Abstract
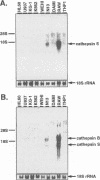
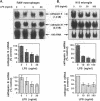
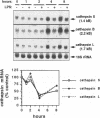
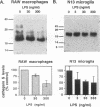
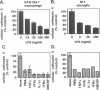
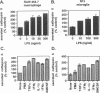
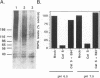
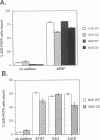
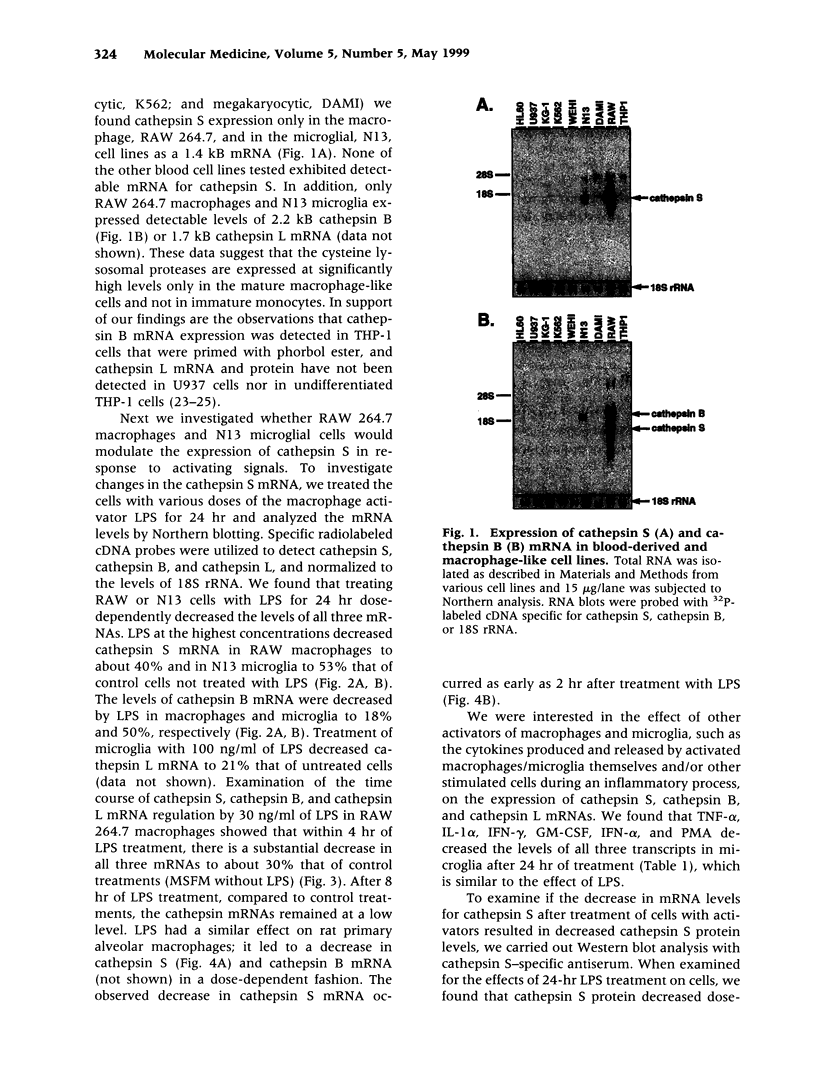
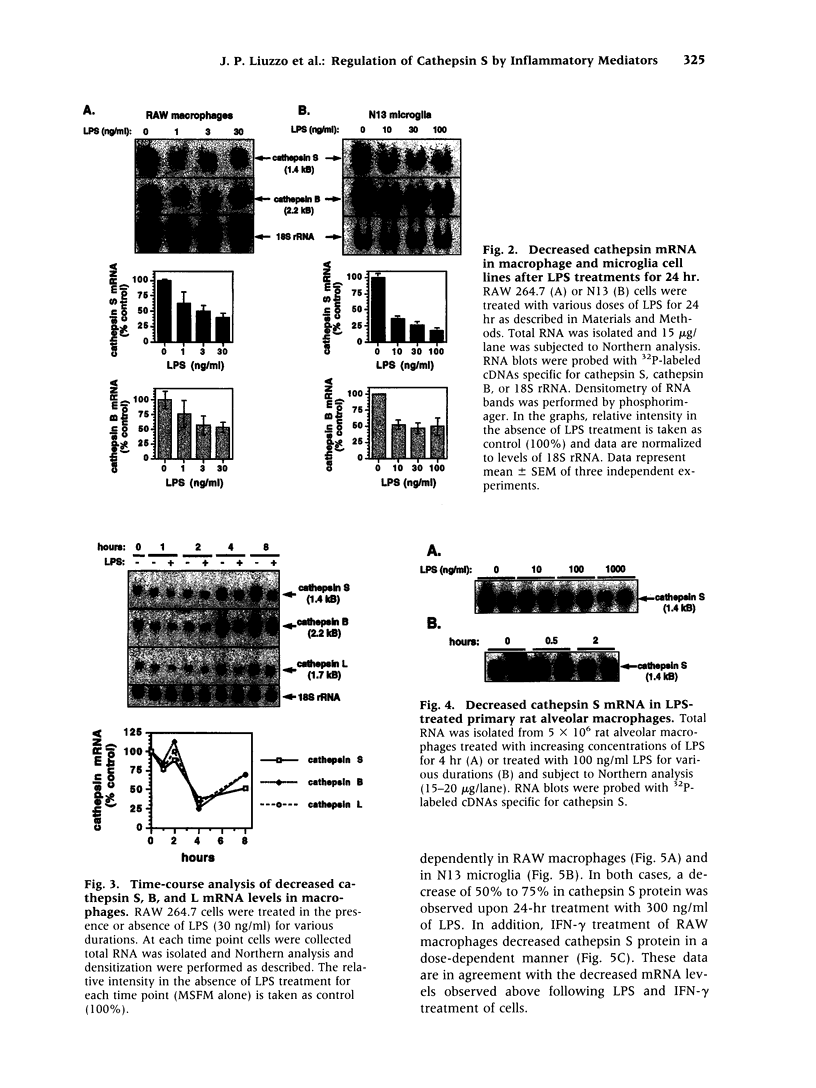
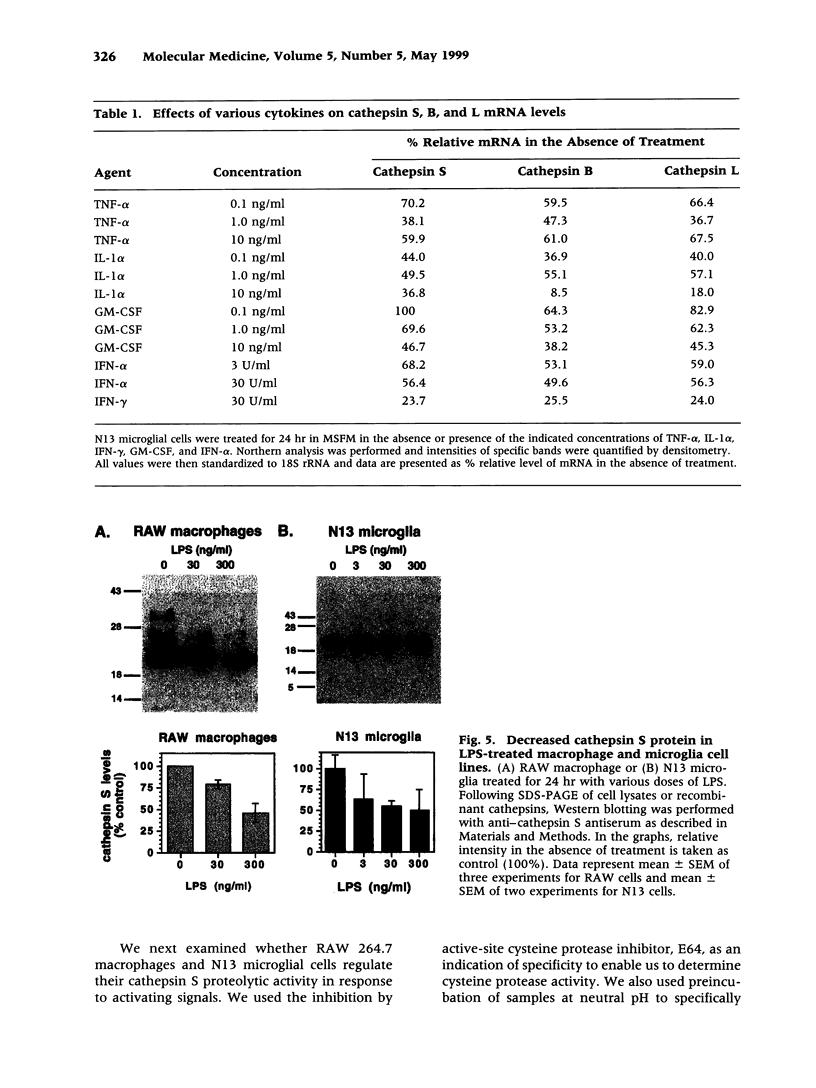
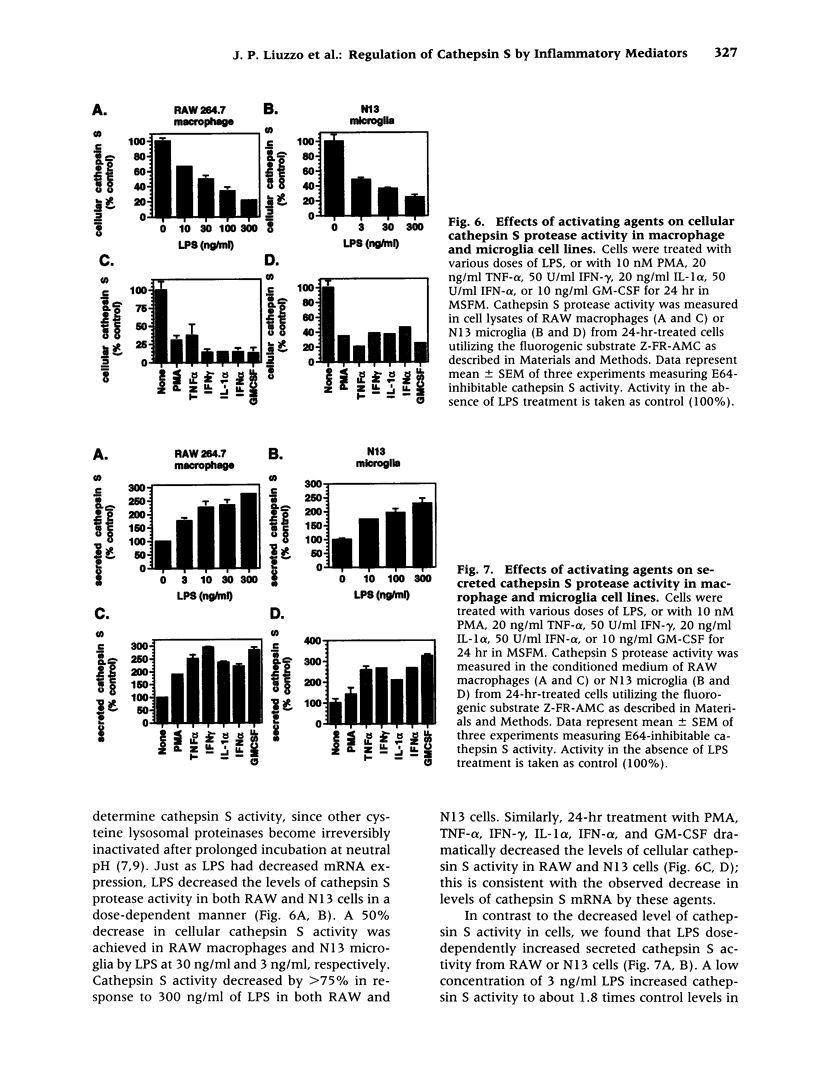
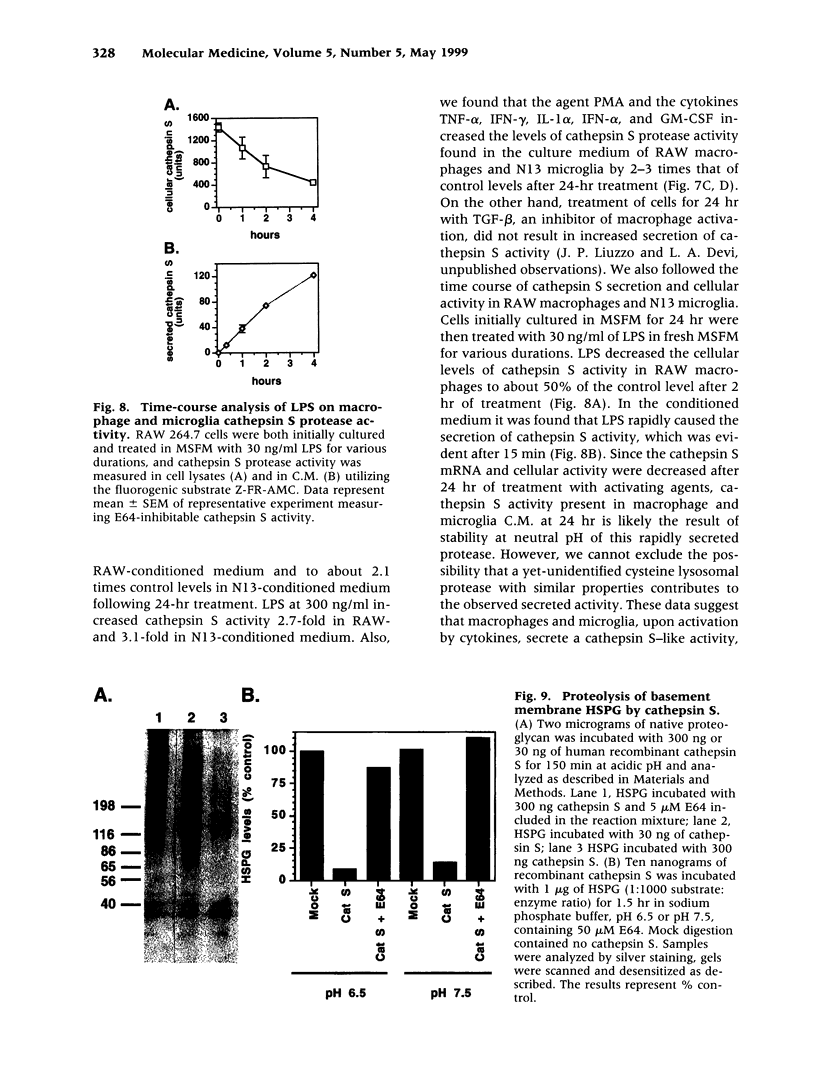
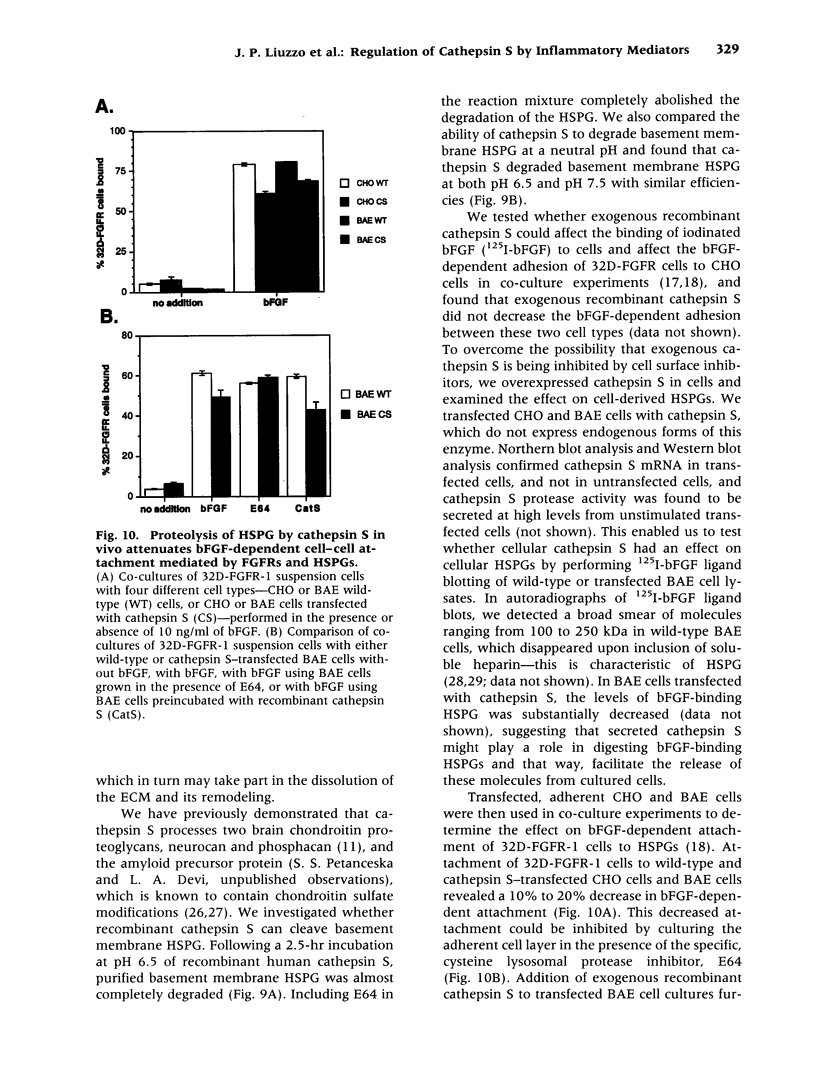
BACKGROUND: Cathepsin S is a member of the family of cysteine lysosomal proteases. The distribution of cathepsin S is restricted to cells from the mononuclear lineage both in the brain and in the periphery. Also, its protease activity is uniquely stable at neutral pH. MATERIALS AND METHODS: We compared the expression of cathepsin S, B, and L mRNAs in various undifferentiated and differentiated cells of mononuclear origin, and examined the modulation of these mRNAs by inflammatory mediators (lipopolysaccharide and various cytokines). In addition, the effect of these agents on cathepsin S protein levels and protease activity was also determined. Lastly, the ability of cathepsin S to process basement membrane components such as heparan sulfate proteoglycans in vitro and in vivo was assessed. RESULTS: Cathepsin S, B, and L mRNAs are expressed in mature macrophages and microglial cells and not in undifferentiated monocytes. Activators of macrophages negatively regulate all three transcripts. Consistent with this, treatment with these agents leads to a decrease in intracellular cathepsin S protein levels and activity. However, the same treatments result in stimulation of secreted cathepsin S activity. Cathepsin S is capable of degrading heparan sulfate proteoglycans in vitro. Also, when expressed in endothelial cells, cathepsin S autocrinely attenuates the basic fibroblast growth factor (bFGF)-mediated binding of FGF receptor containing cells to endothelial cells, by acting on basement membrane proteoglycans. CONCLUSIONS: Taken together, these data imply that cathepsin S is a regulatable cysteine protease that plays a role in the degradation of extracellular proteins, whose secretion from macrophages and microglia is increased by signals that lead to activation of these cells, and may be important in regulating extracellular matrix interactions. http://link.springer-ny. com/link/service/journals/00020/bibs/5n5p320.html
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Aviezer D., Hecht D., Safran M., Eisinger M., David G., Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994 Dec 16;79(6):1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Basilico C., Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Harlan J. M., Adamson J. W. Interleukin 1 stimulates human endothelial cells to produce granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor. J Immunol. 1987 Jul 15;139(2):464–468. [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner G., Gabrilove J., Rifkin D. B., Wilson E. L. Phospholipase C release of basic fibroblast growth factor from human bone marrow cultures as a biologically active complex with a phosphatidylinositol-anchored heparan sulfate proteoglycan. J Cell Biol. 1991 Sep;114(6):1275–1283. doi: 10.1083/jcb.114.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brömme D., Okamoto K., Wang B. B., Biroc S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J Biol Chem. 1996 Jan 26;271(4):2126–2132. doi: 10.1074/jbc.271.4.2126. [DOI] [PubMed] [Google Scholar]
- Brömme D., Steinert A., Friebe S., Fittkau S., Wiederanders B., Kirschke H. The specificity of bovine spleen cathepsin S. A comparison with rat liver cathepsins L and B. Biochem J. 1989 Dec 1;264(2):475–481. doi: 10.1042/bj2640475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M. R., Karustis D. G., Day N. A., Honn K. V., Sloane B. F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem J. 1992 Feb 15;282(Pt 1):273–278. doi: 10.1042/bj2820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément B., Yamada Y. A Mr 80K hepatocyte surface protein(s) interacts with basement membrane components. Exp Cell Res. 1990 Apr;187(2):320–323. doi: 10.1016/0014-4827(90)90098-u. [DOI] [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. The major excreted protein of transformed fibroblasts is an activable acid-protease. J Biol Chem. 1986 Feb 5;261(4):1760–1765. [PubMed] [Google Scholar]
- Gelb B. D., Shi G. P., Heller M., Weremowicz S., Morton C., Desnick R. J., Chapman H. A. Structure and chromosomal assignment of the human cathepsin K gene. Genomics. 1997 Apr 15;41(2):258–262. doi: 10.1006/geno.1997.4631. [DOI] [PubMed] [Google Scholar]
- Giulian D., Corpuz M. Microglial secretion products and their impact on the nervous system. Adv Neurol. 1993;59:315–320. [PubMed] [Google Scholar]
- Heremans A., De Cock B., Cassiman J. J., Van den Berghe H., David G. The core protein of the matrix-associated heparan sulfate proteoglycan binds to fibronectin. J Biol Chem. 1990 May 25;265(15):8716–8724. [PubMed] [Google Scholar]
- Isemura M., Yosizawa Z., Takahashi K., Kosaka H., Kojima N., Ono T. Characterization of porcine plasma fibronectin and its fragmentation by porcine liver cathepsin B. J Biochem. 1981 Jul;90(1):1–9. doi: 10.1093/oxfordjournals.jbchem.a133437. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Kembhavi A. A., Bohley P., Barrett A. J. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982 Feb 1;201(2):367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Wiederanders B., Brömme D., Rinne A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochem J. 1989 Dec 1;264(2):467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Wiederanders B. Cathepsin S and related lysosomal endopeptidases. Methods Enzymol. 1994;244:500–511. doi: 10.1016/0076-6879(94)44036-0. [DOI] [PubMed] [Google Scholar]
- Li Q, Ding L, Bever CT., Jr Interferon-ç induces cathepsin B expression in a human macrophage-like cell line by increasing both transcription and mRNA stability. Int J Mol Med. 1998 Aug;2(2):181–186. [PubMed] [Google Scholar]
- Liuzzo J. P., Moscatelli D. Human leukemia cell lines bind basic fibroblast growth factor (FGF) on FGF receptors and heparan sulfates: downmodulation of FGF receptors by phorbol ester. Blood. 1996 Jan 1;87(1):245–255. [PubMed] [Google Scholar]
- Maciewicz R. A., Etherington D. J. A comparison of four cathepsins (B, L, N and S) with collagenolytic activity from rabbit spleen. Biochem J. 1988 Dec 1;256(2):433–440. doi: 10.1042/bj2560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Johnson D. A., Barrett A. J., Chapman H. A. Elastinolytic activity of human cathepsin L. Biochem J. 1986 Feb 1;233(3):925–927. doi: 10.1042/bj2330925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. I., Barrett A. J., Dingle J. T., Prior D. Cathepsins BI and D. Action on human cartilage proteoglycans. Biochim Biophys Acta. 1973 Apr 12;302(2):411–419. doi: 10.1016/0005-2744(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Mørland B., Pedersen A. Cathepsin B activity in stimulated mouse peritoneal macrophages. Lab Invest. 1979 Nov;41(5):379–384. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. The macrophage as a secretory cell. Int Rev Cytol. 1978;52:119–157. doi: 10.1016/s0074-7696(08)60755-x. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Andersson P. B., Gordon S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993 Jul;16(7):268–273. doi: 10.1016/0166-2236(93)90180-t. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–244. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- Petanceska S., Burke S., Watson S. J., Devi L. Differential distribution of messenger RNAs for cathepsins B, L and S in adult rat brain: an in situ hybridization study. Neuroscience. 1994 Apr;59(3):729–738. doi: 10.1016/0306-4522(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Petanceska S., Canoll P., Devi L. A. Expression of rat cathepsin S in phagocytic cells. J Biol Chem. 1996 Feb 23;271(8):4403–4409. doi: 10.1074/jbc.271.8.4403. [DOI] [PubMed] [Google Scholar]
- Petanceska S., Devi L. Sequence analysis, tissue distribution, and expression of rat cathepsin S. J Biol Chem. 1992 Dec 25;267(36):26038–26043. [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Rappolee D. A., Werb Z. Macrophage-derived growth factors. Curr Top Microbiol Immunol. 1992;181:87–140. doi: 10.1007/978-3-642-77377-8_4. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Ott V. L. Molecular interactions of the syndecan core proteins. Curr Opin Cell Biol. 1998 Oct;10(5):620–628. doi: 10.1016/s0955-0674(98)80038-0. [DOI] [PubMed] [Google Scholar]
- Recklies A. D., Mort J. S., Poole A. R. Secretion of a thiol proteinase from mouse mammary carcinomas and its characterization. Cancer Res. 1982 Mar;42(3):1026–1032. [PubMed] [Google Scholar]
- Reddy V. Y., Zhang Q. Y., Weiss S. J. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. J., Jr, Chen P., Sailor L. Z., Mason R. W., Chapman H. A., Jr Uptake of extracellular enzyme by a novel pathway is a major determinant of cathepsin L levels in human macrophages. J Clin Invest. 1990 Jul;86(1):176–183. doi: 10.1172/JCI114682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. J., Jr, Mason R. W., Chen P., Joseph L. J., Sukhatme V. P., Yee R., Chapman H. A., Jr Synthesis and processing of cathepsin L, an elastase, by human alveolar macrophages. Biochem J. 1989 Jan 15;257(2):493–498. doi: 10.1042/bj2570493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C., Liuzzo J. P., Moscatelli D. Fibroblast growth factor-2 can mediate cell attachment by linking receptors and heparan sulfate proteoglycans on neighboring cells. J Biol Chem. 1995 Oct 13;270(41):24188–24196. doi: 10.1074/jbc.270.41.24188. [DOI] [PubMed] [Google Scholar]
- Roghani M., Mansukhani A., Dell'Era P., Bellosta P., Basilico C., Rifkin D. B., Moscatelli D. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding. J Biol Chem. 1994 Feb 11;269(6):3976–3984. [PubMed] [Google Scholar]
- Schlessinger J., Lax I., Lemmon M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 1995 Nov 3;83(3):357–360. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Shi G. P., Munger J. S., Meara J. P., Rich D. H., Chapman H. A. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem. 1992 Apr 15;267(11):7258–7262. [PubMed] [Google Scholar]
- Shi G. P., Webb A. C., Foster K. E., Knoll J. H., Lemere C. A., Munger J. S., Chapman H. A. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem. 1994 Apr 15;269(15):11530–11536. [PubMed] [Google Scholar]
- Shioi J., Anderson J. P., Ripellino J. A., Robakis N. K. Chondroitin sulfate proteoglycan form of the Alzheimer's beta-amyloid precursor. J Biol Chem. 1992 Jul 15;267(20):13819–13822. [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Hassell J. R. Extracellular matrix fibers containing fibronectin and basement membrane heparan sulfate proteoglycan coalign with focal contacts and microfilament bundles in stationary fibroblasts. Exp Cell Res. 1987 Dec;173(2):558–571. doi: 10.1016/0014-4827(87)90295-3. [DOI] [PubMed] [Google Scholar]
- Sloane B. F. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990 Apr;1(2):137–152. [PubMed] [Google Scholar]
- Warfel A. H., Zucker-Franklin D., Frangione B., Ghiso J. Constitutive secretion of cystatin C (gamma-trace) by monocytes and macrophages and its downregulation after stimulation. J Exp Med. 1987 Dec 1;166(6):1912–1917. doi: 10.1084/jem.166.6.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]