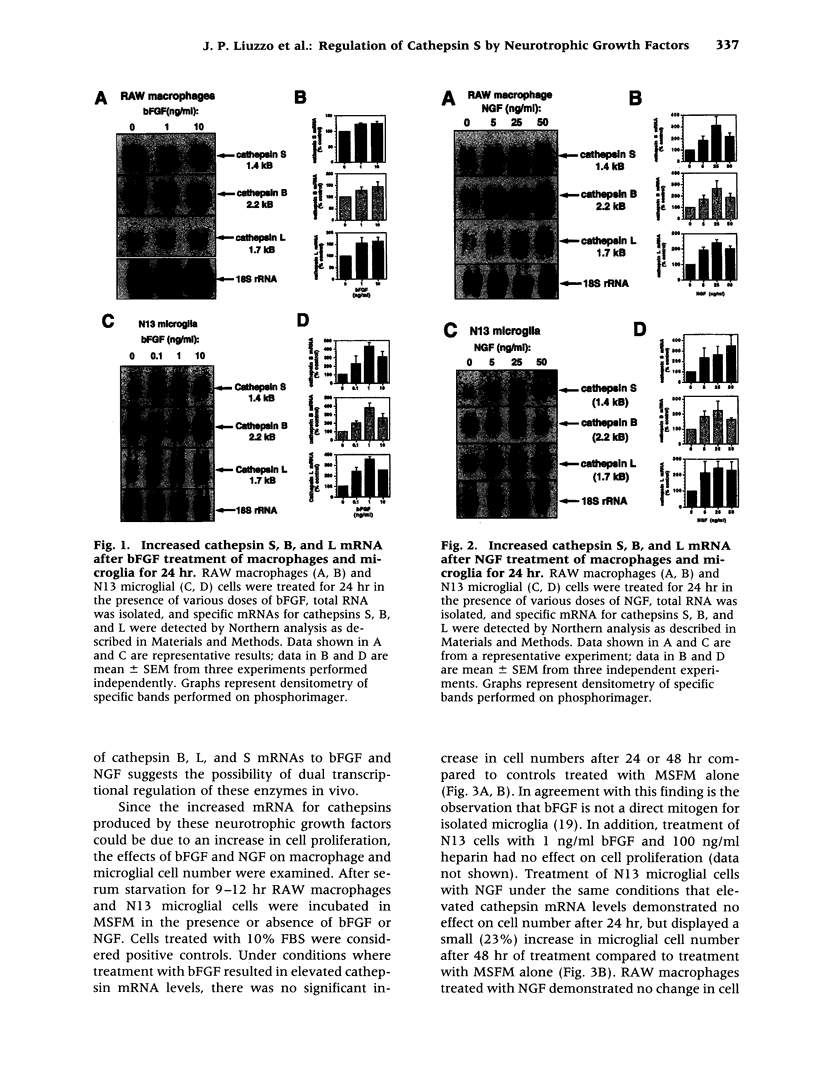
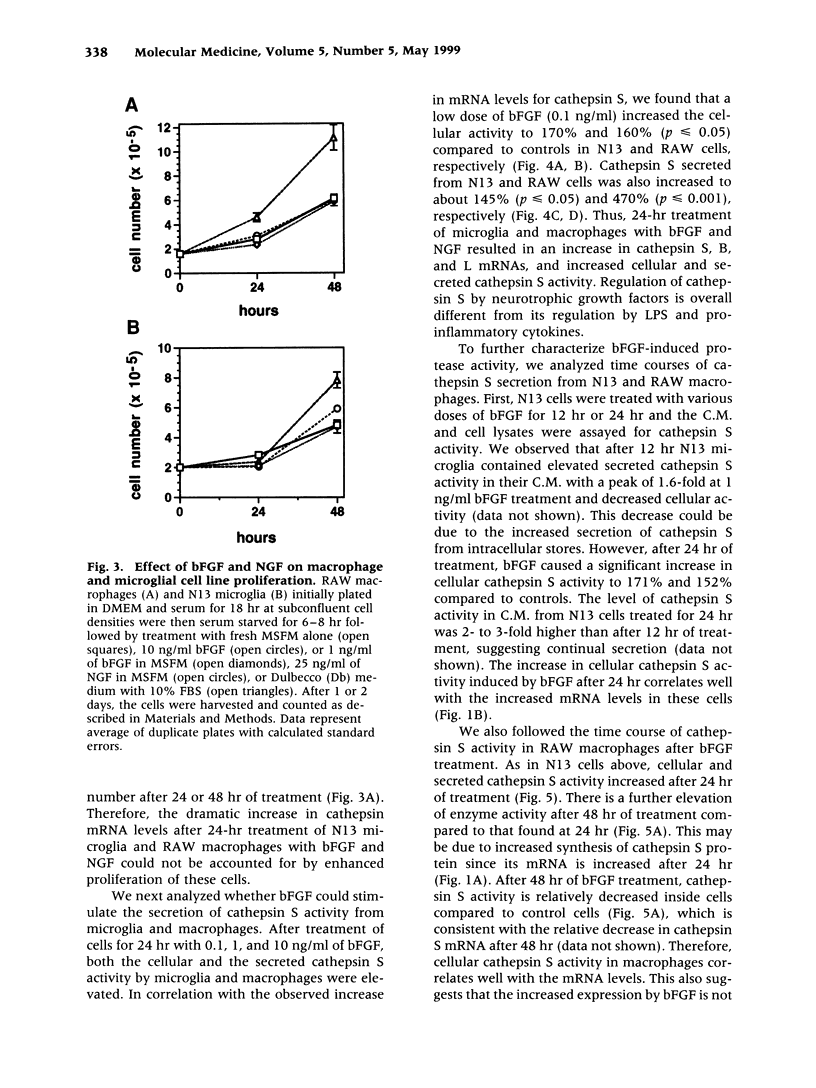
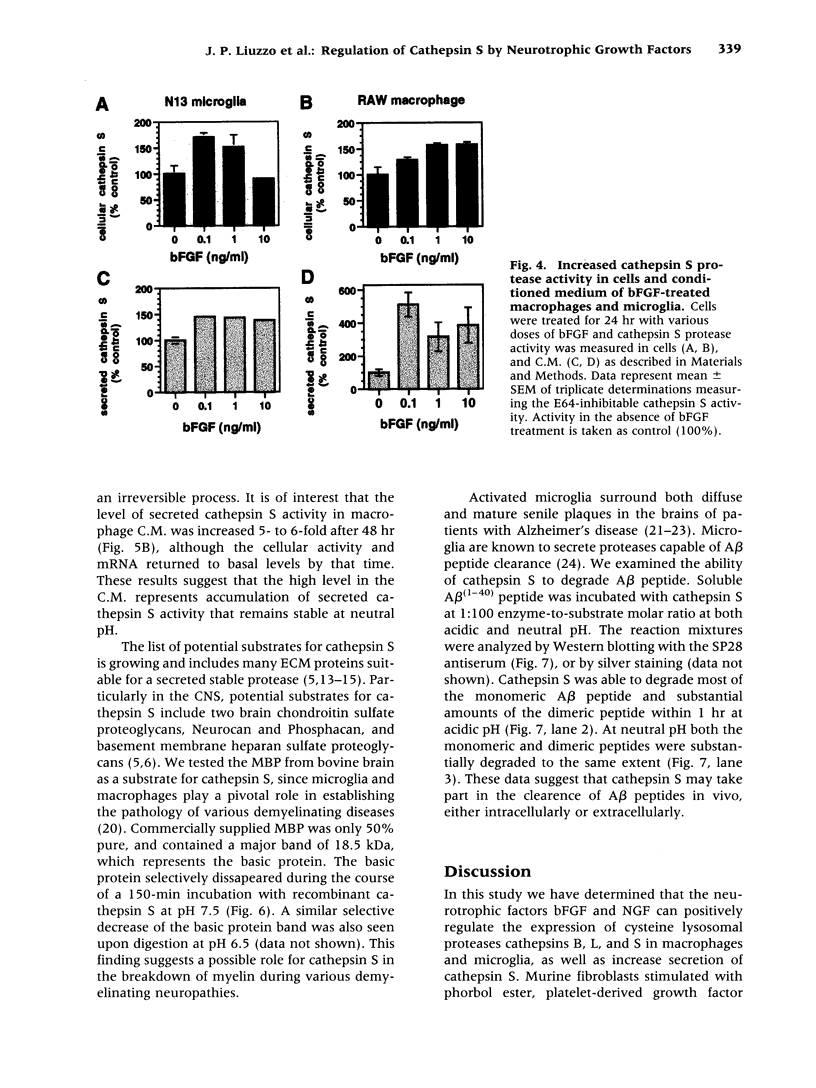
Abstract
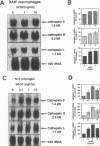
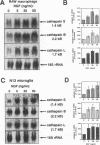
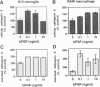
BACKGROUND: Cathepsin S is a member of the family of cysteine lysosomal proteases preferentially expressed in macrophages and microglia and is active after prolonged incubation in neutral pH. Upon activation of macrophages by a number of inflammatory mediators, there is an increase in secreted cathepsin S activity accompanied by a decrease in cellular cathepsin S activity and protein content, as well as a decrease in cathepsin S mRNA. The decrease in cathepsin S mRNA and protein at the cellular level is in contrast to the response observed in some in vivo scenarios. MATERIALS AND METHODS: We investigated the effect of basic fibroblast growth factor (bFGF) and nerve growth factor (NGF), two growth factors present during cell injury and inflammation but not known to activate macrophages and microglia, on the expression of cathepsin S, cathepsin B, and cathepsin L mRNAs in these cells, and on cathepsin S activity. We then tested the ability of cathepsin S to degrade myelin basic protein, and amyloid beta peptide at both acidic and neutral pH. RESULTS: Basic FGF and NGF treatment of macrophages and microglia significantly increased the levels of cathepsin S, B, and L mRNAs (2- to 5-fold). Basic FGF also increased cathepsin S activity intra- and extracellularly. Recombinant human cathepsin S was able to degrade myelin basic protein and monomeric and dimeric amyloid beta peptide at both acidic and neutral pH, as well as to process human amyloid precursor protein generating amyloidogenic fragments. CONCLUSIONS: These data suggest that bFGF and NGF may be the molecular signals that positively regulate the expression and activity of cysteine lysosomal proteases (cathepsin S in particular) in macrophages and microglia in vivo, and that there is an interplay between these factors and the activators of inflammation. Disruption of the balance between these two categories of signals may underlie the pathological changes that involve cysteine proteases. http://link.springer-ny.com/link/service/journals/00020/bibs /5n5p334. html
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Araujo D. M., Cotman C. W. Basic FGF in astroglial, microglial, and neuronal cultures: characterization of binding sites and modulation of release by lymphokines and trophic factors. J Neurosci. 1992 May;12(5):1668–1678. doi: 10.1523/JNEUROSCI.12-05-01668.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaci L., Presta M., Ennas M. G., Dell'Era P., Sogos V., Lauro G., Gremo F. Differential expression of fibroblast growth factor receptors by human neurones, astrocytes and microglia. Neuroreport. 1994 Dec 30;6(1):197–200. doi: 10.1097/00001756-199412300-00050. [DOI] [PubMed] [Google Scholar]
- Banik N. L. Pathogenesis of myelin breakdown in demyelinating diseases: role of proteolytic enzymes. Crit Rev Neurobiol. 1992;6(4):257–271. [PubMed] [Google Scholar]
- Brown M. C., Perry V. H., Lunn E. R., Gordon S., Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron. 1991 Mar;6(3):359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Brömme D., Okamoto K., Wang B. B., Biroc S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J Biol Chem. 1996 Jan 26;271(4):2126–2132. doi: 10.1074/jbc.271.4.2126. [DOI] [PubMed] [Google Scholar]
- Brömme D., Steinert A., Friebe S., Fittkau S., Wiederanders B., Kirschke H. The specificity of bovine spleen cathepsin S. A comparison with rat liver cathepsins L and B. Biochem J. 1989 Dec 1;264(2):475–481. doi: 10.1042/bj2640475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Frick K. K., Doherty P. J., Gottesman M. M., Scher C. D. Regulation of the transcript for a lysosomal protein: evidence for a gene program modified by platelet-derived growth factor. Mol Cell Biol. 1985 Oct;5(10):2582–2589. doi: 10.1128/mcb.5.10.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J., Lannes-Vieira J., Wekerle H. Differential expression of fibroblast growth factor-2 and receptor by glial cells in experimental autoimmune encephalomyelitis (EAE) Glia. 1996 Feb;16(2):93–100. doi: 10.1002/(SICI)1098-1136(199602)16:2<93::AID-GLIA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Gilad G. M., Gilad V. H. Chemotaxis and accumulation of nerve growth factor by microglia and macrophages. J Neurosci Res. 1995 Aug 1;41(5):594–602. doi: 10.1002/jnr.490410505. [DOI] [PubMed] [Google Scholar]
- Giulian D., Ingeman J. E. Colony-stimulating factors as promoters of ameboid microglia. J Neurosci. 1988 Dec;8(12):4707–4717. doi: 10.1523/JNEUROSCI.08-12-04707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F., Lee J. W., Cotman C. W. Basic FGF in adult rat brain: cellular distribution and response to entorhinal lesion and fimbria-fornix transection. J Neurosci. 1992 Jan;12(1):345–355. doi: 10.1523/JNEUROSCI.12-01-00345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Wiederanders B., Brömme D., Rinne A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochem J. 1989 Dec 1;264(2):467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Wiederanders B. Cathepsin S and related lysosomal endopeptidases. Methods Enzymol. 1994;244:500–511. doi: 10.1016/0076-6879(94)44036-0. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Liu H. M., Chen H. H. Correlation between fibroblast growth factor expression and cell proliferation in experimental brain infarct: studied with proliferating cell nuclear antigen immunohistochemistry. J Neuropathol Exp Neurol. 1994 Mar;53(2):118–126. doi: 10.1097/00005072-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Liuzzo J. P., Petanceska S. S., Moscatelli D., Devi L. A. Inflammatory mediators regulate cathepsin S in macrophages and microglia: A role in attenuating heparan sulfate interactions. Mol Med. 1999 May;5(5):320–333. [PMC free article] [PubMed] [Google Scholar]
- Logan A., Frautschy S. A., Baird A. Basic fibroblast growth factor and central nervous system injury. Ann N Y Acad Sci. 1991;638:474–476. doi: 10.1111/j.1749-6632.1991.tb49073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R. A., Etherington D. J. A comparison of four cathepsins (B, L, N and S) with collagenolytic activity from rabbit spleen. Biochem J. 1988 Dec 1;256(2):433–440. doi: 10.1042/bj2560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M., Houlgatte R., Brachet P., Prochiantz A. Lipopolysaccharide-stimulated rat brain macrophages release NGF in vitro. Dev Biol. 1989 May;133(1):309–311. doi: 10.1016/0012-1606(89)90322-9. [DOI] [PubMed] [Google Scholar]
- Marks N., Grynbaum A., Lajtha A. Pentapeptide (pepstatin) inhibition of brain acid proteinase. Science. 1973 Sep 7;181(4103):949–951. doi: 10.1126/science.181.4103.949. [DOI] [PubMed] [Google Scholar]
- Morrison R. S. Fibroblast growth factors: potential neurotrophic agents in the central nervous system. J Neurosci Res. 1987;17(2):99–101. doi: 10.1002/jnr.490170202. [DOI] [PubMed] [Google Scholar]
- Munger J. S., Haass C., Lemere C. A., Shi G. P., Wong W. S., Teplow D. B., Selkoe D. J., Chapman H. A. Lysosomal processing of amyloid precursor protein to A beta peptides: a distinct role for cathepsin S. Biochem J. 1995 Oct 1;311(Pt 1):299–305. doi: 10.1042/bj3110299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nilsen-Hamilton M., Hamilton R. T., Allen W. R., Massoglia S. L. Stimulation of the release of two glycoproteins from mouse 3T3 cells by growth factors and by agents that increase intralysosomal pH. Biochem Biophys Res Commun. 1981 Jul 30;101(2):411–417. doi: 10.1016/0006-291x(81)91275-4. [DOI] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. The macrophage as a secretory cell. Int Rev Cytol. 1978;52:119–157. doi: 10.1016/s0074-7696(08)60755-x. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Andersson P. B., Gordon S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993 Jul;16(7):268–273. doi: 10.1016/0166-2236(93)90180-t. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–244. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- Petanceska S., Canoll P., Devi L. A. Expression of rat cathepsin S in phagocytic cells. J Biol Chem. 1996 Feb 23;271(8):4403–4409. doi: 10.1074/jbc.271.8.4403. [DOI] [PubMed] [Google Scholar]
- Petanceska S., Devi L. Sequence analysis, tissue distribution, and expression of rat cathepsin S. J Biol Chem. 1992 Dec 25;267(36):26038–26043. [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Presta M., Urbinati C., Dell'era P., Lauro G. M., Sogos V., Balaci L., Ennas M. G., Gremo F. Expression of basic fibroblast growth factor and its receptors in human fetal microglia cells. Int J Dev Neurosci. 1995 Feb;13(1):29–39. doi: 10.1016/0736-5748(94)00065-b. [DOI] [PubMed] [Google Scholar]
- Shaffer L. M., Dority M. D., Gupta-Bansal R., Frederickson R. C., Younkin S. G., Brunden K. R. Amyloid beta protein (A beta) removal by neuroglial cells in culture. Neurobiol Aging. 1995 Sep-Oct;16(5):737–745. doi: 10.1016/0197-4580(95)00055-j. [DOI] [PubMed] [Google Scholar]
- Shi G. P., Munger J. S., Meara J. P., Rich D. H., Chapman H. A. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem. 1992 Apr 15;267(11):7258–7262. [PubMed] [Google Scholar]
- Shi G. P., Webb A. C., Foster K. E., Knoll J. H., Lemere C. A., Munger J. S., Chapman H. A. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem. 1994 Apr 15;269(15):11530–11536. [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996 May 3;85(3):299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Thomas W. E. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992 Jan-Apr;17(1):61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Gage F. H. Fibroblast growth factors stimulate nerve growth factor synthesis and secretion by astrocytes. Brain Res. 1991 Jan 4;538(1):118–126. doi: 10.1016/0006-8993(91)90385-9. [DOI] [PubMed] [Google Scholar]