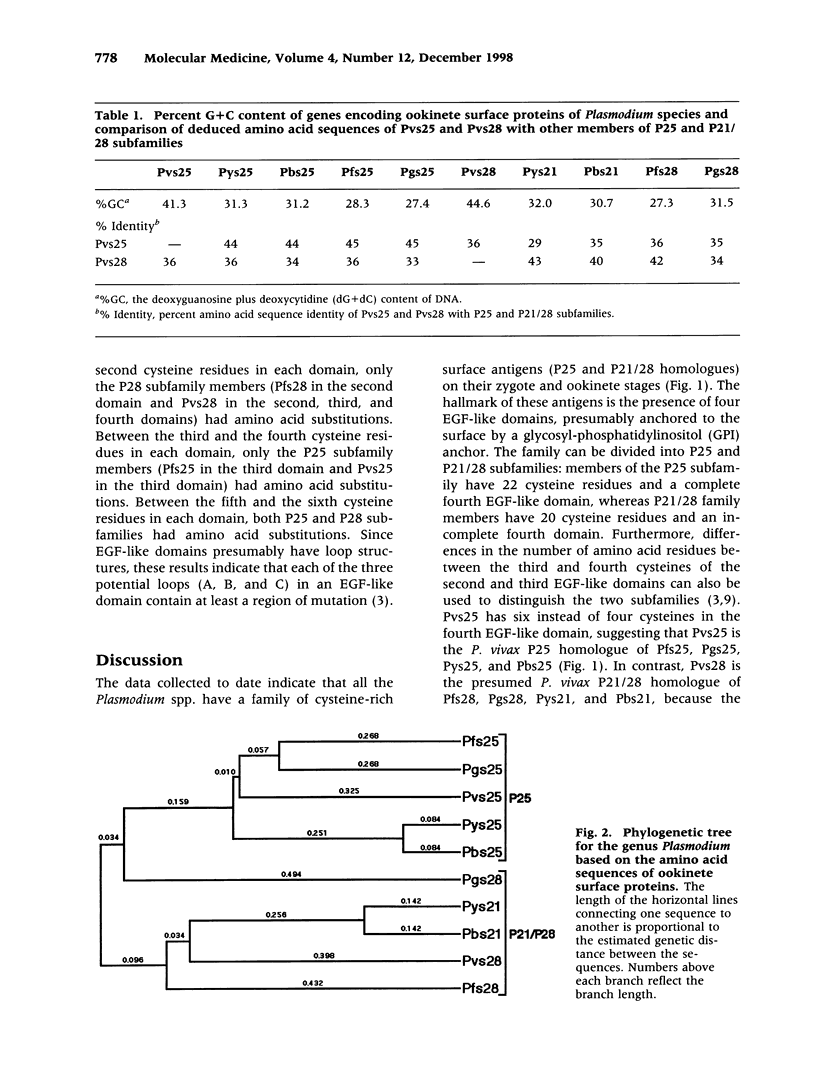
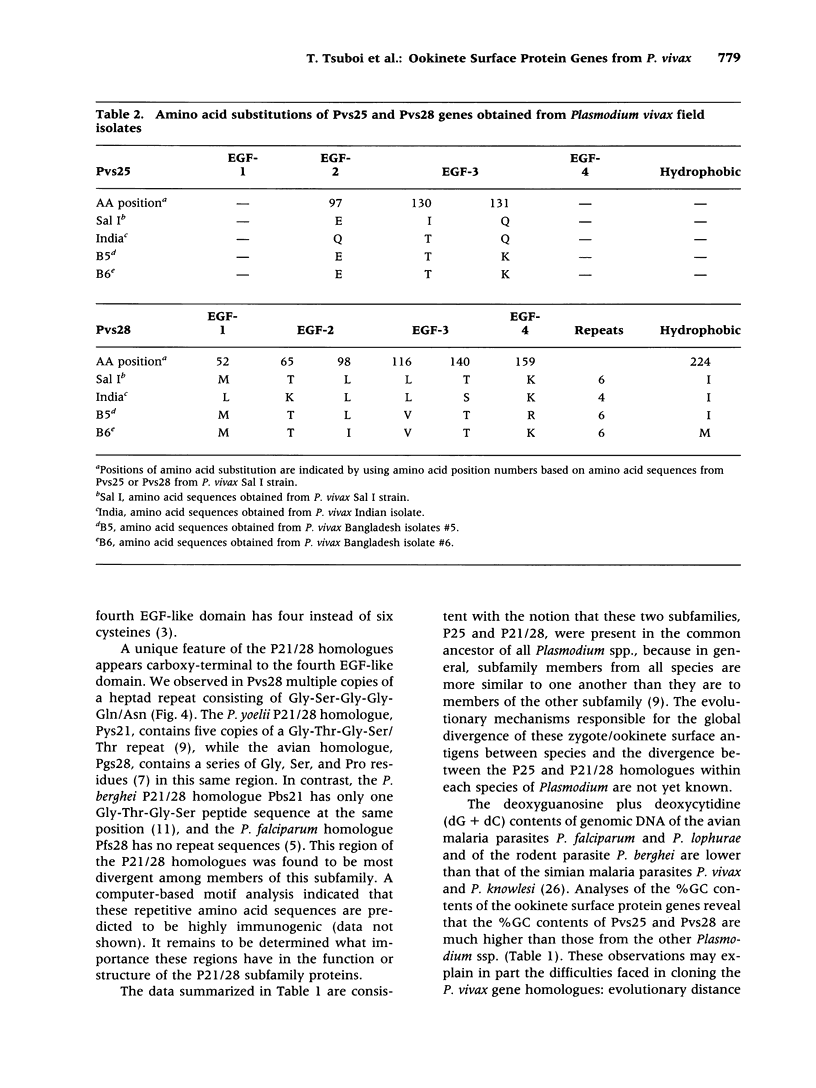
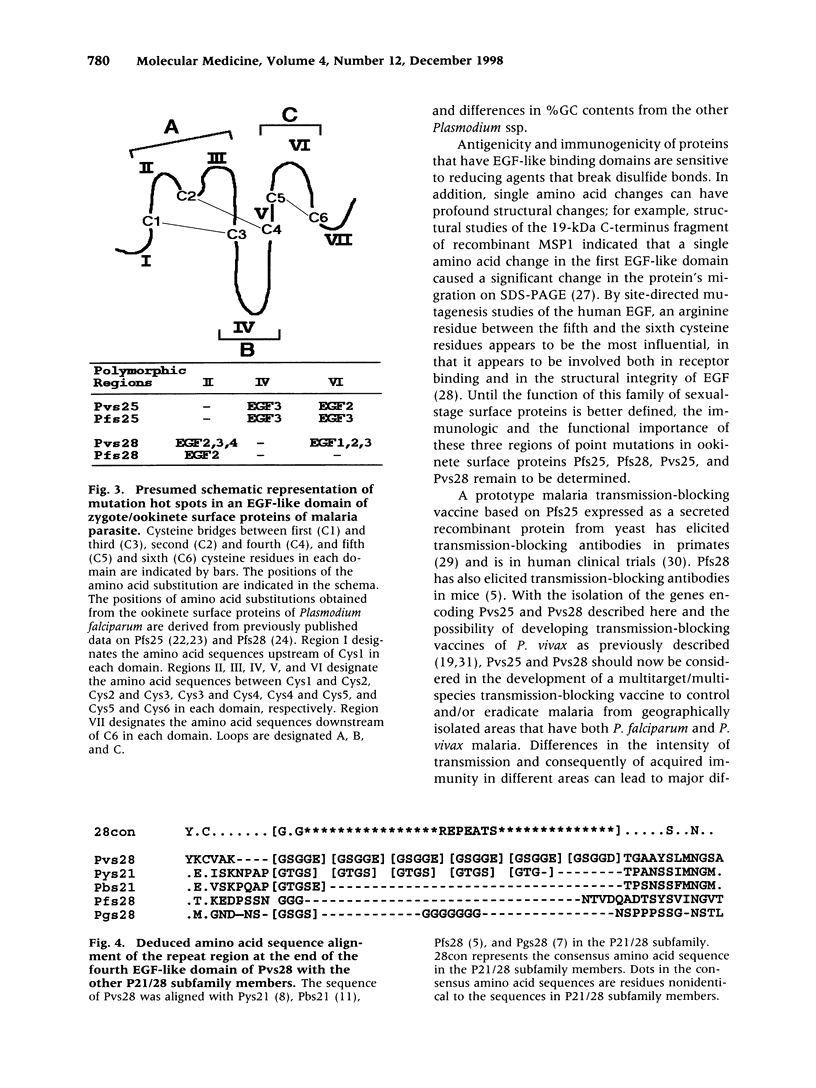
Abstract
BACKGROUND: For many malarious regions outside of Africa, development of effective transmission-blocking vaccines will require coverage against both Plasmodium falciparum and P. vivax. Work on P. vivax transmission-blocking vaccines has been hampered by the inability to clone the vaccine candidate genes from this parasite. Materials and METHODS: To search for genes encoding the ookinete surface proteins from P. vivax, the DNA sequences of the eight known proteins in the P25 subfamily (Pfs25, Pgs25, Pys25, Pbs25) and in the P21/28 subfamily (Pfs28, Pgs28, Pys21, Pbs21) of zygote/ookinete surface proteins were aligned. Regions of highest identity were used to design degenerate PCR oligonucleotides. Genomic DNA from the Sal I strain of P. vivax and genomic and splinkerette DNA libraries were used as PCR templates. To characterize the polymorphisms of Pvs25 and Pvs28, these two genes were PCR amplified and the DNA sequences were determined from genomic DNA extracted from patients infected with P. vivax. RESULTS: Analysis of the deduced amino acid sequence of Pvs28 revealed a secretory signal sequence, four epidermal growth factor (EGF)-like domains, six copies of the heptad amino acid repeat (GSGGE/D), and a short hydrophobic region. Because the fourth EGF-like domain has four rather than six cysteines, the gene designated Pvs28 is the presumed homologue of P21/28 subfamily members. Analysis of the deduced amino acid sequence of Pvs25 revealed a similar structure to that of Pvs28. The presence of six rather than four cysteines in the fourth EGF-like domain suggested that Pvs25 is the homologue of P25 subfamily members. Several regions of genetic polymorphisms in Pvs25 and Pvs28 were identified in field isolates of P. vivax. CONCLUSIONS: The genes encoding two ookinete surface proteins, Pvs28 and Pvs25, from P. vivax have been isolated and sequenced. Comparison of the primary structures of Pvs25, Pvs28, Pfs25, and Pfs28 suggest that there are regions of genetic polymorphism in the P25 and P21/28 subfamilies.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker G. C., Rodríguez M. H., Sinden R. E. Attempted isolation of the gene encoding the 21 Kd Plasmodium berghei ookinete transmission blocking antigen from Plasmodium yoelli and Plasmodium vivax. Mem Inst Oswaldo Cruz. 1994;89 (Suppl 2):37–41. doi: 10.1590/s0074-02761994000600010. [DOI] [PubMed] [Google Scholar]
- Campbell C. C., Collins W. E., Chin W., Roberts J. M., Broderson J. R. Studies of the Sal I strain of Plasmodium vivax in the squirrel monkey (Saimiri sciureus). J Parasitol. 1983 Jun;69(3):598–601. [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P. E., Kaslow D. C. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997 Mar;65(3):1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P. E., Pimenta P., Kaslow D. C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993 Feb 1;177(2):505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers H. D., Godal T. Malaria vaccine development: current status. Parasitol Today. 1998 Feb;14(2):56–64. doi: 10.1016/s0169-4758(97)01184-8. [DOI] [PubMed] [Google Scholar]
- Fried M., Gwadz R. W., Kaslow D. C. Identification of two cysteine-rich, lipophilic proteins on the surface of Plasmodium knowlesi ookinetes: Pks20 and Pks24. Exp Parasitol. 1994 May;78(3):326–330. doi: 10.1006/expr.1994.1034. [DOI] [PubMed] [Google Scholar]
- Gamage-Mendis A. C., Rajakaruna J., Carter R., Mendis K. N. Infectious reservoir of Plasmodium vivax and Plasmodium falciparum malaria in an endemic region of Sri Lanka. Am J Trop Med Hyg. 1991 Oct;45(4):479–487. doi: 10.4269/ajtmh.1991.45.479. [DOI] [PubMed] [Google Scholar]
- Golenda C. F., Li J., Rosenberg R. Continuous in vitro propagation of the malaria parasite Plasmodium vivax. Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6786–6791. doi: 10.1073/pnas.94.13.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P. M., Burkot T. R., Carter R., Cattani J. A., Lagog M., Parker J., Brabin B. J., Gibson F. D., Bradley D. J., Alpers M. P. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology. 1988 Apr;96(Pt 2):251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- Hafalla J. C., Santiago M. L., Pasay M. C., Ramirez B. L., Gozar M. M., Saul A., Kaslow D. C. Minimal variation in the Pfs28 ookinete antigen from Philippine field isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1997 Jul;87(1):97–99. doi: 10.1016/s0166-6851(97)00042-x. [DOI] [PubMed] [Google Scholar]
- Hommel U., Dudgeon T. J., Fallon A., Edwards R. M., Campbell I. D. Structure-function relationships in human epidermal growth factor studied by site-directed mutagenesis and 1H NMR. Biochemistry. 1991 Sep 10;30(36):8891–8898. doi: 10.1021/bi00100a024. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Bathurst I. C., Lensen T., Ponnudurai T., Barr P. J., Keister D. B. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994 Dec;62(12):5576–5580. doi: 10.1128/iai.62.12.5576-5580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow D. C., Hui G., Kumar S. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1(19)) variants secreted from Saccharomyces cerevisiae. Mol Biochem Parasitol. 1994 Feb;63(2):283–289. doi: 10.1016/0166-6851(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Quakyi I. A., Keister D. B. Minimal variation in a vaccine candidate from the sexual stage of Plasmodium falciparum. Mol Biochem Parasitol. 1989 Jan 1;32(1):101–103. doi: 10.1016/0166-6851(89)90134-5. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Quakyi I. A., Syin C., Raum M. G., Keister D. B., Coligan J. E., McCutchan T. F., Miller L. H. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988 May 5;333(6168):74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Syin C., McCutchan T. F., Miller L. H. Comparison of the primary structure of the 25 kDa ookinete surface antigens of Plasmodium falciparum and Plasmodium gallinaceum reveal six conserved regions. Mol Biochem Parasitol. 1989 Mar 15;33(3):283–287. doi: 10.1016/0166-6851(89)90090-x. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C. Transmission-blocking immunity against malaria and other vector-borne diseases. Curr Opin Immunol. 1993 Aug;5(4):557–565. doi: 10.1016/0952-7915(93)90037-s. [DOI] [PubMed] [Google Scholar]
- METSELAAR D. Relative increase in the prevalence of Plasmodium falciparum some years after the beginning of a house-spraying campaign in Netherlands New Guinea. Trans R Soc Trop Med Hyg. 1960 Nov;54:523–528. doi: 10.1016/0035-9203(60)90026-2. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Dame J. B., Miller L. H., Barnwell J. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science. 1984 Aug 24;225(4664):808–811. doi: 10.1126/science.6382604. [DOI] [PubMed] [Google Scholar]
- Mendis K. N., Munesinghe Y. D., de Silva Y. N., Keragalla I., Carter R. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun. 1987 Feb;55(2):369–372. doi: 10.1128/iai.55.2.369-372.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. S., Basri H., Purnomo, Andersen E. M., Bangs M. J., Mount D. L., Gorden J., Lal A. A., Purwokusumo A. R., Harjosuwarno S. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet. 1993 Jan 9;341(8837):96–100. doi: 10.1016/0140-6736(93)92568-e. [DOI] [PubMed] [Google Scholar]
- Paton M. G., Barker G. C., Matsuoka H., Ramesar J., Janse C. J., Waters A. P., Sinden R. E. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol Biochem Parasitol. 1993 Jun;59(2):263–275. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- Premawansa S., Peiris J. S., Perera K. L., Ariyaratne G., Carter R., Mendis K. N. Target antigens of transmission blocking immunity of Plasmodium vivax malaria. Characterization and polymorphism in natural parasite isolates. J Immunol. 1990 Jun 1;144(11):4376–4383. [PubMed] [Google Scholar]
- Shi Y. P., Alpers M. P., Povoa M. M., Lal A. A. Single amino acid variation in the ookinete vaccine antigen from field isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Jan;50(1):179–180. doi: 10.1016/0166-6851(92)90254-h. [DOI] [PubMed] [Google Scholar]
- Snewin V. A., Premawansa S., Kapilananda G. M., Ratnayaka L., Udagama P. V., Mattei D. M., Khouri E., Del Giudice G., Peiris J. S., Mendis K. N. Transmission blocking immunity in Plasmodium vivax malaria: antibodies raised against a peptide block parasite development in the mosquito vector. J Exp Med. 1995 Jan 1;181(1):357–362. doi: 10.1084/jem.181.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Tsuboi T., Cao Y. M., Hitsumoto Y., Yanagi T., Kanbara H., Torii M. Two antigens on zygotes and ookinetes of Plasmodium yoelii and Plasmodium berghei that are distinct targets of transmission-blocking immunity. Infect Immun. 1997 Jun;65(6):2260–2264. doi: 10.1128/iai.65.6.2260-2264.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T., Cao Y. M., Kaslow D. C., Shiwaku K., Torii M. Primary structure of a novel ookinete surface protein from Plasmodium berghei. Mol Biochem Parasitol. 1997 Mar;85(1):131–134. doi: 10.1016/s0166-6851(96)02821-6. [DOI] [PubMed] [Google Scholar]
- Tsuboi T., Kaslow D. C., Cao Y. M., Shiwaku K., Torii M. Comparison of Plasmodium yoelii ookinete surface antigens with human and avian malaria parasite homologues reveals two highly conserved regions. Mol Biochem Parasitol. 1997 Jul;87(1):107–111. doi: 10.1016/s0166-6851(97)00049-2. [DOI] [PubMed] [Google Scholar]
- Waters A. P., Higgins D. G., McCutchan T. F. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3140–3144. doi: 10.1073/pnas.88.8.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]



