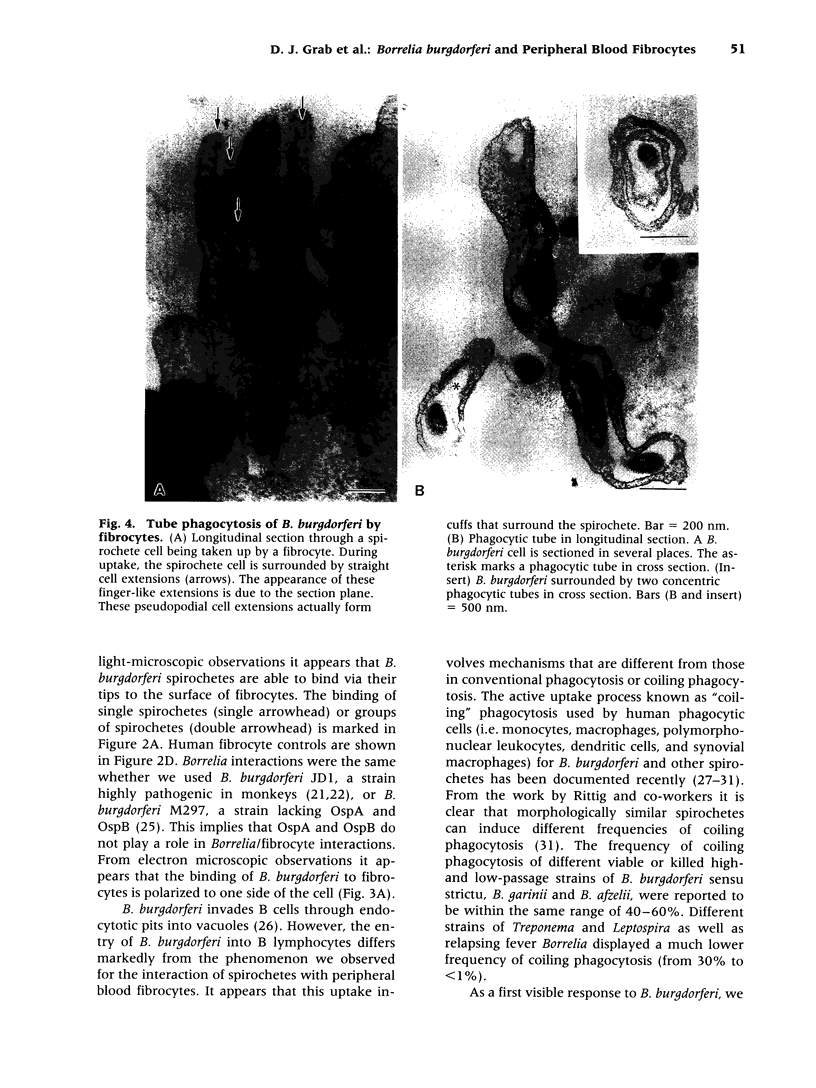
BACKGROUND: Borrelia Burgdorferi has a predilection for collagenous tissue and can interact with fibronectin and cellular collagens. While the molecular mechanisms of how B. burgdorferi targets connective tissues and causes arthritis are not understood, the spirochetes can bind to a number of different cell types, including fibroblasts. A novel circulating fibroblast-like cell called the peripheral blood fibrocyte has recently been described. Fibrocytes express collagen types I and III as well as fibronectin. Besides playing a role in wound healing, fibrocytes have the potential to target to connective tissue and the functional capacity to recruit, activate, and present antigen to CD4(+) T cells. MATERIALS AND METHODS: Rhesus monkey fibrocytes were isolated and characterized by flow cytometry. B. burgdorferi were incubated with human or monkey fibrocyte cultures in vitro and the cellular interactions analyzed by light and electron microscopy. The two strains of B. burgdorferi studied included JD1, which is highly pathogenic for monkeys, and M297, which lacks the cell surface OspA and OspB proteins. RESULTS: In this study, we demonstrate that B. burgdorferi binds to both human and monkey (rhesus) fibrocytes in vitro. This process does not require OspA or OspB. In addition, the spirochetes are not phagocytosed but are taken into deep recesses of the cell membrane, a process that may protect them from the immune system. CONCLUSIONS: This interaction between B. burgdorferi and peripheral blood fibrocytes provides a potential explanation for the targeting of spirochetes to joint connective tissue and may contribute to the inflammatory process in Lyme arthritis.
Full text
PDF

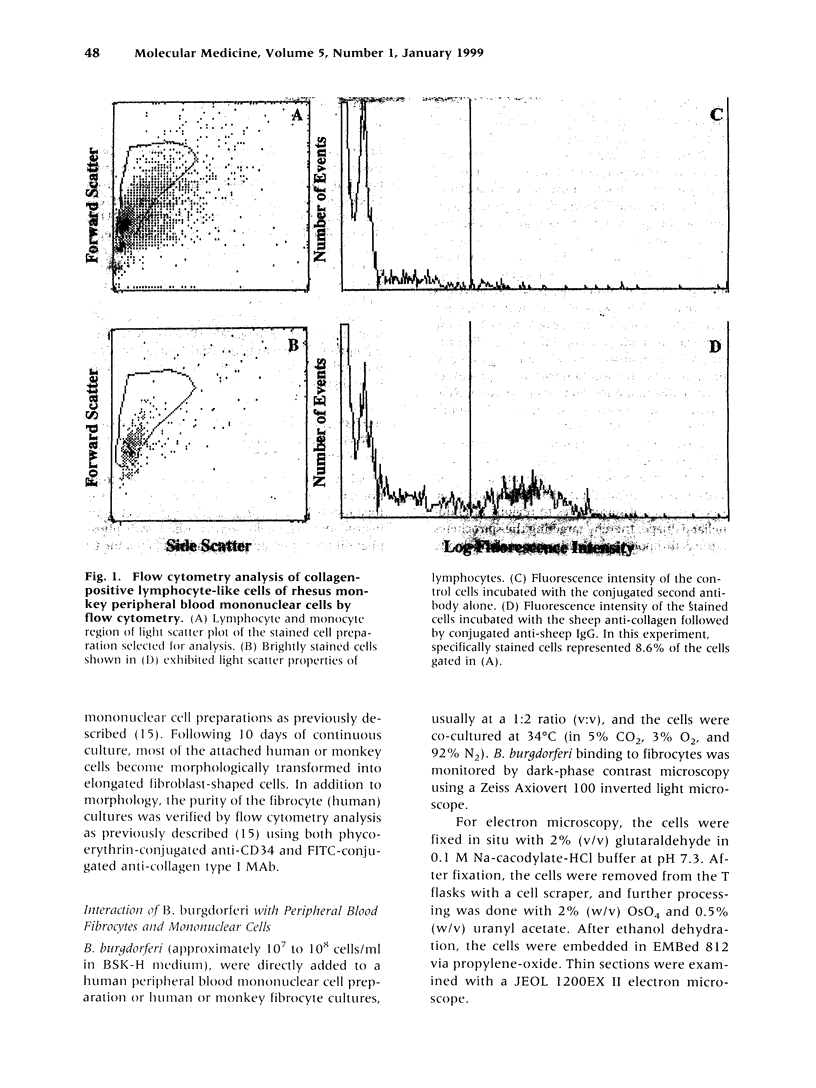



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Persing D. H., Armstrong A. L., Peeples R. A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991 Aug;139(2):263–273. [PMC free article] [PubMed] [Google Scholar]
- Chesney J., Bacher M., Bender A., Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J., Metz C., Stavitsky A. B., Bacher M., Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998 Jan 1;160(1):419–425. [PubMed] [Google Scholar]
- Coburn J., Leong J. M., Erban J. K. Integrin alpha IIb beta 3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7059–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Sellati T. J., Testa J. E., Kew R. R., Furie M. B., Benach J. L. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995 Jul;63(7):2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs H., Wallich R., Simon M. M., Kramer M. D. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgilis K., Peacocke M., Klempner M. S. Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro. J Infect Dis. 1992 Aug;166(2):440–444. doi: 10.1093/infdis/166.2.440. [DOI] [PubMed] [Google Scholar]
- Grab D. J., Givens C., Kennedy R. Fibronectin-binding activity in Borrelia burgdorferi1. Biochim Biophys Acta. 1998 Aug 14;1407(2):135–145. doi: 10.1016/s0925-4439(98)00038-6. [DOI] [PubMed] [Google Scholar]
- Grab D. J., Kennedy R., Philipp M. T. Borrelia burgdorferi possesses a collagenolytic activity. FEMS Microbiol Lett. 1996 Oct 15;144(1):39–45. doi: 10.1111/j.1574-6968.1996.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Hu L. T., Perides G., Noring R., Klempner M. S. Binding of human plasminogen to Borrelia burgdorferi. Infect Immun. 1995 Sep;63(9):3491–3496. doi: 10.1128/iai.63.9.3491-3496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Engstrom S. M., Coleman L. A., Kodner C. B., Johnson R. C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993 Dec;61(12):5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häupl T., Hahn G., Rittig M., Krause A., Schoerner C., Schönherr U., Kalden J. R., Burmester G. R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993 Nov;36(11):1621–1626. doi: 10.1002/art.1780361118. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Noring R., Epstein M. P., McCloud B., Hu R., Limentani S. A., Rogers R. A. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1995 May;171(5):1258–1265. doi: 10.1093/infdis/171.5.1258. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Noring R., Rogers R. A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1993 May;167(5):1074–1081. doi: 10.1093/infdis/167.5.1074. [DOI] [PubMed] [Google Scholar]
- Montgomery R. R., Malawista S. E. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect Immun. 1996 Jul;64(7):2867–2872. doi: 10.1128/iai.64.7.2867-2872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachner A. R., Basta J., Delaney E., Hulinska D. Localization of Borrelia burgdorferi in murine Lyme borreliosis by electron microscopy. Am J Trop Med Hyg. 1995 Feb;52(2):128–133. doi: 10.4269/ajtmh.1995.52.128. [DOI] [PubMed] [Google Scholar]
- Philipp M. T., Aydintug M. K., Bohm R. P., Jr, Cogswell F. B., Dennis V. A., Lanners H. N., Lowrie R. C., Jr, Roberts E. D., Conway M. D., Karaçorlu M. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993 Jul;61(7):3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig M. G., Häupl T., Krause A., Kressel M., Groscurth P., Burmester G. R. Borrelia burgdorferi-induced ultrastructural alterations in human phagocytes: a clue to pathogenicity? J Pathol. 1994 Jul;173(3):269–282. doi: 10.1002/path.1711730311. [DOI] [PubMed] [Google Scholar]
- Rittig M. G., Jagoda J. C., Wilske B., Murgia R., Cinco M., Repp R., Burmester G. R., Krause A. Coiling phagocytosis discriminates between different spirochetes and is enhanced by phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor. Infect Immun. 1998 Feb;66(2):627–635. doi: 10.1128/iai.66.2.627-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig M. G., Kuhn K. H., Dechant C. A., Gauckler A., Modolell M., Ricciardi-Castagnoli P., Krause A., Burmester G. R. Phagocytes from both vertebrate and invertebrate species use "coiling" phagocytosis. Dev Comp Immunol. 1996 Nov-Dec;20(6):393–406. doi: 10.1016/s0145-305x(96)00023-7. [DOI] [PubMed] [Google Scholar]
- Roberts E. D., Bohm R. P., Jr, Cogswell F. B., Lanners H. N., Lowrie R. C., Jr, Povinelli L., Piesman J., Philipp M. T. Chronic lyme disease in the rhesus monkey. Lab Invest. 1995 Feb;72(2):146–160. [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Suhonen J., Hartiala K., Viljanen M. K. Tube phagocytosis, a novel way for neutrophils to Phagocytize borrelia burgdorferi. Infect Immun. 1998 Jul;66(7):3433–3435. doi: 10.1128/iai.66.7.3433-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski A., Furie M. B., Benach J. L., Lane B. P., Fleit H. B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990 May;85(5):1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. R., Austin F. E. Hemolytic activity of Borrelia burgdorferi. Infect Immun. 1992 Aug;60(8):3224–3230. doi: 10.1128/iai.60.8.3224-3230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]