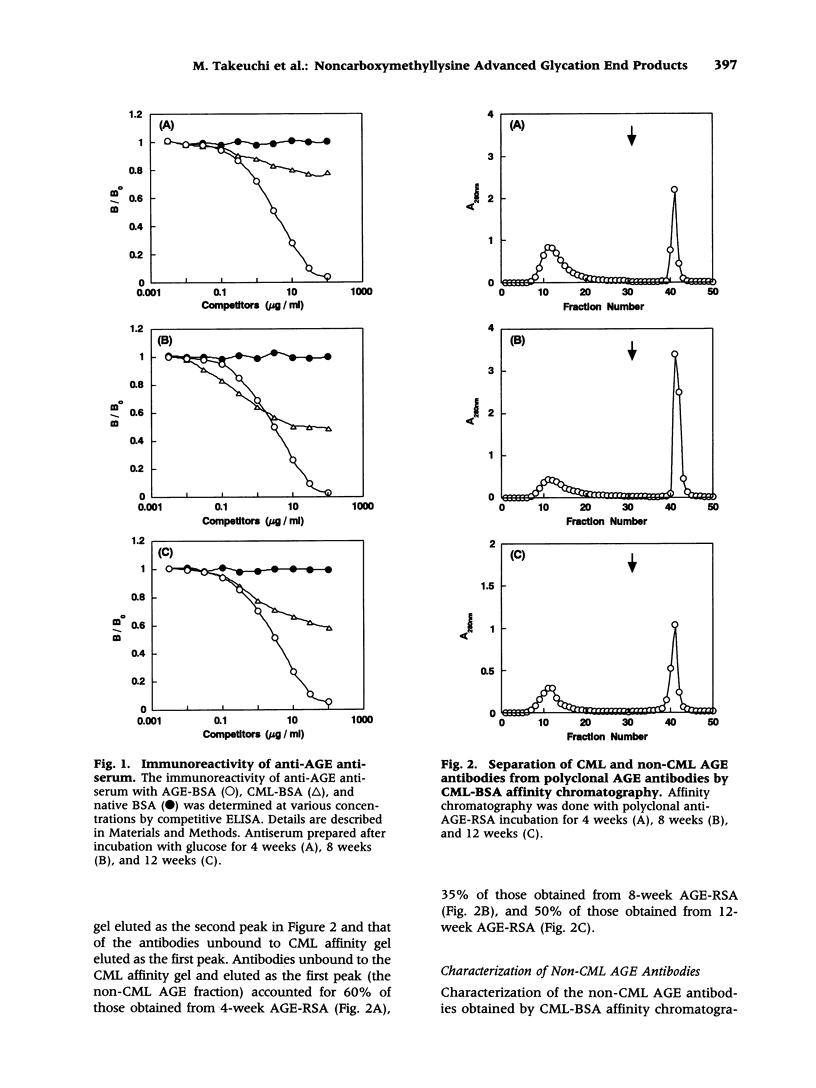
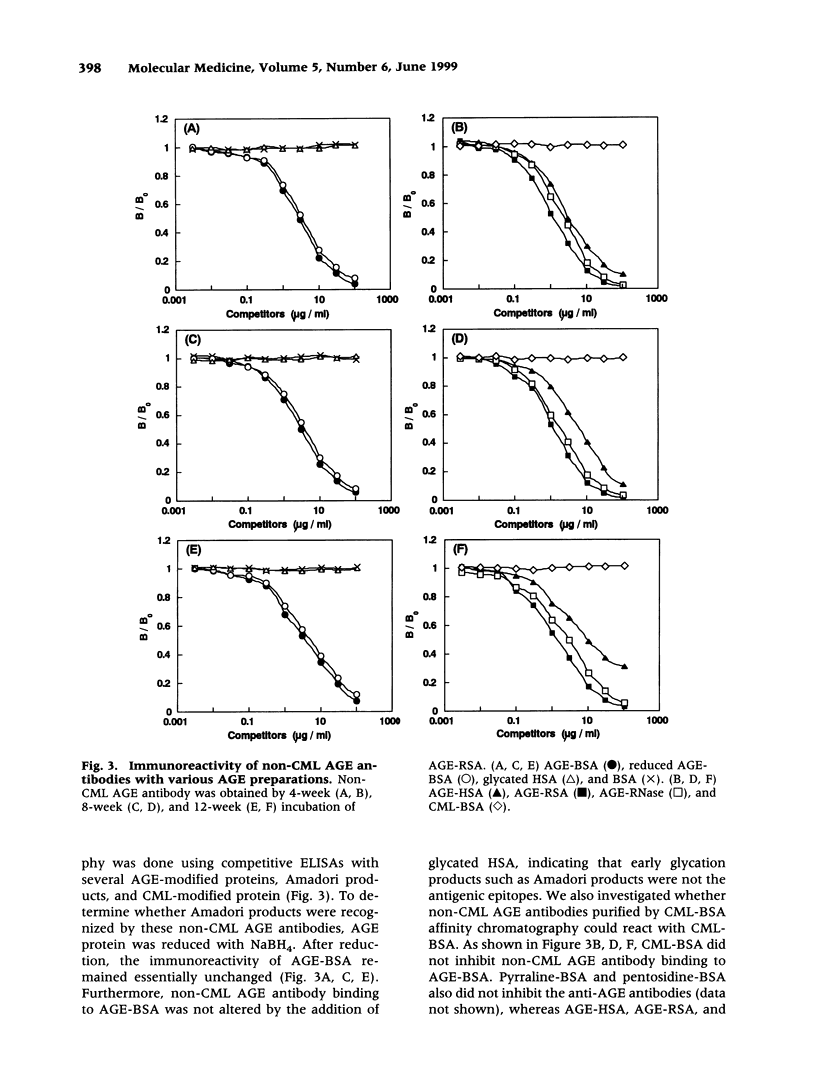
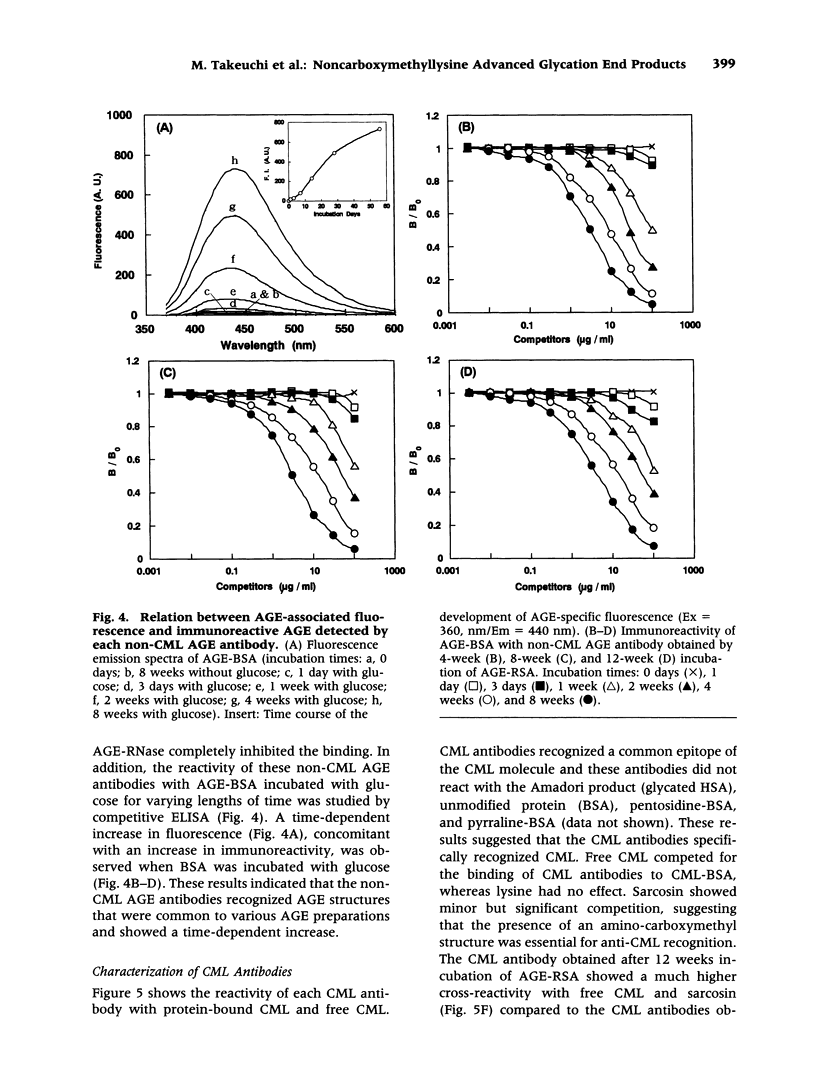
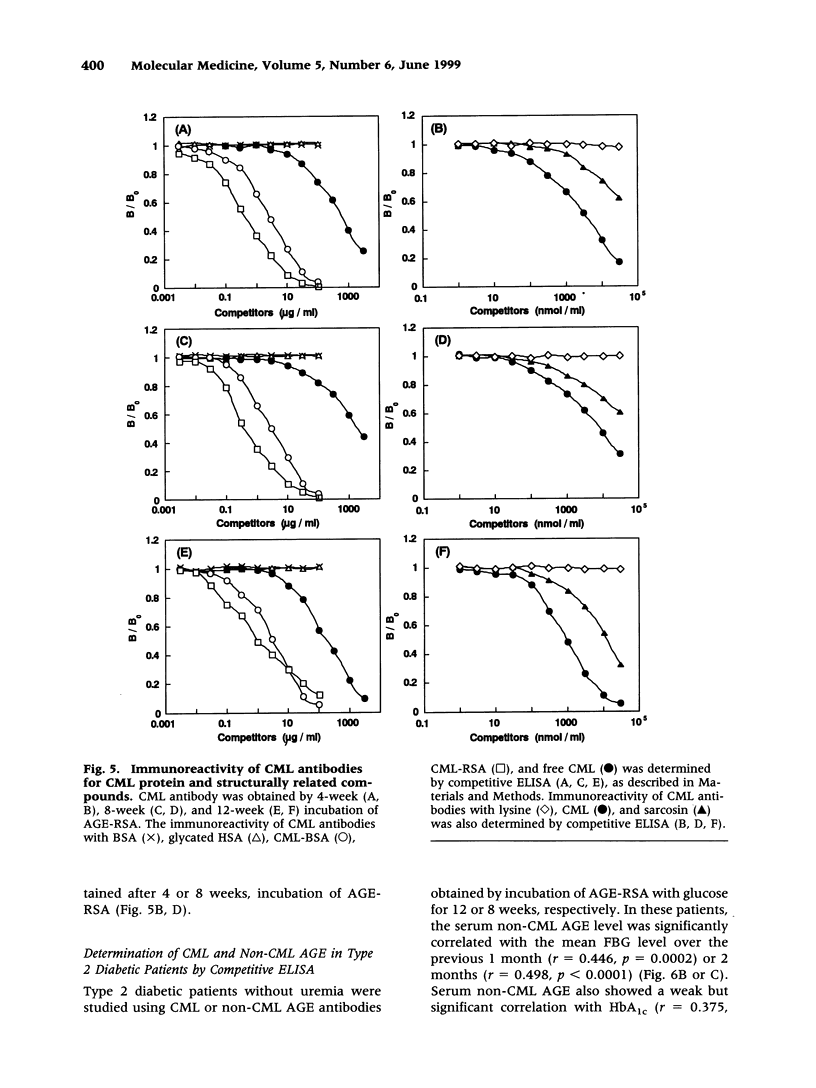
Abstract
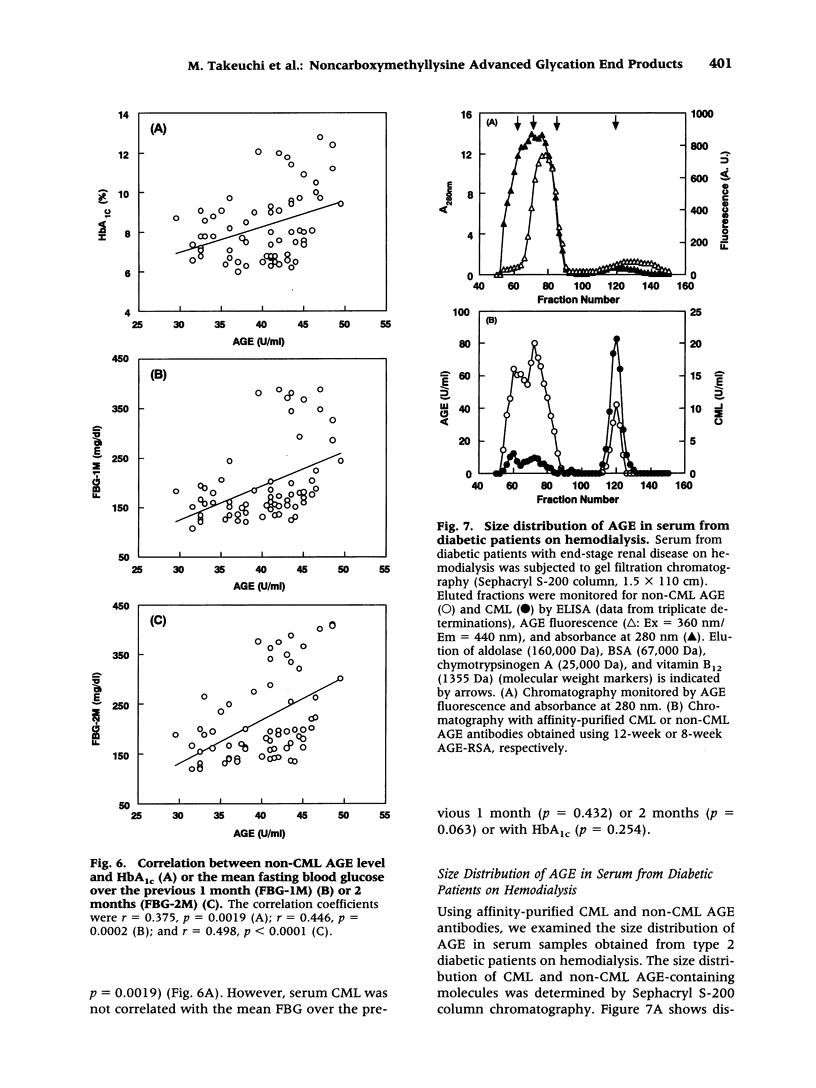
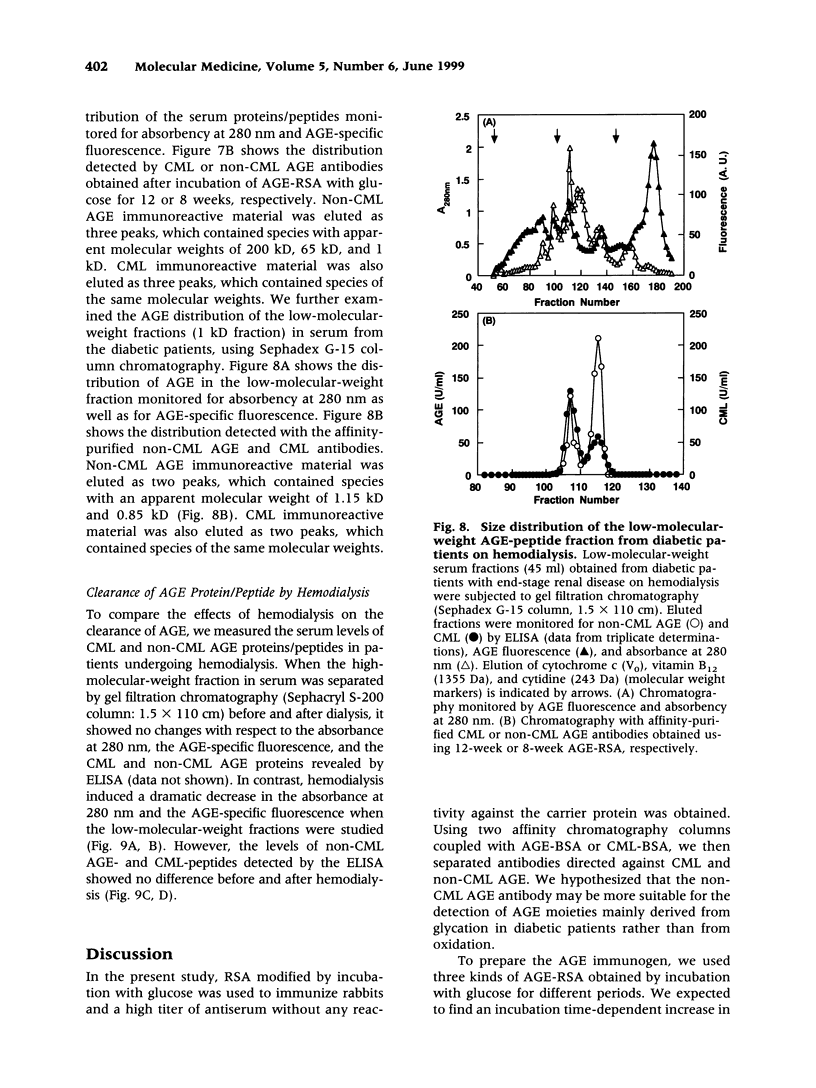
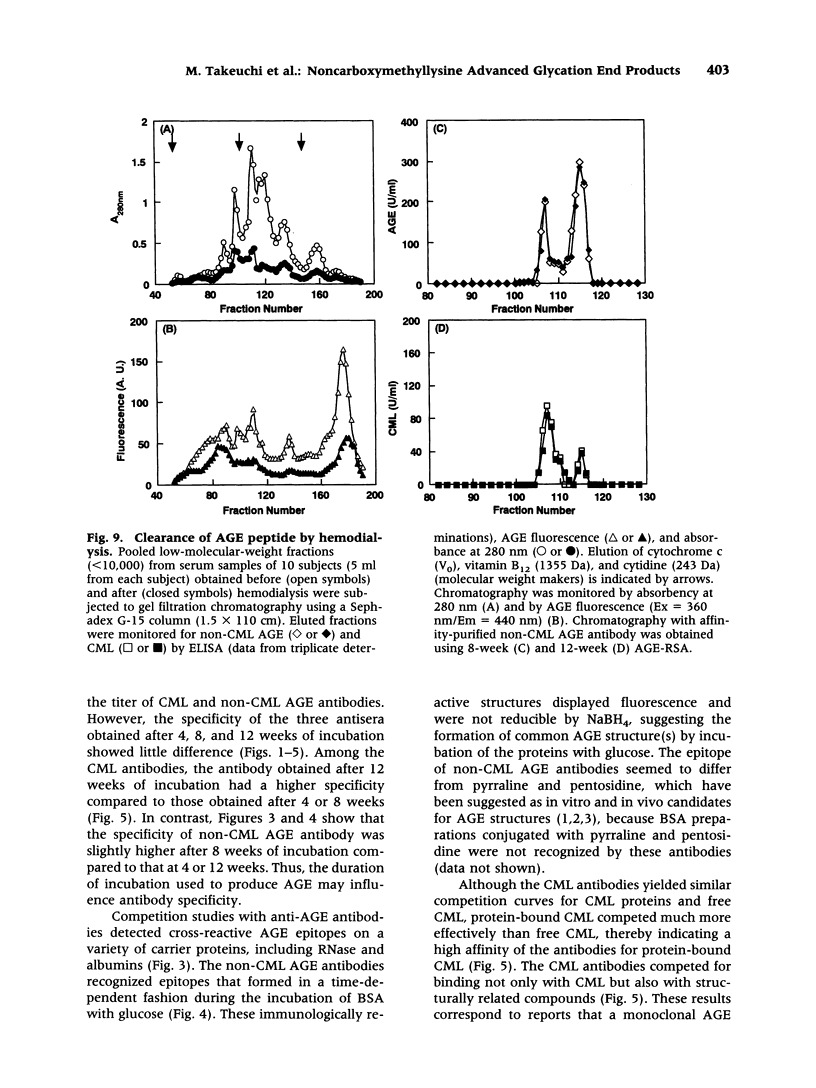
BACKGROUND: The advanced stage of the Maillard reaction, which leads to the formation of advanced glycation end products (AGE), plays an important role in the pathogenesis of angiopathy in diabetic patients and in the aging process. N(epsilon)-(carboxymethyl)lysine (CML) is thought to be an important epitope for many of currently available AGE antibodies. However, recent findings have indicated that a major source of CML may be by pathways other than glycation. A distinction between CML and non-CML AGE may increase our understanding of AGE formation in vivo. In the present study, we prepared antibodies directed against CML and non-CML AGE. MATERIALS AND METHODS: AGE-rabbit serum albumin prepared by 4, 8, and 12 weeks of incubation with glucose was used to immunize rabbits, and a high-titer AGE-specific antiserum was obtained without affinity for the carrier protein. To separate CML and non-CML AGE antibodies, the anti-AGE antiserum was subjected to affinity chromatography on a column coupled with AGE-BSA and CML-BSA. Two different antibodies were obtained, one reacting specifically with CML and the other reacting with non-CML AGE. Circulating levels of CML and non-CML AGE were measured in 66 type 2 diabetic patients without uremia by means of the competitive ELISA. Size distribution and clearance by hemodialysis detected by non-CML AGE and CML were assessed in serum from diabetic patients on hemodialysis. RESULTS: The serum non-CML AGE level in type 2 diabetic patients was significantly correlated with the mean fasting blood glucose level over the previous 2 months (r = 0.498, p < 0.0001) or the previous 1 month (r = 0.446, p = 0. 0002) and with HbA(1c) (r = 0.375, p = 0.0019), but the CML AGE level was not correlated with these clinical parameters. The CML and non-CML AGE were detected as four peaks with apparent molecular weights of 200, 65, 1.15, and 0.85 kD. The hemodialysis treatment did not affect the high-molecular-weight protein fractions. Although the low-molecular-weight peptide fractions (absorbance at 280 nm and fluorescence) were decreased by hemodialysis, there was no difference before and after dialysis in the non-CML AGE- and CML-peptide fractions (1.15 and 0.85 kD fractions). CONCLUSIONS: We propose that both CML and non-CML AGE are present in the blood and that non-CML AGE rather than CML AGE should be more closely evaluated when investigating the pathophysiology of AGE-related diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg T. J., Clausen J. T., Torjesen P. A., Dahl-Jørgensen K., Bangstad H. J., Hanssen K. F. The advanced glycation end product Nepsilon-(carboxymethyl)lysine is increased in serum from children and adolescents with type 1 diabetes. Diabetes Care. 1998 Nov;21(11):1997–2002. doi: 10.2337/diacare.21.11.1997. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dunn J. A., McCance D. R., Thorpe S. R., Lyons T. J., Baynes J. W. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991 Feb 5;30(5):1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- Fu M. X., Requena J. R., Jenkins A. J., Lyons T. J., Baynes J. W., Thorpe S. R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996 Apr 26;271(17):9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- Hayase F., Nagaraj R. H., Miyata S., Njoroge F. G., Monnier V. M. Aging of proteins: immunological detection of a glucose-derived pyrrole formed during maillard reaction in vivo. J Biol Chem. 1989 Mar 5;264(7):3758–3764. [PubMed] [Google Scholar]
- Ikeda K., Higashi T., Sano H., Jinnouchi Y., Yoshida M., Araki T., Ueda S., Horiuchi S. N (epsilon)-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry. 1996 Jun 18;35(24):8075–8083. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- Makita Z., Bucala R., Rayfield E. J., Friedman E. A., Kaufman A. M., Korbet S. M., Barth R. H., Winston J. A., Fuh H., Manogue K. R. Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet. 1994 Jun 18;343(8912):1519–1522. doi: 10.1016/s0140-6736(94)92935-1. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Monnier V. M., Kohn R. R., Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Taneda S., Kuwajima S., Aoki S., Kuroda Y., Misawa K., Nakagawa S. Production and characterization of antibodies to advanced glycation products on proteins. Biochem Biophys Res Commun. 1989 Jul 31;162(2):740–745. doi: 10.1016/0006-291x(89)92372-3. [DOI] [PubMed] [Google Scholar]
- Niwa T., Katsuzaki T., Miyazaki S., Miyazaki T., Ishizaki Y., Hayase F., Tatemichi N., Takei Y. Immunohistochemical detection of imidazolone, a novel advanced glycation end product, in kidneys and aortas of diabetic patients. J Clin Invest. 1997 Mar 15;99(6):1272–1280. doi: 10.1172/JCI119285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasiou P., Grass L., Rodela H., Patrikarea A., Oreopoulos D., Diamandis E. P. Immunological quantification of advanced glycosylation end-products in the serum of patients on hemodialysis or CAPD. Kidney Int. 1994 Jul;46(1):216–222. doi: 10.1038/ki.1994.262. [DOI] [PubMed] [Google Scholar]
- Reddy S., Bichler J., Wells-Knecht K. J., Thorpe S. R., Baynes J. W. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995 Aug 29;34(34):10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- Schleicher E. D., Wagner E., Nerlich A. G. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997 Feb 1;99(3):457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Vasan S., Zhang X., Zhang X., Kapurniotu A., Bernhagen J., Teichberg S., Basgen J., Wagle D., Shih D., Terlecky I. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996 Jul 18;382(6588):275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- Wells-Knecht M. C., Lyons T. J., McCance D. R., Thorpe S. R., Baynes J. W. Age-dependent increase in ortho-tyrosine and methionine sulfoxide in human skin collagen is not accelerated in diabetes. Evidence against a generalized increase in oxidative stress in diabetes. J Clin Invest. 1997 Aug 15;100(4):839–846. doi: 10.1172/JCI119599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffenbuttel B. H., Giordano D., Founds H. W., Bucala R. Long-term assessment of glucose control by haemoglobin-AGE measurement. Lancet. 1996 Feb 24;347(9000):513–515. doi: 10.1016/s0140-6736(96)91141-1. [DOI] [PubMed] [Google Scholar]


