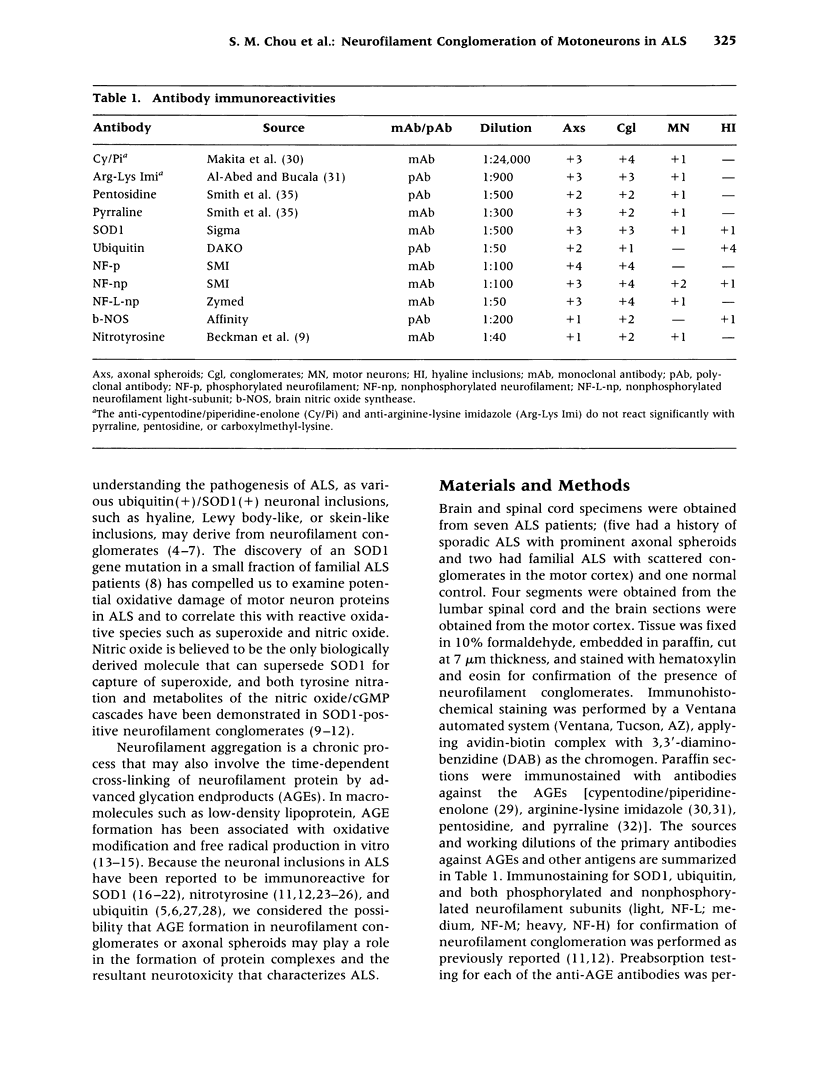
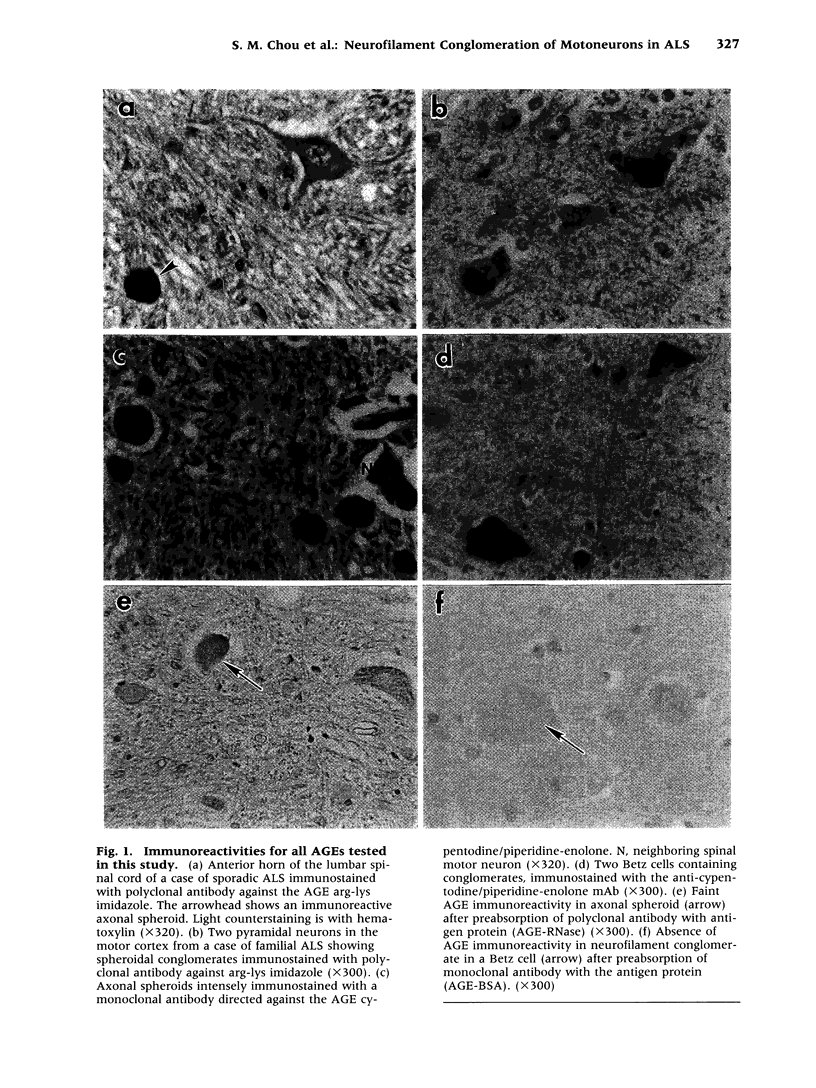
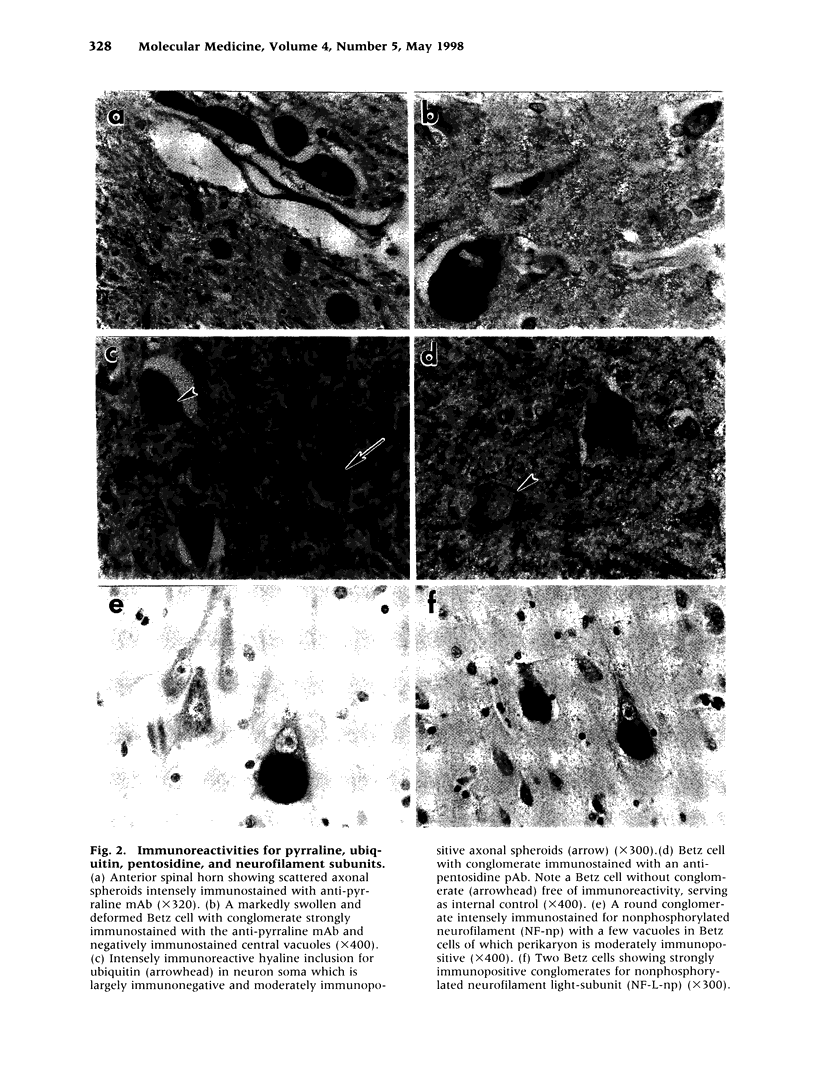
Abstract
BACKGROUND: Massive neurofilament conglomeration in motor neurons has been described to occur in the early stages of both familial and sporadic amyotrophic lateral sclerosis (ALS). Previously, neurofilament conglomerates were immunolabeled for both superoxide dismutase (SOD1) and nitrotyrosine, suggesting the potential for oxidative nitration damage to neurofilament protein by peroxynitrite. Long-lived neurofilaments may also undergo modification by advanced glycation endproducts (AGEs) with concomitant generation of free radicals, including superoxide. This radical species may then react with nitric oxide to form the potent oxidant, peroxynitrite, which in turn can nitrate neurofilament protein. Such a glycated and nitrated neurofilament protein may become resistant to proteolytic systems, forming high-molecular-weight protein complexes and cytotoxic, neuronal inclusions. MATERIALS AND METHODS: Paraffin sections containing both neurofilament conglomerates and neuronal inclusions were obtained from patients with sporadic (n = 5) and familial (n = 2) ALS and were probed with specific antibodies directed against the AGEs cypentodine/piperidine-enolone, arginine-lysine imidazole, pentosidine, and pyrraline. RESULTS: Neurofilament conglomerates, but not neuronal inclusions, were intensely immunolabeled with each of the anti-AGE antibodies tested. The immunoreactivity was selective for neurofilament conglomerates and suggested that AGEs may form inter- or intramolecular cross-links in neurofilament proteins. CONCLUSIONS: These data support the hypothesis that AGE formation affects neurofilament proteins in vivo and is associated with the concomitant induction of SODI and protein nitration in neurofilament conglomerates. AGE formation in neurofilament protein may not only cause covalent cross-linking but also generate superoxide and block nitric oxide-mediated responses, thereby perpetuating neuronal toxicity in patients with ALS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Pan L. H., Watanabe M., Konno H., Kato T., Itoyama Y. Upregulation of protein-tyrosine nitration in the anterior horn cells of amyotrophic lateral sclerosis. Neurol Res. 1997 Apr;19(2):124–128. doi: 10.1080/01616412.1997.11740784. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Ferrante R. J., Browne S. E., Matthews R. T., Kowall N. W., Brown R. H., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997 Oct;42(4):644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Carson M., Smith C. D., Koppenol W. H. ALS, SOD and peroxynitrite. Nature. 1993 Aug 12;364(6438):584–584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Ye Y. Z., Chen J., Conger K. A. The interactions of nitric oxide with oxygen radicals and scavengers in cerebral ischemic injury. Adv Neurol. 1996;71:339–354. [PubMed] [Google Scholar]
- Brett J., Schmidt A. M., Yan S. D., Zou Y. S., Weidman E., Pinsky D., Nowygrod R., Neeper M., Przysiecki C., Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993 Dec;143(6):1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Bruijn L. I., Beal M. F., Becher M. W., Schulz J. B., Wong P. C., Price D. L., Cleveland D. W. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn L. I., Becher M. W., Lee M. K., Anderson K. L., Jenkins N. A., Copeland N. G., Sisodia S. S., Rothstein J. D., Borchelt D. R., Price D. L. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997 Feb;18(2):327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Carpenter S. Proximal axonal enlargement in motor neuron disease. Neurology. 1968 Sep;18(9):841–851. doi: 10.1212/wnl.18.9.841. [DOI] [PubMed] [Google Scholar]
- Chou S. M. Neuropathology of amyotrophic lateral sclerosis: new perspectives on an old disease. J Formos Med Assoc. 1997 Jul;96(7):488–498. [PubMed] [Google Scholar]
- Chou S. M., Wang H. S., Komai K. Colocalization of NOS and SOD1 in neurofilament accumulation within motor neurons of amyotrophic lateral sclerosis: an immunohistochemical study. J Chem Neuroanat. 1996 Jun;10(3-4):249–258. doi: 10.1016/0891-0618(96)00137-8. [DOI] [PubMed] [Google Scholar]
- Chou S. M., Wang H. S., Taniguchi A. Role of SOD-1 and nitric oxide/cyclic GMP cascade on neurofilament aggregation in ALS/MND. J Neurol Sci. 1996 Aug;139 (Suppl):16–26. doi: 10.1016/0022-510x(96)00090-1. [DOI] [PubMed] [Google Scholar]
- Collard J. F., Côté F., Julien J. P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995 May 4;375(6526):61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- Côté F., Collard J. F., Houle D., Julien J. P. Copy-dependent and correct developmental expression of the human neurofilament heavy gene in transgenic mice. Brain Res Mol Brain Res. 1994 Oct;26(1-2):99–105. doi: 10.1016/0169-328x(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Gurney M. E. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am J Pathol. 1994 Dec;145(6):1271–1279. [PMC free article] [PubMed] [Google Scholar]
- Dong D. L., Xu Z. S., Chevrier M. R., Cotter R. J., Cleveland D. W., Hart G. W. Glycosylation of mammalian neurofilaments. Localization of multiple O-linked N-acetylglucosamine moieties on neurofilament polypeptides L and M. J Biol Chem. 1993 Aug 5;268(22):16679–16687. [PubMed] [Google Scholar]
- Dong D. L., Xu Z. S., Hart G. W., Cleveland D. W. Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J Biol Chem. 1996 Aug 23;271(34):20845–20852. doi: 10.1074/jbc.271.34.20845. [DOI] [PubMed] [Google Scholar]
- Fu M. X., Requena J. R., Jenkins A. J., Lyons T. J., Baynes J. W., Thorpe S. R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996 Apr 26;271(17):9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- Fu M. X., Wells-Knecht K. J., Blackledge J. A., Lyons T. J., Thorpe S. R., Baynes J. W. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 1994 May;43(5):676–683. doi: 10.2337/diab.43.5.676. [DOI] [PubMed] [Google Scholar]
- Gou J. P., Eyer J., Leterrier J. F. Progressive hyperphosphorylation of neurofilament heavy subunits with aging: possible involvement in the mechanism of neurofilament accumulation. Biochem Biophys Res Commun. 1995 Oct 4;215(1):368–376. doi: 10.1006/bbrc.1995.2475. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Greis K. D., Dong L. Y., Blomberg M. A., Chou T. Y., Jiang M. S., Roquemore E. P., Snow D. M., Kreppel L. K., Cole R. N. O-linked N-acetylglucosamine: the "yin-yang" of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol. 1995;376:115–123. [PubMed] [Google Scholar]
- Julien J. P., Côté F., Collard J. F. Mice overexpressing the human neurofilament heavy gene as a model of ALS. Neurobiol Aging. 1995 May-Jun;16(3):487–492. doi: 10.1016/0197-4580(94)00169-2. [DOI] [PubMed] [Google Scholar]
- Lee M. K., Marszalek J. R., Cleveland D. W. A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron. 1994 Oct;13(4):975–988. doi: 10.1016/0896-6273(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Leigh P. N., Anderton B. H., Dodson A., Gallo J. M., Swash M., Power D. M. Ubiquitin deposits in anterior horn cells in motor neurone disease. Neurosci Lett. 1988 Nov 11;93(2-3):197–203. doi: 10.1016/0304-3940(88)90081-x. [DOI] [PubMed] [Google Scholar]
- Ma D., Descarries L., Julien J. P., Doucet G. Abnormal perikaryal accumulation of neurofilament light protein in the brain of mice transgenic for the human protein: sequence of postnatal development. Neuroscience. 1995 Sep;68(1):135–149. doi: 10.1016/0306-4522(95)00088-z. [DOI] [PubMed] [Google Scholar]
- Makita Z., Bucala R., Rayfield E. J., Friedman E. A., Kaufman A. M., Korbet S. M., Barth R. H., Winston J. A., Fuh H., Manogue K. R. Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet. 1994 Jun 18;343(8912):1519–1522. doi: 10.1016/s0140-6736(94)92935-1. [DOI] [PubMed] [Google Scholar]
- McLean W. G., Pekiner C., Cullum N. A., Casson I. F. Posttranslational modifications of nerve cytoskeletal proteins in experimental diabetes. Mol Neurobiol. 1992 Summer-Fall;6(2-3):225–237. doi: 10.1007/BF02780555. [DOI] [PubMed] [Google Scholar]
- Migheli A., Attanasio A., Schiffer D. Ubiquitin and neurofilament expression in anterior horn cells in amyotrophic lateral sclerosis: possible clues to the pathogenesis. Neuropathol Appl Neurobiol. 1994 Jun;20(3):282–289. doi: 10.1111/j.1365-2990.1994.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Sell D. R., Nagaraj R. H., Miyata S., Grandhee S., Odetti P., Ibrahim S. A. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992 Oct;41 (Suppl 2):36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- Murayama S., Mori H., Ihara Y., Bouldin T. W., Suzuki K., Tomonaga M. Immunocytochemical and ultrastructural studies of lower motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 1990 Feb;27(2):137–148. doi: 10.1002/ana.410270208. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Horii Y., Nishino T., Shiiki H., Sakaguchi Y., Kagoshima T., Dohi K., Makita Z., Vlassara H., Bucala R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol. 1993 Dec;143(6):1649–1656. [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Murayama S., Shimizu J., Ohya Y., Kwak S., Asayama K., Kanazawa I. Cu/Zn superoxide dismutase-like immunoreactivity is present in Lewy bodies from Parkinson disease: a light and electron microscopic immunocytochemical study. Acta Neuropathol. 1995;89(6):471–474. doi: 10.1007/BF00571500. [DOI] [PubMed] [Google Scholar]
- Pardo C. A., Xu Z., Borchelt D. R., Price D. L., Sisodia S. S., Cleveland D. W. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rouleau G. A., Clark A. W., Rooke K., Pramatarova A., Krizus A., Suchowersky O., Julien J. P., Figlewicz D. SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann Neurol. 1996 Jan;39(1):128–131. doi: 10.1002/ana.410390119. [DOI] [PubMed] [Google Scholar]
- Ryle C., Donaghy M. Non-enzymatic glycation of peripheral nerve proteins in human diabetics. J Neurol Sci. 1995 Mar;129(1):62–68. doi: 10.1016/0022-510x(94)00251-i. [DOI] [PubMed] [Google Scholar]
- Ryle C., Leow C. K., Donaghy M. Nonenzymatic glycation of peripheral and central nervous system proteins in experimental diabetes mellitus. Muscle Nerve. 1997 May;20(5):577–584. doi: 10.1002/(sici)1097-4598(199705)20:5<577::aid-mus6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Schiffer D., Autilio-Gambetti L., Chiò A., Gambetti P., Giordana M. T., Gullotta F., Migheli A., Vigliani M. C. Ubiquitin in motor neuron disease: study at the light and electron microscope. J Neuropathol Exp Neurol. 1991 Jul;50(4):463–473. doi: 10.1097/00005072-199107000-00007. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Hori O., Brett J., Yan S. D., Wautier J. L., Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994 Oct;14(10):1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- Shibata N., Hirano A., Kobayashi M., Sasaki S., Kato T., Matsumoto S., Shiozawa Z., Komori T., Ikemoto A., Umahara T. Cu/Zn superoxide dismutase-like immunoreactivity in Lewy body-like inclusions of sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1994 Sep 26;179(1-2):149–152. doi: 10.1016/0304-3940(94)90956-3. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Richey Harris P. L., Sayre L. M., Beckman J. S., Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997 Apr 15;17(8):2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Taneda S., Richey P. L., Miyata S., Yan S. D., Stern D., Sayre L. M., Monnier V. M., Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt A. W., Li Y. M., Gardiner T. A., Bucala R., Archer D. B., Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997 Feb;150(2):523–531. [PMC free article] [PubMed] [Google Scholar]
- Tu P. H., Raju P., Robinson K. A., Gurney M. E., Trojanowski J. Q., Lee V. M. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Bucala R., Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994 Feb;70(2):138–151. [PubMed] [Google Scholar]
- Wu W. Expression of nitric-oxide synthase (NOS) in injured CNS neurons as shown by NADPH diaphorase histochemistry. Exp Neurol. 1993 Apr;120(2):153–159. doi: 10.1006/exnr.1993.1050. [DOI] [PubMed] [Google Scholar]
- Wu Y., Li Y., Liu H., Wu W. Induction of nitric oxide synthase and motoneuron death in newborn and early postnatal rats following spinal root avulsion. Neurosci Lett. 1995 Jul 14;194(1-2):109–112. doi: 10.1016/0304-3940(95)11741-e. [DOI] [PubMed] [Google Scholar]
- Xu Z., Cork L. C., Griffin J. W., Cleveland D. W. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993 Apr 9;73(1):23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- Yagihashi S. Axonal cytoskeleton and diabetic neuropathy. Diabet Med. 1993;10 (Suppl 2):107S–109S. doi: 10.1111/j.1464-5491.1993.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Yim H. S., Kang S. O., Hah Y. C., Chock P. B., Yim M. B. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. J Biol Chem. 1995 Nov 24;270(47):28228–28233. doi: 10.1074/jbc.270.47.28228. [DOI] [PubMed] [Google Scholar]
- Yim H. S., Kang S. O., Hah Y. C., Chock P. B., Yim M. B. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. J Biol Chem. 1995 Nov 24;270(47):28228–28233. doi: 10.1074/jbc.270.47.28228. [DOI] [PubMed] [Google Scholar]
- Yu W. H. Nitric oxide synthase in motor neurons after axotomy. J Histochem Cytochem. 1994 Apr;42(4):451–457. doi: 10.1177/42.4.7510317. [DOI] [PubMed] [Google Scholar]
- Zhu M., Spink D. C., Yan B., Bank S., DeCaprio A. P. Formation and structure of cross-linking and monomeric pyrrole autoxidation products in 2,5-hexanedione-treated amino acids, peptides, and protein. Chem Res Toxicol. 1994 Jul-Aug;7(4):551–558. doi: 10.1021/tx00040a011. [DOI] [PubMed] [Google Scholar]