Abstract
BACKGROUND: The development of effective adjuvant therapies for the treatment of high-risk melanoma patients is critical for the prevention of metastatic disease and improvement of patient survival. Active specific immunotherapy has been tested as an adjuvant treatment in numerous clinical trials with overall limited, but occasionally promising, success rates. Newcastle disease virus (NDV) oncolysate has been utilized as an adjunctive immunotherapeutic agent in the postsurgical management of these patients. A phase II study initiated in 1975 using adjuvant vaccine therapy composed of allogeneic and autologous human melanoma cells infected with live NDV (NDV oncolysate) in patients with AJCC stage III melanoma following therapeutic lymph node dissection has shown >60% survival rate at 10 years with no adverse effects. Continued long-term analysis of trials with promising early results as well as assessment of immunologic responses generated in these patients may result in improved therapeutic decisions for clinical trials in the future. MATERIALS AND METHODS: We analyzed the 15-year survival of patients treated postsurgically with NDV oncolysate in the phase II study described above. In an attempt to understand the immunological effects of this treatment, we have also carried out a comprehensive analysis of the peripheral blood T cell repertoire in these patients. RESULTS: The overall 15-year survival of this group of patients is 55%. Previous studies have suggested that improved outcome in patients undergoing immunotherapy is correlated with increased numbers of CD8(+)CD57(+) cells. In surviving patients, we observed a striking oligoclonality in the CD8(+) T cell population in peripheral blood, which reflects clonal expansions in the CD8(+)CD57(+) subset. CONCLUSIONS: The data suggest that adjuvant vaccination with NDV oncolysates is associated with prolonged survival of patients with lymph node-positive malignant melanoma and that CD8(+) T cells may be an important component of therapeutic efficacy.
Full text
PDF



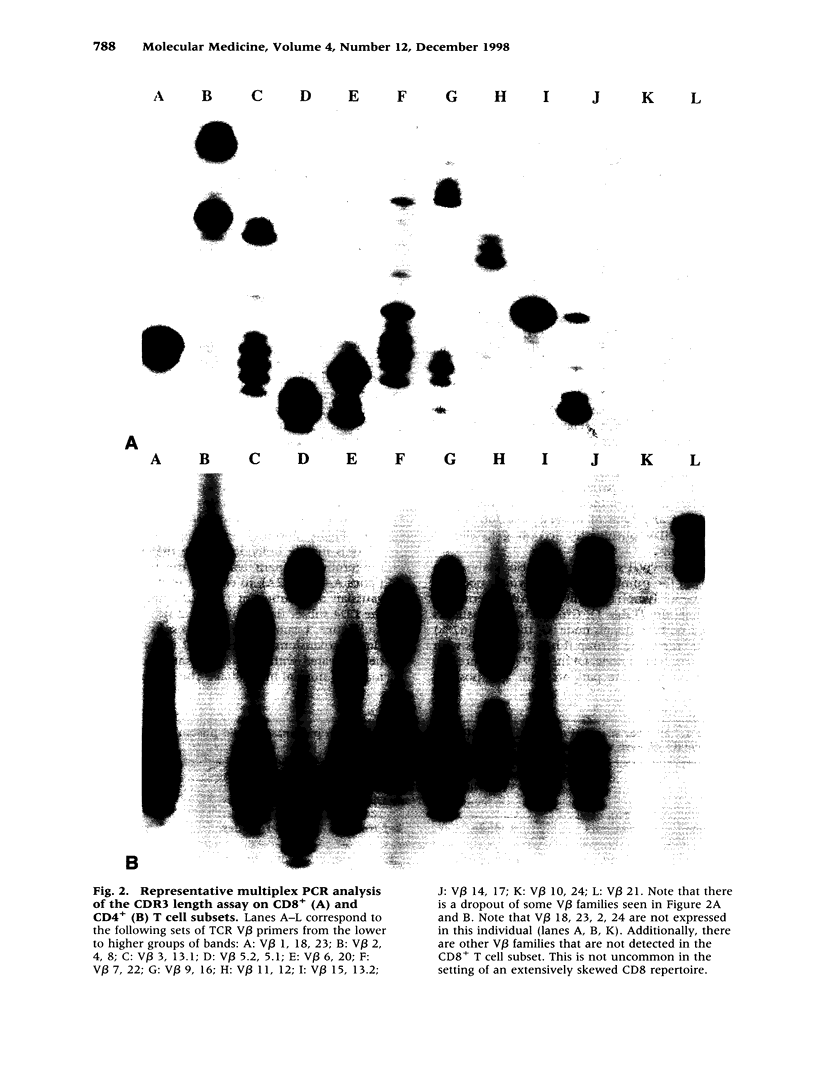
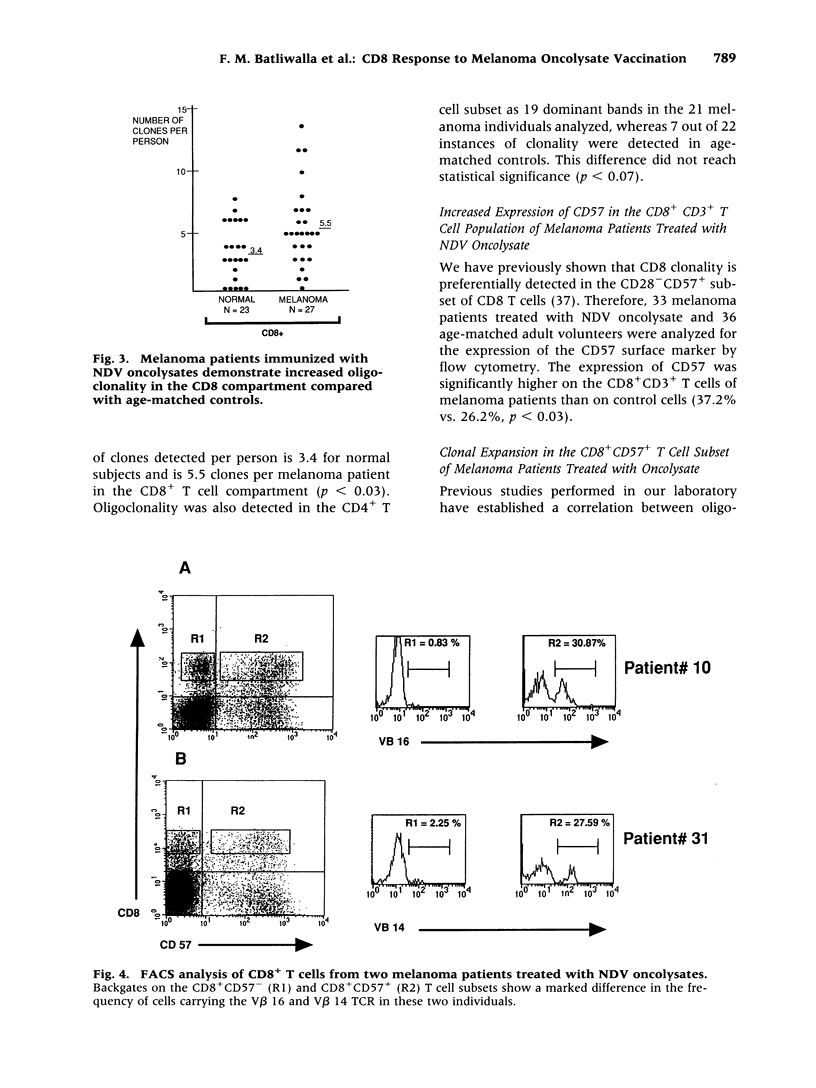




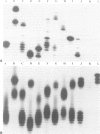
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed I. Malignant melanoma: prognostic indicators. Mayo Clin Proc. 1997 Apr;72(4):356–361. doi: 10.4065/72.4.356. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Murad T. M., Ingalls A. L., Maddox W. A. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II). Ann Surg. 1981 Mar;193(3):377–388. doi: 10.1097/00000658-198103000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A., Morton D. L. The role of adjuvant therapy in melanoma management. Cancer. 1995 Jan 15;75(2 Suppl):726–734. doi: 10.1002/1097-0142(19950115)75:2+<726::aid-cncr2820751417>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Barth A., Morton D. L. The role of adjuvant therapy in melanoma management. Cancer. 1995 Jan 15;75(2 Suppl):726–734. doi: 10.1002/1097-0142(19950115)75:2+<726::aid-cncr2820751417>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Berd D., Maguire H. C., Jr, Schuchter L. M., Hamilton R., Hauck W. W., Sato T., Mastrangelo M. J. Autologous hapten-modified melanoma vaccine as postsurgical adjuvant treatment after resection of nodal metastases. J Clin Oncol. 1997 Jun;15(6):2359–2370. doi: 10.1200/JCO.1997.15.6.2359. [DOI] [PubMed] [Google Scholar]
- CASSEL W. A., GARRETT R. E. NEWCASTLE DISEASE VIRUS AS AN ANTINEOPLASTIC AGENT. Cancer. 1965 Jul;18:863–868. doi: 10.1002/1097-0142(196507)18:7<863::aid-cncr2820180714>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Callan M. F., Reyburn H. T., Bowness P., Ottenhoff T. H., Engel I., Klausner R. D., Bell J. I., McMichael A. A method for producing monoclonal antibodies to human T-cell-receptor beta-chain variable regions. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10454–10458. doi: 10.1073/pnas.90.22.10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callery C., Cochran A. J., Roe D. J., Rees W., Nathanson S. D., Benedetti J. K., Elashoff R. M., Morton D. L. Factors prognostic for survival in patients with malignant melanoma spread to the regional lymph nodes. Ann Surg. 1982 Jul;196(1):69–75. doi: 10.1097/00000658-198207000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartei G., Sala P. G., Sanzari M., Ceschia V., Clocchiatti L., Sibau A., Donà S., Giovannoni M., Vigevani E. Reduced lymphocyte subpopulations in patients with advanced or disseminated melanoma. J Am Acad Dermatol. 1993 May;28(5 Pt 1):738–744. doi: 10.1016/0190-9622(93)70103-z. [DOI] [PubMed] [Google Scholar]
- Cassel W. A., Murray D. R. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother. 1992;9(4):169–171. doi: 10.1007/BF02987752. [DOI] [PubMed] [Google Scholar]
- Cassel W. A., Murray D. R., Torbin A. H., Olkowski Z. L., Moore M. E. Viral oncolysate in the management of malignant melanoma. I. Preparation of the oncolysate and measurement of immunologic responses. Cancer. 1977 Aug;40(2):672–679. doi: 10.1002/1097-0142(197708)40:2<672::aid-cncr2820400213>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cassel W. A., Murray D. R. Treatment of stage II malignant melanoma patients with a Newcastle disease virus oncolysate. Nat Immun Cell Growth Regul. 1988;7(5-6):351–352. [PubMed] [Google Scholar]
- Cassel W. A., Weidenheim K. M., Campbell W. G., Jr, Murray D. R. Malignant melanoma. Inflammatory mononuclear cell infiltrates in cerebral metastases during concurrent therapy with viral oncolysate. Cancer. 1986 Apr 1;57(7):1302–1312. doi: 10.1002/1097-0142(19860401)57:7<1302::aid-cncr2820570709>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Deaths from melanoma--United States, 1973-1992. MMWR Morb Mortal Wkly Rep. 1995 May 5;44(17):337, 343-7. [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Lafferty J., White J., Pigeon M., Kubo R., Kappler J., Marrack P. A method for production of antibodies to human T-cell receptor beta-chain variable regions. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8357–8361. doi: 10.1073/pnas.88.19.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Cancer vaccines get a shot in the arm. Science. 1993 Nov 5;262(5135):841–843. doi: 10.1126/science.8235605. [DOI] [PubMed] [Google Scholar]
- Day C. L., Jr, Mihm M. C., Jr, Lew R. A., Kopf A. W., Sober A. J., Fitzpatrick T. B. Cutaneous malignant melanoma: prognostic guidelines for physicians and patients. CA Cancer J Clin. 1982 Mar-Apr;32(2):113–122. doi: 10.3322/canjclin.32.2.113. [DOI] [PubMed] [Google Scholar]
- Elliott G. T., McLeod R. A., Perez J., Von Eschen K. B. Interim results of a phase II multicenter clinical trial evaluating the activity of a therapeutic allogeneic melanoma vaccine (theraccine) in the treatment of disseminated malignant melanoma. Semin Surg Oncol. 1993 May-Jun;9(3):264–272. [PubMed] [Google Scholar]
- Ettinghausen S. E., Rosenberg S. A. Immunotherapy and gene therapy of cancer. Adv Surg. 1995;28:223–254. [PubMed] [Google Scholar]
- Fortner J. G., Woodruff J., Schottenfeld D., Maclean B. Biostatistical basis of elective node dissection for malignant melanoma. Ann Surg. 1977 Jul;186(1):101–103. doi: 10.1097/00000658-197707000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Hingorani R., Monteiro J. Oligoclonality in the CD8+ T-cell population. Analysis using a multiplex PCR assay for CDR3 length. Ann N Y Acad Sci. 1995 Jul 7;756:19–27. doi: 10.1111/j.1749-6632.1995.tb44479.x. [DOI] [PubMed] [Google Scholar]
- Hingorani R., Choi I. H., Akolkar P., Gulwani-Akolkar B., Pergolizzi R., Silver J., Gregersen P. K. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993 Nov 15;151(10):5762–5769. [PubMed] [Google Scholar]
- Karakousis C. P., Seddiq M. K., Moore R. Prognostic value of lymph node dissection in malignant melanoma. Arch Surg. 1980 Jun;115(6):719–722. doi: 10.1001/archsurg.1980.01380060021006. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. M., Strawderman M. H., Ernstoff M. S., Smith T. J., Borden E. C., Blum R. H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996 Jan;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- Koh H. K. Cutaneous melanoma. N Engl J Med. 1991 Jul 18;325(3):171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- Liebrich W., Schlag P., Manasterski M., Lehner B., Stöhr M., Möller P., Schirrmacher V. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer. 1991;27(6):703–710. doi: 10.1016/0277-5379(91)90170-i. [DOI] [PubMed] [Google Scholar]
- Livingston P. O., Wong G. Y., Adluri S., Tao Y., Padavan M., Parente R., Hanlon C., Calves M. J., Helling F., Ritter G. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994 May;12(5):1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- Lorence R. M., Reichard K. W., Katubig B. B., Reyes H. M., Phuangsab A., Mitchell B. R., Cascino C. J., Walter R. J., Peeples M. E. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst. 1994 Aug 17;86(16):1228–1233. doi: 10.1093/jnci/86.16.1228. [DOI] [PubMed] [Google Scholar]
- Lorence R. M., Rood P. A., Kelley K. W. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J Natl Cancer Inst. 1988 Oct 19;80(16):1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Monteiro J., Hingorani R., Choi I. H., Silver J., Pergolizzi R., Gregersen P. K. Oligoclonality in the human CD8+ T cell repertoire in normal subjects and monozygotic twins: implications for studies of infectious and autoimmune diseases. Mol Med. 1995 Sep;1(6):614–624. [PMC free article] [PubMed] [Google Scholar]
- Morley J. K., Batliwalla F. M., Hingorani R., Gregersen P. K. Oligoclonal CD8+ T cells are preferentially expanded in the CD57+ subset. J Immunol. 1995 Jun 1;154(11):6182–6190. [PubMed] [Google Scholar]
- Morton D. L., Barth A. Vaccine therapy for malignant melanoma. CA Cancer J Clin. 1996 Jul-Aug;46(4):225–244. doi: 10.3322/canjclin.46.4.225. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Wanek L., Nizze J. A., Elashoff R. M., Wong J. H. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991 Oct;214(4):491–501. doi: 10.1097/00000658-199110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji B., Chakraborty N. G. Immunobiology and immunotherapy of melanoma. Curr Opin Oncol. 1995 Mar;7(2):175–184. doi: 10.1097/00001622-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Murray D. R., Cassel W. A., Torbin A. H., Olkowski Z. L., Moore M. E. Viral oncolysate in the management of malignant melanoma. II. Clinical studies. Cancer. 1977 Aug;40(2):680–686. doi: 10.1002/1097-0142(197708)40:2<680::aid-cncr2820400214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nishimura M. I., Kawakami Y., Charmley P., O'Neil B., Shilyansky J., Yannelli J. R., Rosenberg S. A., Hood L. T-cell receptor repertoire in tumor-infiltrating lymphocytes. Analysis of melanoma-specific long-term lines. J Immunother Emphasis Tumor Immunol. 1994 Aug;16(2):85–94. doi: 10.1097/00002371-199408000-00002. [DOI] [PubMed] [Google Scholar]
- Padovan E., Casorati G., Dellabona P., Meyer S., Brockhaus M., Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993 Oct 15;262(5132):422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- Plaksin D., Porgador A., Vadai E., Feldman M., Schirrmacher V., Eisenbach L. Effective anti-metastatic melanoma vaccination with tumor cells transfected with MHC genes and/or infected with Newcastle disease virus (NDV). Int J Cancer. 1994 Dec 15;59(6):796–801. doi: 10.1002/ijc.2910590615. [DOI] [PubMed] [Google Scholar]
- Reynolds S. R., Oratz R., Shapiro R. L., Hao P., Yun Z., Fotino M., Vukmanović S., Bystryn J. C. Stimulation of CD8+ T cell responses to MAGE-3 and Melan A/MART-1 by immunization to a polyvalent melanoma vaccine. Int J Cancer. 1997 Sep 17;72(6):972–976. doi: 10.1002/(sici)1097-0215(19970917)72:6<972::aid-ijc9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Rigel D. S. Malignant melanoma: perspectives on incidence and its effects on awareness, diagnosis, and treatment. CA Cancer J Clin. 1996 Jul-Aug;46(4):195–198. doi: 10.3322/canjclin.46.4.195. [DOI] [PubMed] [Google Scholar]
- Sato T., McCue P., Masuoka K., Salwen S., Lattime E. C., Mastrangelo M. J., Berd D. Interleukin 10 production by human melanoma. Clin Cancer Res. 1996 Aug;2(8):1383–1390. [PubMed] [Google Scholar]
- Sensi M., Farina C., Maccalli C., Lupetti R., Nicolini G., Anichini A., Parmiani G., Berd D. Clonal expansion of T lymphocytes in human melanoma metastases after treatment with a hapten-modified autologous tumor vaccine. J Clin Invest. 1997 Feb 15;99(4):710–717. doi: 10.1172/JCI119215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkovics J. G. Viral oncolysates as human tumor vaccines. Int Rev Immunol. 1991;7(4):259–287. doi: 10.3109/08830189109114875. [DOI] [PubMed] [Google Scholar]
- Sinkovics J., Horvath J. New developments in the virus therapy of cancer: a historical review. Intervirology. 1993;36(4):193–214. doi: 10.1159/000150339. [DOI] [PubMed] [Google Scholar]
- Sondak V. K., Wolfe J. A. Adjuvant therapy for melanoma. Curr Opin Oncol. 1997 Mar;9(2):189–204. doi: 10.1097/00001622-199703000-00015. [DOI] [PubMed] [Google Scholar]
- Stoeck M., Marland-Noske C., Manasterski M., Zawatzky R., Horn S., Möbus V., Schlag P., Schirrmacher V. In vitro expansion and analysis of T lymphocyte microcultures obtained from the vaccination sites of cancer patients undergoing active specific immunization with autologous Newcastle-disease-virus-modified tumour cells. Cancer Immunol Immunother. 1993 Sep;37(4):240–244. doi: 10.1007/BF01518517. [DOI] [PMC free article] [PubMed] [Google Scholar]