Abstract
BACKGROUND: Early loss of neurites followed by delayed damage of neuronal somata is a feature of several neurodegenerative diseases. Death by apoptosis would ensure the rapid removal of injured neurons, whereas conditions that prevent apoptosis may facilitate the persistence of damaged cells and favor inflammation and disease progression. MATERIALS AND METHODS: Cultures of cerebellar granule cells (CGC) were treated with microtubule disrupting agents. These compounds induced an early degeneration of neurites followed by apoptotic destruction of neuronal somata. The fate of injured neurons was followed after co-exposure to caspase inhibitors or agents that decrease intracellular ATP (deoxyglucose, S-nitrosoglutathione, 1-methyl-4-phenylpyridinium). We examined the implications of energy loss for caspase activation, exposure of phagocytosis markers, and long-term persistence of damaged cells. RESULTS: In CGC exposed to colchicine or nocodazole, axodendritic degeneration preceded caspase activation and apoptosis. ATP-depleting agents or protein synthesis inhibition prevented caspase activation, translocation of the phagocytosis marker, phosphatidylserine, and apoptotic death. However, they did not affect the primary neurite loss. Repletion of ATP by enhanced glycolysis restored all apoptotic features. Peptide inhibitors of caspases also prevented the apoptotic changes in the cell bodies, although the axodendritic net was lost. Under this condition cell demise still occurred 48 hr later in a caspase-independent manner and involved plasma membrane lysis at the latest stage. CONCLUSIONS: Inhibition of the apoptotic machinery by drugs, energy deprivation, or endogenous mediators may result in the persistence and subsequent lysis of injured neurons. In vivo, this may favor the onset of inflammatory processes and perpetuate neurodegeneration.
Full text
PDF



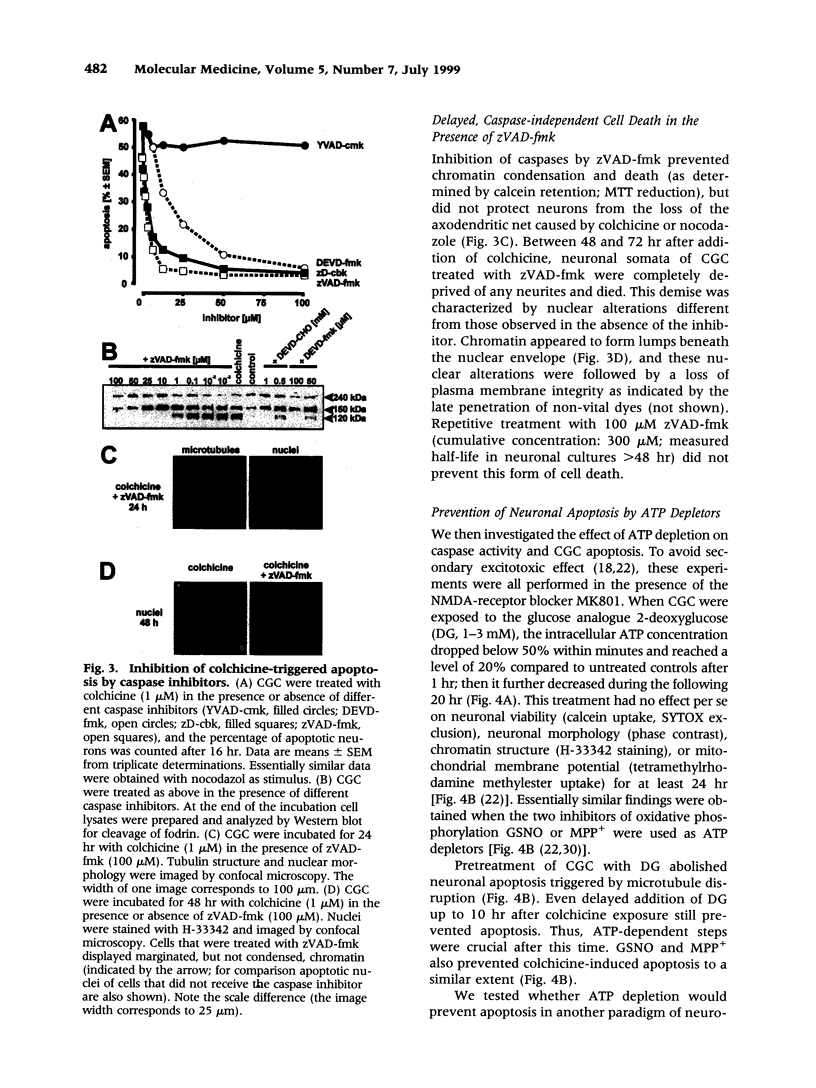
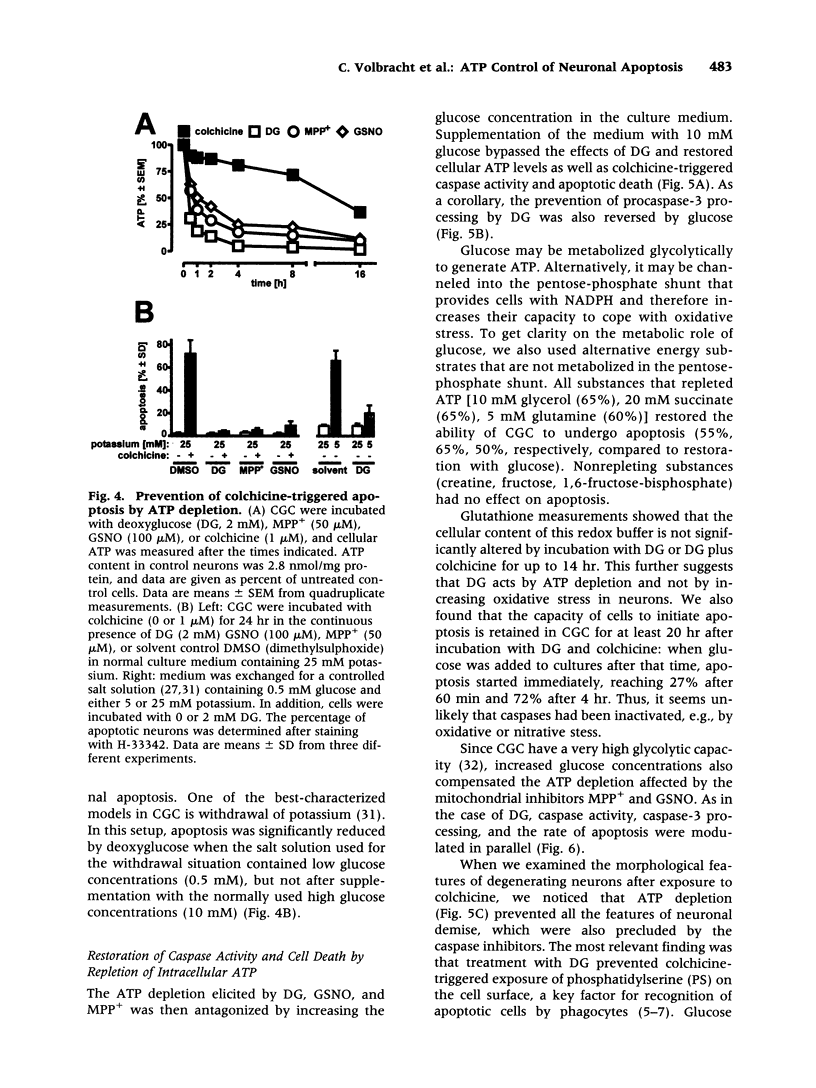
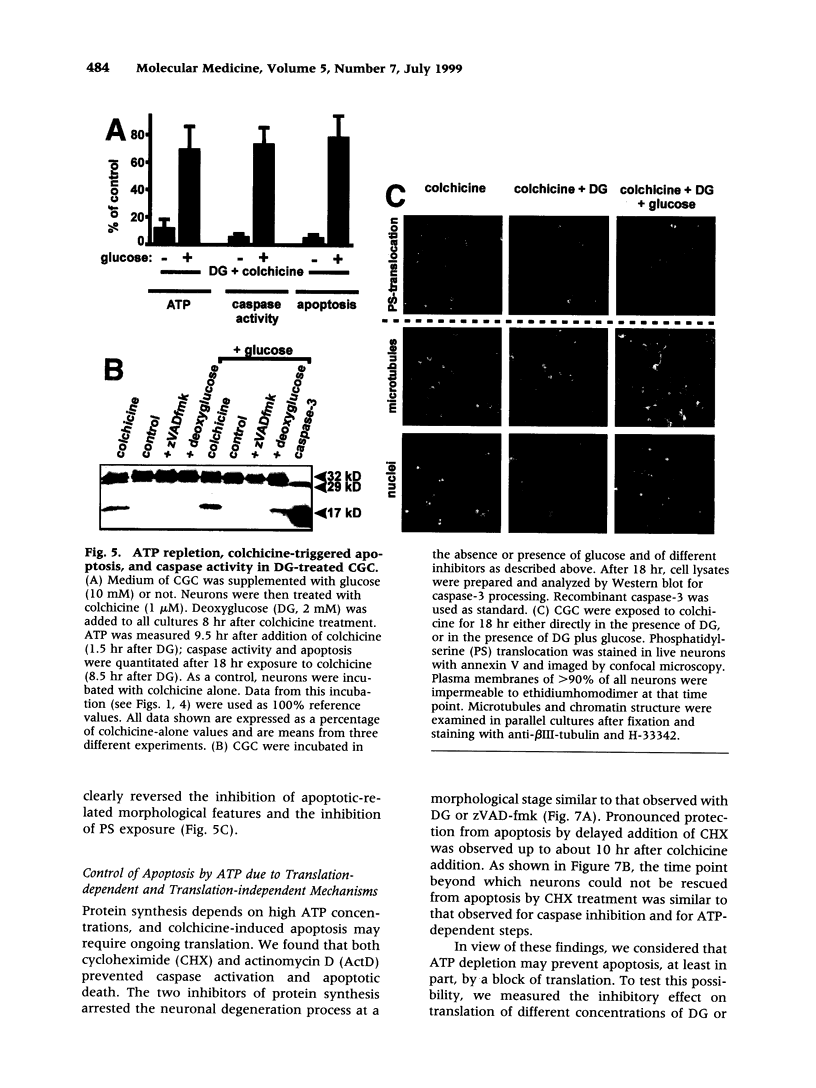
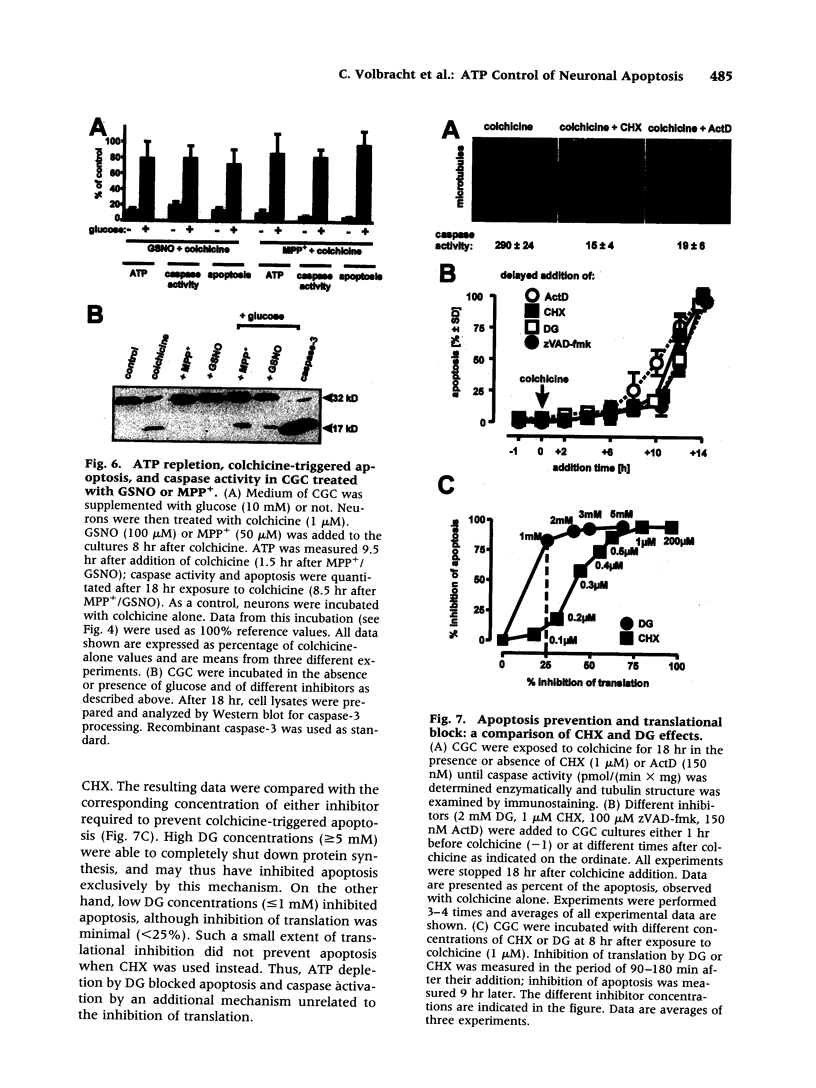




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankarcrona M., Dypbukt J. M., Bonfoco E., Zhivotovsky B., Orrenius S., Lipton S. A., Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995 Oct;15(4):961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Barinaga M. Is apoptosis key in Alzheimer's disease? Science. 1998 Aug 28;281(5381):1303–1304. doi: 10.1126/science.281.5381.1303. [DOI] [PubMed] [Google Scholar]
- Basu A., Haldar S. Microtubule-damaging drugs triggered bcl2 phosphorylation-requirement of phosphorylation on both serine-70 and serine-87 residues of bcl2 protein. Int J Oncol. 1998 Oct;13(4):659–664. doi: 10.3892/ijo.13.4.659. [DOI] [PubMed] [Google Scholar]
- Beal M. F. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996 Oct;6(5):661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
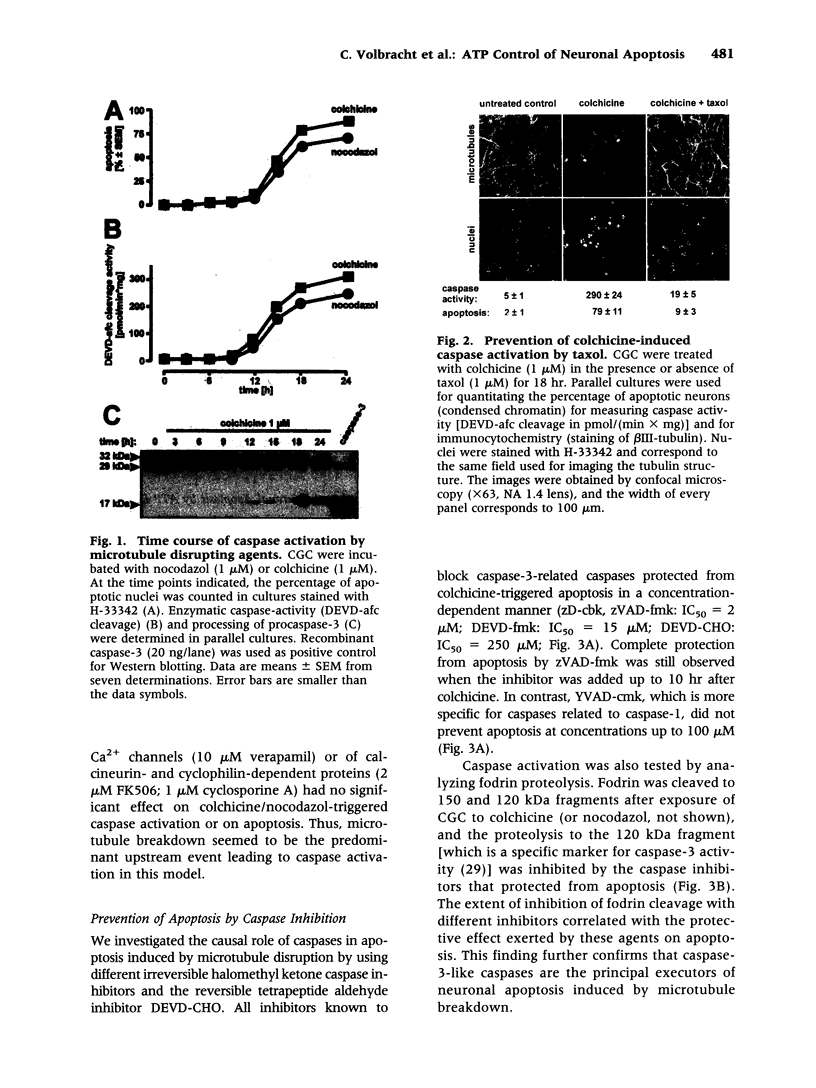
- Bonfoco E., Ceccatelli S., Manzo L., Nicotera P. Colchicine induces apoptosis in cerebellar granule cells. Exp Cell Res. 1995 May;218(1):189–200. doi: 10.1006/excr.1995.1147. [DOI] [PubMed] [Google Scholar]
- Budd S. L., Nicholls D. G. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996 Jan;66(1):403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S., Ahlbom E., Diana A., Zhivotovsky B. Apoptosis in rat hippocampal dentate gyrus after intraventricular colchicine. Neuroreport. 1997 Dec 1;8(17):3779–3783. doi: 10.1097/00001756-199712010-00025. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S., Ernfors P., Villar M. J., Persson H., Hökfelt T. Expanded distribution of mRNA for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the rat brain after colchicine treatment. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10352–10356. doi: 10.1073/pnas.88.22.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccatelli S., Villar M. J., Goldstein M., Hökfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9569–9573. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994 May 23;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Clementi E., Brown G. C., Feelisch M., Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998 Jun 23;95(13):7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S., Zeiher A. M. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide. 1997 Aug;1(4):275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Shimizu S., Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997 May 15;57(10):1835–1840. [PubMed] [Google Scholar]
- Evtodienko Y. V., Teplova V. V., Sidash S. S., Ichas F., Mazat J. P. Microtubule-active drugs suppress the closure of the permeability transition pore in tumour mitochondria. FEBS Lett. 1996 Sep 9;393(1):86–88. doi: 10.1016/0014-5793(96)00875-7. [DOI] [PubMed] [Google Scholar]
- Green D., Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998 Jul;8(7):267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- Hartley A., Stone J. M., Heron C., Cooper J. M., Schapira A. H. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson's disease. J Neurochem. 1994 Nov;63(5):1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- Hirsch T., Marchetti P., Susin S. A., Dallaporta B., Zamzami N., Marzo I., Geuskens M., Kroemer G. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene. 1997 Sep 25;15(13):1573–1581. doi: 10.1038/sj.onc.1201324. [DOI] [PubMed] [Google Scholar]
- Hsia A. Y., Masliah E., McConlogue L., Yu G. Q., Tatsuno G., Hu K., Kholodenko D., Malenka R. C., Nicoll R. A., Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci U S A. 1999 Mar 16;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke R. U., Ng P., Sprengart M. L., Porter A. G. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998 Jun 19;273(25):15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- Kharlamov E., Cagnoli C. M., Atabay C., Ikonomović S., Grayson D. R., Manev H. Opposite effect of protein synthesis inhibitors on potassium deficiency-induced apoptotic cell death in immature and mature neuronal cultures. J Neurochem. 1995 Sep;65(3):1395–1398. doi: 10.1046/j.1471-4159.1995.65031395.x. [DOI] [PubMed] [Google Scholar]
- Krahling S., Callahan M. K., Williamson P., Schlegel R. A. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999 Feb;6(2):183–189. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- Leist M., Fava E., Montecucco C., Nicotera P. Peroxynitrite and nitric oxide donors induce neuronal apoptosis by eliciting autocrine excitotoxicity. Eur J Neurosci. 1997 Jul;9(7):1488–1498. doi: 10.1111/j.1460-9568.1997.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Leist M., Gantner F., Bohlinger I., Germann P. G., Tiegs G., Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994 Aug 15;153(4):1778–1788. [PubMed] [Google Scholar]
- Leist M., Gantner F., Künstle G., Wendel A. Cytokine-mediated hepatic apoptosis. Rev Physiol Biochem Pharmacol. 1998;133:109–155. doi: 10.1007/BFb0000614. [DOI] [PubMed] [Google Scholar]
- Leist M., Nicotera P. Apoptosis, excitotoxicity, and neuropathology. Exp Cell Res. 1998 Mar 15;239(2):183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- Leist M., Single B., Castoldi A. F., Kühnle S., Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997 Apr 21;185(8):1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M., Single B., Künstle G., Volbracht C., Hentze H., Nicotera P. Apoptosis in the absence of poly-(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1997 Apr 17;233(2):518–522. doi: 10.1006/bbrc.1997.6491. [DOI] [PubMed] [Google Scholar]
- Leist M., Single B., Naumann H., Fava E., Simon B., Kühnle S., Nicotera P. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp Cell Res. 1999 Jun 15;249(2):396–403. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- Leist M., Single B., Naumann H., Fava E., Simon B., Kühnle S., Nicotera P. Nitric oxide inhibits execution of apoptosis at two distinct ATP-dependent steps upstream and downstream of mitochondrial cytochrome c release. Biochem Biophys Res Commun. 1999 Apr 29;258(1):215–221. doi: 10.1006/bbrc.1999.0491. [DOI] [PubMed] [Google Scholar]
- Leist M., Volbracht C., Fava E., Nicotera P. 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Mol Pharmacol. 1998 Nov;54(5):789–801. doi: 10.1124/mol.54.5.789. [DOI] [PubMed] [Google Scholar]
- Leist M., Volbracht C., Kühnle S., Fava E., Ferrando-May E., Nicotera P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol Med. 1997 Nov;3(11):750–764. [PMC free article] [PubMed] [Google Scholar]
- Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997 Nov 14;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996 Jul 12;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- Mattson M. P. Mother's legacy: mitochondrial DNA mutations and Alzheimer's disease. Trends Neurosci. 1997 Sep;20(9):373–375. doi: 10.1016/s0166-2236(97)01114-4. [DOI] [PubMed] [Google Scholar]
- Nicklas W. J., Vyas I., Heikkila R. E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985 Jul 1;36(26):2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Leist M., Manzo L. Neuronal cell death: a demise with different shapes. Trends Pharmacol Sci. 1999 Feb;20(2):46–51. doi: 10.1016/s0165-6147(99)01304-8. [DOI] [PubMed] [Google Scholar]
- Ren Y., Savill J. Apoptosis: the importance of being eaten. Cell Death Differ. 1998 Jul;5(7):563–568. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Schulz J. B., Weller M., Klockgether T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. J Neurosci. 1996 Aug 1;16(15):4696–4706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994 Oct 14;204(1):169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- Srivastava R. K., Srivastava A. R., Korsmeyer S. J., Nesterova M., Cho-Chung Y. S., Longo D. L. Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol. 1998 Jun;18(6):3509–3517. doi: 10.1128/mcb.18.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C., Brück W., Bancher C., Jellinger K., Lassmann H. Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol. 1998 May;57(5):456–464. doi: 10.1097/00005072-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Stroh C., Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998 Dec;5(12):997–1000. doi: 10.1038/sj.cdd.4400451. [DOI] [PubMed] [Google Scholar]
- Tenneti L., D'Emilia D. M., Lipton S. A. Suppression of neuronal apoptosis by S-nitrosylation of caspases. Neurosci Lett. 1997 Nov 7;236(3):139–142. doi: 10.1016/s0304-3940(97)00780-5. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A. Interleukin-1 beta converting enzyme. Methods Enzymol. 1994;244:615–631. doi: 10.1016/0076-6879(94)44045-x. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tipton K. F., Singer T. P. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem. 1993 Oct;61(4):1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial diseases in man and mouse. Science. 1999 Mar 5;283(5407):1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Brohee H. Prevention of nuclear damage in irradiated rat thymocytes by post-irradiation oxygen deprivation. Nature. 1966 Aug 13;211(5050):775–776. doi: 10.1038/211775a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]