Abstract
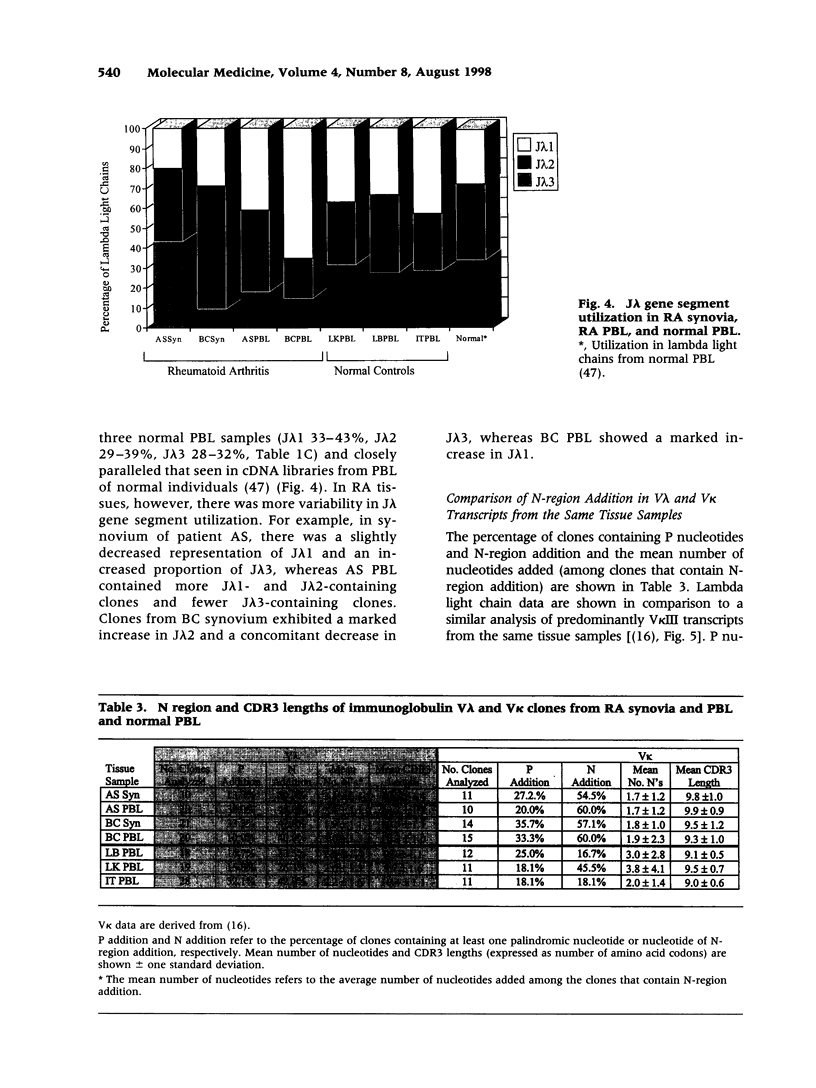
BACKGROUND: In rheumatoid arthritis (RA), B-lineage cells in the synovial membrane secrete large amounts of immunoglobulin that contribute to tissue destruction. The CDR3 of an immunoglobulin light chain is formed by rearrangements of VL and JL gene segments. Addition of non-germline-encoded (N) nucleotides at V(D)J joins by the enzyme terminal deoxynucleotidyl transferase (TdT) enhances antibody diversity. TdT was previously thought to be active in B cells only during heavy chain rearrangement, but we and others reported unexpectedly high levels of N addition in kappa light chains. We also found clonally related kappa chains bearing unusually long CDR3 intervals in RA synovium, suggesting oligoclonal expansion of a set of atypical B lymphocytes. In this study, we analyzed lambda light chain expression to determine if N addition occurs throughout immunoglobulin gene rearrangement and to compare CDR3 lengths of lambda and kappa light chains in RA patients and normal individuals. MATERIALS AND METHODS: Reverse transcription-polymerase chain reaction (RT-PCR) amplification of V lambda III transcripts was performed on RA synovia and peripheral blood lymphocytes (PBL) and normal PBL for which kappa repertoires were previously analyzed. Representative lambda + PCR products were cloned and sequenced. RESULTS: Analysis of 161 cDNA clones revealed that N addition occurs in lambda light chains of RA patients and normal controls. The lambda light chain repertoires in RA were enriched for long CDR3 intervals. In both RA and controls, CDR3 lengths were strongly influenced by which V lambda gene segment was present in the rearrangement. Five sets of clonally related sequences were found in RA synovia and PBL; one set was found in normal PBL. CONCLUSIONS: In humans, unlike mice, N addition enhances antibody diversity at all stages of immunoglobulin assembly, and the structural diversity of lambda CDR3 intervals is greater than that of kappa light chains. Clonally related V lambda gene segments in RA support an antigen-driven B-cell response.
Full text
PDF



























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P. The pathophysiology and treatment of rheumatoid arthritis. Arthritis Rheum. 1997 Apr;40(4):595–597. doi: 10.1002/art.1780400402. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. The fidelity of Taq polymerase catalyzing PCR is improved by an N-terminal deletion. Gene. 1992 Mar 1;112(1):29–35. doi: 10.1016/0378-1119(92)90299-5. [DOI] [PubMed] [Google Scholar]
- Bauer T. R., Jr, Blomberg B. The human lambda L chain Ig locus. Recharacterization of JC lambda 6 and identification of a functional JC lambda 7. J Immunol. 1991 Apr 15;146(8):2813–2820. [PubMed] [Google Scholar]
- Blomberg B. B., Glozak M. A., Donohoe M. E. Regulation of human lambda light chain gene expression. Ann N Y Acad Sci. 1995 Sep 29;764:84–98. [PubMed] [Google Scholar]
- Bridges S. L., Jr, Clausen B. E., Lavelle J. C., Fowler P. G., Koopman W. J., Schroeder H. W., Jr Analysis of immunoglobulin gamma heavy chains from rheumatoid arthritis synovium. Evidence of antigen-driven selection. Ann N Y Acad Sci. 1995 Sep 29;764:450–452. doi: 10.1111/j.1749-6632.1995.tb55862.x. [DOI] [PubMed] [Google Scholar]
- Bridges S. L., Jr, Lavelle J. C., Lee S. K., Byer S., Schroeder H. W., Jr CDR3 fingerprinting of immunoglobulin kappa light chains expressed in rheumatoid arthritis. Evidence of antigenic selection or dysregulation of gene rearrangement in B cells. Ann N Y Acad Sci. 1997 Apr 5;815:423–426. doi: 10.1111/j.1749-6632.1997.tb52093.x. [DOI] [PubMed] [Google Scholar]
- Bridges S. L., Jr, Lee S. K., Johnson M. L., Lavelle J. C., Fowler P. G., Koopman W. J., Schroeder H. W., Jr Somatic mutation and CDR3 lengths of immunoglobulin kappa light chains expressed in patients with rheumatoid arthritis and in normal individuals. J Clin Invest. 1995 Aug;96(2):831–841. doi: 10.1172/JCI118129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges S. L., Jr, Lee S. K., Koopman W. J., Schroeder H. W., Jr Analysis of immunoglobulin gamma heavy chain expression in synovial tissue of a patient with rheumatoid arthritis. Arthritis Rheum. 1993 May;36(5):631–641. [PubMed] [Google Scholar]
- Brown K. A., Perry M. E., Mustafa Y., Wood S. K., Crawley M., Taub N., Dumonde D. C. The distribution and abnormal morphology of plasma cells in rheumatoid synovium. Scand J Immunol. 1995 May;41(5):509–517. doi: 10.1111/j.1365-3083.1995.tb03600.x. [DOI] [PubMed] [Google Scholar]
- Campbell M. J., Zelenetz A. D., Levy S., Levy R. Use of family specific leader region primers for PCR amplification of the human heavy chain variable region gene repertoire. Mol Immunol. 1992 Feb;29(2):193–203. doi: 10.1016/0161-5890(92)90100-c. [DOI] [PubMed] [Google Scholar]
- Ch'ang L. Y., Schell M., Ringelberg C., Weiss D. T., Solomon A. Molecular characterization of a human V lambda VIII germline gene. Mol Immunol. 1995 Jan;32(1):49–55. doi: 10.1016/0161-5890(94)00135-n. [DOI] [PubMed] [Google Scholar]
- Ch'ang L. Y., Yen C. P., Besl L., Schell M., Solomon A. Identification and characterization of a functional human Ig V lambda VI germline gene. Mol Immunol. 1994 May;31(7):531–536. doi: 10.1016/0161-5890(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Chen C., Prak E. L., Weigert M. Editing disease-associated autoantibodies. Immunity. 1997 Jan;6(1):97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chuchana P., Blancher A., Brockly F., Alexandre D., Lefranc G., Lefranc M. P. Definition of the human immunoglobulin variable lambda (IGLV) gene subgroups. Eur J Immunol. 1990 Jun;20(6):1317–1325. doi: 10.1002/eji.1830200618. [DOI] [PubMed] [Google Scholar]
- Clausen B. E., Bridges S. L., Jr, Lavelle J. C., Fowler P. G., Gay S., Koopman W. J., Schroeder H. W., Jr Clonally-related immunoglobulin VH domains and nonrandom use of DH gene segments in rheumatoid arthritis synovium. Mol Med. 1998 Apr;4(4):240–257. [PMC free article] [PubMed] [Google Scholar]
- Combriato G., Klobeck H. G. V lambda and J lambda-C lambda gene segments of the human immunoglobulin lambda light chain locus are separated by 14 kb and rearrange by a deletion mechanism. Eur J Immunol. 1991 Jun;21(6):1513–1522. doi: 10.1002/eji.1830210627. [DOI] [PubMed] [Google Scholar]
- Daley M. D., Olee T., Peng H. Q., Soto-Gil R. W., Chen P. P., Siminovitch K. A. Molecular characterization of the human immunoglobulin V lambda I germline gene repertoire. Mol Immunol. 1992 Sep;29(9):1031–1042. doi: 10.1016/0161-5890(92)90034-u. [DOI] [PubMed] [Google Scholar]
- Daley M. D., Peng H. Q., Misener V., Liu X. Y., Chen P. P., Siminovitch K. A. Molecular analysis of human immunoglobulin V lambda germline genes: subgroups V lambda III and V lambda IV. Mol Immunol. 1992 Dec;29(12):1515–1518. doi: 10.1016/0161-5890(92)90226-n. [DOI] [PubMed] [Google Scholar]
- Dariavach P., Lefranc G., Lefranc M. P. Human immunoglobulin C lambda 6 gene encodes the Kern+Oz-lambda chain and C lambda 4 and C lambda 5 are pseudogenes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9074–9078. doi: 10.1073/pnas.84.24.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftos M., Soto-Gil R., Quan M., Olee T., Chen P. P. Utilization of a potentially universal downstream primer in the rapid identification and characterization of V lambda genes from two new human V lambda gene families. Scand J Immunol. 1994 Jan;39(1):95–103. doi: 10.1111/j.1365-3083.1994.tb03345.x. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Dreyer W. J., Bennett J. C. The molecular basis of antibody formation: a paradox. Proc Natl Acad Sci U S A. 1965 Sep;54(3):864–869. doi: 10.1073/pnas.54.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgavish R. A., Schroeder H. W., Jr SAW: a graphical user interface for the analysis of immunoglobulin variable domain sequences. Biotechniques. 1993 Dec;15(6):1066–1071. [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Ermel R. W., Kenny T. P., Chen P. P., Robbins D. L. Molecular analysis of rheumatoid factors derived from rheumatoid synovium suggests an antigen-driven response in inflamed joints. Arthritis Rheum. 1993 Mar;36(3):380–388. doi: 10.1002/art.1780360314. [DOI] [PubMed] [Google Scholar]
- Ermel R. W., Kenny T. P., Wong A., Solomon A., Chen P. P., Robbins D. L. Preferential utilization of a novel V lambda 3 gene in monoclonal rheumatoid factors derived from the synovial cells of rheumatoid arthritis patients. Arthritis Rheum. 1994 Jun;37(6):860–868. doi: 10.1002/art.1780370614. [DOI] [PubMed] [Google Scholar]
- Fang Q., Kannapell C. C., Gaskin F., Solomon A., Koopman W. J., Fu S. M. Human rheumatoid factors with restrictive specificity for rabbit immunoglobulin G: auto- and multi-reactivity, diverse VH gene segment usage and preferential usage of V lambda IIIb. J Exp Med. 1994 May 1;179(5):1445–1456. doi: 10.1084/jem.179.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freni M. A., Artuso D., Gerken G., Spanti C., Marafioti T., Alessi N., Spadaro A., Ajello A., Ferraù O. Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology. 1995 Aug;22(2):389–394. [PubMed] [Google Scholar]
- Frippiat J. P., Dard P., Marsh S., Winter G., Lefranc M. P. Immunoglobulin lambda light chain orphons on human chromosome 8q11.2. Eur J Immunol. 1997 May;27(5):1260–1265. doi: 10.1002/eji.1830270530. [DOI] [PubMed] [Google Scholar]
- Frippiat J. P., Lefranc M. P. Genomic organisation of 34 kb of the human immunoglobulin lambda locus (IGLV): restriction map and sequences of new V lambda III genes. Mol Immunol. 1994 Jun;31(9):657–670. doi: 10.1016/0161-5890(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Frippiat J. P., Williams S. C., Tomlinson I. M., Cook G. P., Cherif D., Le Paslier D., Collins J. E., Dunham I., Winter G., Lefranc M. P. Organization of the human immunoglobulin lambda light-chain locus on chromosome 22q11.2. Hum Mol Genet. 1995 Jun;4(6):983–991. doi: 10.1093/hmg/4.6.983. [DOI] [PubMed] [Google Scholar]
- Gellert M. Recent advances in understanding V(D)J recombination. Adv Immunol. 1997;64:39–64. doi: 10.1016/s0065-2776(08)60886-x. [DOI] [PubMed] [Google Scholar]
- Goossens T., Klein U., Küppers R. Frequent occurrence of deletions and duplications during somatic hypermutation: implications for oncogene translocations and heavy chain disease. Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2463–2468. doi: 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Dillon S. R., Zheng B., Shimoda M., Schlissel M. S., Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997 Oct 10;278(5336):301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- Han S., Zheng B., Schatz D. G., Spanopoulou E., Kelsoe G. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996 Dec 20;274(5295):2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- Harindranath N., Goldfarb I. S., Ikematsu H., Burastero S. E., Wilder R. L., Notkins A. L., Casali P. Complete sequence of the genes encoding the VH and VL regions of low- and high-affinity monoclonal IgM and IgA1 rheumatoid factors produced by CD5+ B cells from a rheumatoid arthritis patient. Int Immunol. 1991 Sep;3(9):865–875. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatovich O., Tomlinson I. M., Jones P. T., Winter G. The creation of diversity in the human immunoglobulin V(lambda) repertoire. J Mol Biol. 1997 Apr 25;268(1):69–77. doi: 10.1006/jmbi.1997.0956. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Ziff M. Electron microscopic study of rheumatoid synovial vasculature. Intimate relationship between tall endothelium and lymphoid aggregation. J Clin Invest. 1986 Feb;77(2):355–361. doi: 10.1172/JCI112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Minoshima S., Nakato E., Shibuya K., Shintani A., Schmeits J. L., Wang J., Shimizu N. One-megabase sequence analysis of the human immunoglobulin lambda gene locus. Genome Res. 1997 Mar;7(3):250–261. doi: 10.1101/gr.7.3.250. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Jaenichen R., Zachau H. G. Expressed human immunoglobulin kappa genes and their hypermutation. Eur J Immunol. 1993 Dec;23(12):3248–3262. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 1997 Feb 15;89(4):1288–1298. [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993 Dec;23(12):3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Cooper M. D., Carroll A. J., Burrows P. D. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumon I. In situ characterization of mononuclear cell phenotype in intrahepatic lymphoid follicles in patients with chronic viral hepatitis. Gastroenterol Jpn. 1992 Oct;27(5):638–645. doi: 10.1007/BF02774979. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Kirkham P. M., Koopman W. J., Schroeder H. W., Jr Evidence of antigen receptor-influenced oligoclonal B lymphocyte expansion in the synovium of a patient with longstanding rheumatoid arthritis. J Clin Invest. 1994 Jan;93(1):361–370. doi: 10.1172/JCI116968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Koopman W. J., Schroeder H. W., Jr The immunoglobulin kappa light chain repertoire expressed in the synovium of a patient with rheumatoid arthritis. Arthritis Rheum. 1992 Aug;35(8):905–913. doi: 10.1002/art.1780350809. [DOI] [PubMed] [Google Scholar]
- Lewis S. M. P nucleotide insertions and the resolution of hairpin DNA structures in mammalian cells. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1332–1336. doi: 10.1073/pnas.91.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen M., Johnsen H. E., Hansen P. W., Christiansen S. E. Isolation of human T and B lymphocytes by E-rosette gradient centrifugation. Characterization of the isolated subpopulations. J Immunol Methods. 1980;33(4):323–336. doi: 10.1016/0022-1759(80)90003-4. [DOI] [PubMed] [Google Scholar]
- Martin T., Blaison G., Levallois H., Pasquali J. L. Molecular analysis of the V kappa III-J kappa junctional diversity of polyclonal rheumatoid factors during rheumatoid arthritis frequently reveals N addition. Eur J Immunol. 1992 Jul;22(7):1773–1779. doi: 10.1002/eji.1830220716. [DOI] [PubMed] [Google Scholar]
- Martin T., Crouzier R., Blaison G., Levallois H., Pasquali J. L. A minor group of rheumatoid factors isolated from a patient with rheumatoid arthritis is derived from somatically mutated Vk1 genes further evidence that rheumatoid factors during autoimmune diseases undergo an antigen driven maturation. Autoimmunity. 1993;15(2):163–170. doi: 10.3109/08916939309043891. [DOI] [PubMed] [Google Scholar]
- McBlane J. F., van Gent D. C., Ramsden D. A., Romeo C., Cuomo C. A., Gellert M., Oettinger M. A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995 Nov 3;83(3):387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- Mosnier J. F., Degott C., Marcellin P., Hénin D., Erlinger S., Benhamou J. P. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology. 1993 Mar;17(3):366–371. [PubMed] [Google Scholar]
- Niewold T. A., Murphy C. L., Weiss D. T., Solomon A. Characterization of a light chain product of the human JC lambda 7 gene complex. J Immunol. 1996 Nov 15;157(10):4474–4477. [PubMed] [Google Scholar]
- Olee T., Lu E. W., Huang D. F., Soto-Gil R. W., Deftos M., Kozin F., Carson D. A., Chen P. P. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen-driven response. J Exp Med. 1992 Mar 1;175(3):831–842. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou F., Casellas R., Suh H., Qin X. F., Besmer E., Pelanda R., Nemazee D., Rajewsky K., Nussenzweig M. C. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997 Oct 10;278(5336):298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- Pelanda R., Schwers S., Sonoda E., Torres R. M., Nemazee D., Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997 Dec;7(6):765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- Prak E. L., Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995 Aug 1;182(2):541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Higuchi R. G., Wilson A. C. Ancient DNA and the polymerase chain reaction. The emerging field of molecular archaeology. J Biol Chem. 1989 Jun 15;264(17):9709–9712. [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Randen I., Brown D., Thompson K. M., Hughes-Jones N., Pascual V., Victor K., Capra J. D., Førre O., Natvig J. B. Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. J Immunol. 1992 May 15;148(10):3296–3301. [PubMed] [Google Scholar]
- Randen I., Thompson K. M., Pascual V., Victor K., Beale D., Coadwell J., Førre O., Capra J. D., Natvig J. B. Rheumatoid factor V genes from patients with rheumatoid arthritis are diverse and show evidence of an antigen-driven response. Immunol Rev. 1992 Aug;128:49–71. doi: 10.1111/j.1600-065x.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Ruff-Jamison S., Glenney J. R., Jr Requirement for both H and L chain V regions, VH and VK joining amino acids, and the unique H chain D region for the high affinity binding of an anti-phosphotyrosine antibody. J Immunol. 1993 Apr 15;150(8 Pt 1):3389–3396. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Schröder A. E., Greiner A., Seyfert C., Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder A. E., Sieper J., Berek C. Antigen-dependent B cell differentiation in the synovial tissue of a patient with reactive arthritis. Mol Med. 1997 Apr;3(4):260–272. [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Duray P. H., Butcher E. C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988 Apr;31(4):487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Udey J. A., Blomberg B. Human lambda light chain locus: organization and DNA sequences of three genomic J regions. Immunogenetics. 1987;25(1):63–70. doi: 10.1007/BF00768834. [DOI] [PubMed] [Google Scholar]
- Vasicek T. J., Leder P. Structure and expression of the human immunoglobulin lambda genes. J Exp Med. 1990 Aug 1;172(2):609–620. doi: 10.1084/jem.172.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor K. D., Capra J. D. An apparently common mechanism of generating antibody diversity: length variation of the VL-JL junction. Mol Immunol. 1994 Jan;31(1):39–46. doi: 10.1016/0161-5890(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Weber J. C., Blaison G., Martin T., Knapp A. M., Pasquali J. L. Evidence that the V kappa III gene usage is nonstochastic in both adult and newborn peripheral B cells and that peripheral CD5+ adult B cells are oligoclonal. J Clin Invest. 1994 May;93(5):2093–2105. doi: 10.1172/JCI117204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., Frippiat J. P., Tomlinson I. M., Ignatovich O., Lefranc M. P., Winter G. Sequence and evolution of the human germline V lambda repertoire. J Mol Biol. 1996 Nov 29;264(2):220–232. doi: 10.1006/jmbi.1996.0636. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Winter G. Cloning and sequencing of human immunoglobulin V lambda gene segments. Eur J Immunol. 1993 Jul;23(7):1456–1461. doi: 10.1002/eji.1830230709. [DOI] [PubMed] [Google Scholar]
- Wilson P. C., de Bouteiller O., Liu Y. J., Potter K., Banchereau J., Capra J. D., Pascual V. Somatic hypermutation introduces insertions and deletions into immunoglobulin V genes. J Exp Med. 1998 Jan 5;187(1):59–70. doi: 10.1084/jem.187.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff M. Relation of cellular infiltration of rheumatoid synovial membrane to its immune response. Arthritis Rheum. 1974 May-Jun;17(3):313–319. doi: 10.1002/art.1780170317. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- van Dinther-Janssen A. C., Pals S. T., Scheper R., Breedveld F., Meijer C. J. Dendritic cells and high endothelial venules in the rheumatoid synovial membrane. J Rheumatol. 1990 Jan;17(1):11–17. [PubMed] [Google Scholar]