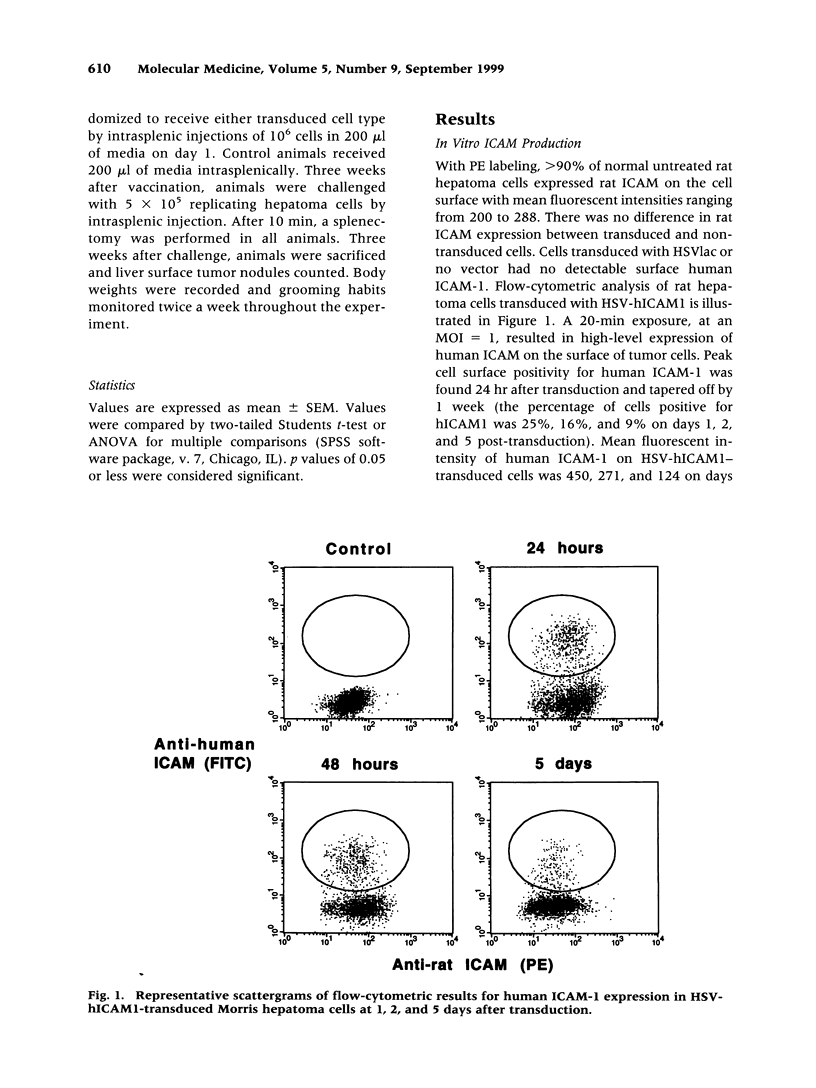
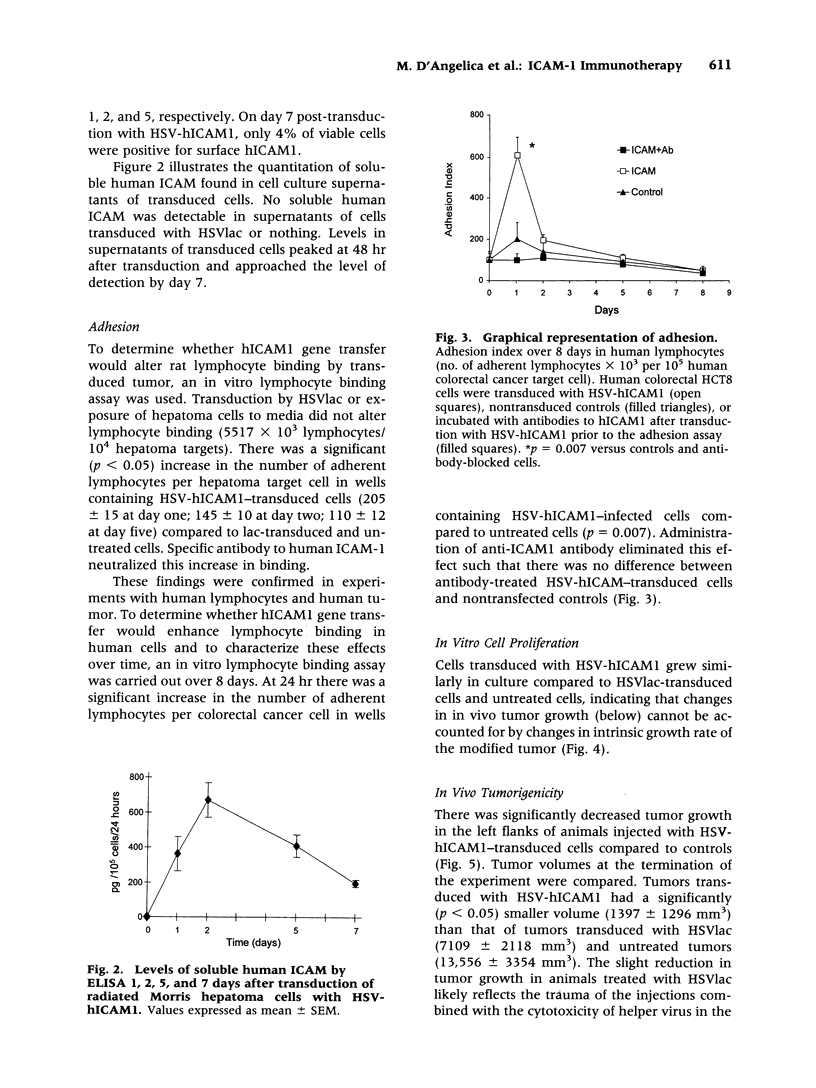
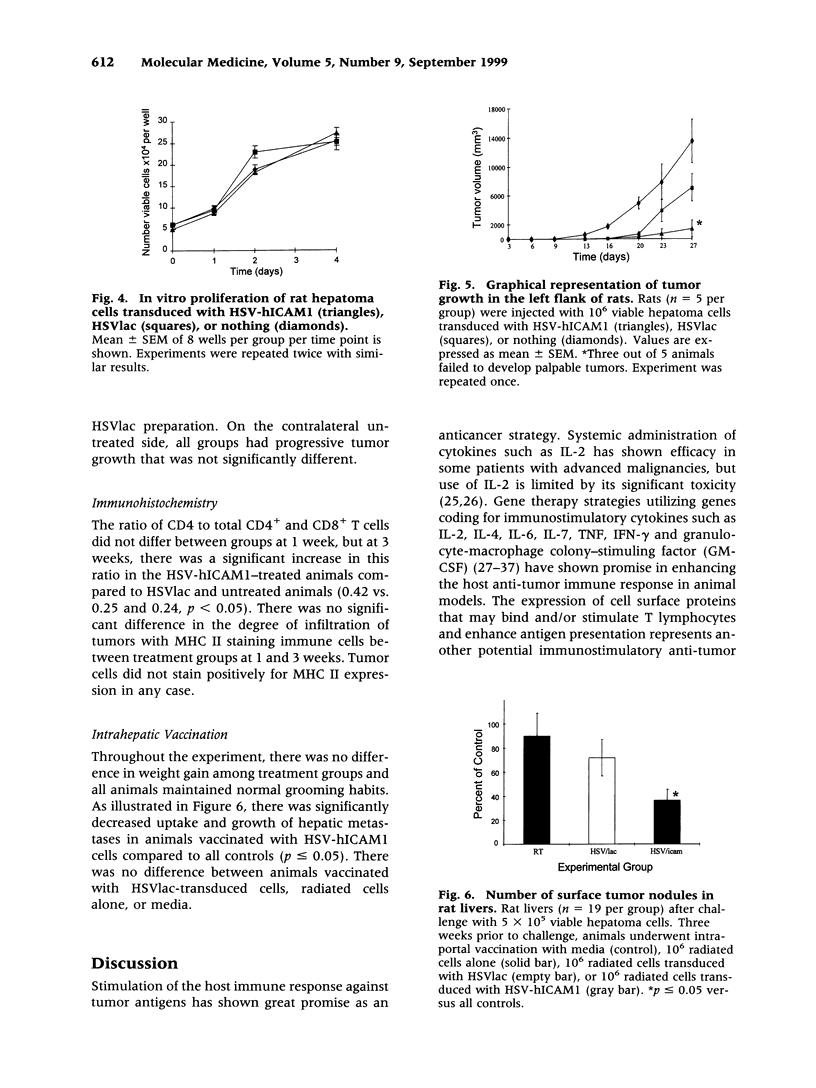
Abstract
BACKGROUND: Costimulatory and cellular adhesion molecules are thought to be essential components of antigen presentation in the immune response to cancer. The current studies examine gene transfer utilizing herpes viral amplicon vectors (HSV) to direct surface expression of adhesion molecules, and specifically evaluate the potential of a tumor-expressing intercellular adhesion molecule-1 (ICAM-1) to elicit an anti-tumor response. MATERIALS AND METHODS: The human ICAM-1 (hICAM1) gene was inserted into an HSV amplicon vector and tested in a transplantable rat hepatocellular carcinoma and in a human colorectal cancer cell line. Cell surface ICAM-1 expression was assessed by flow cytometry. Lymphocyte binding to HSV-hICAM1-transduced cells was compared with that to cells transduced with HSV not carrying the ICAM gene. Tumorigenicity of HSV-hICAM1-transduced tumor cells were tested in syngeneic Buffalo rats. Additionally, immunization with irradiated (10,000 rads) HSV-hICAM1-transduced tumor cells was performed to determine its effect on tumor growth. RESULTS: A 20-min exposure of tumor cells at a multiplicity of infection (MOI) of 1 resulted in high-level cell surface expression of human ICAM in approximately 25% of tumor cells. Transduced rat or human tumor cells exhibited significantly enhanced binding of lymphocytes (p < 0.05). HSV-hICAM1-transduced cells elicited an increase in infiltration by CD4(+) lymphocytes in vivo and exhibited decreased tumorigenicity. Immunization with irradiated HSV-hICAM1-transduced cells protected against growth of subsequent injected parental tumor cells. CONCLUSIONS: HSV amplicon-mediated gene transfer is an efficient method for modifying the cell surface expression of adhesion molecules. Increased tumor expression of ICAM-1 represents a promising immune anti-cancer strategy.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allione A., Consalvo M., Nanni P., Lollini P. L., Cavallo F., Giovarelli M., Forni M., Gulino A., Colombo M. P., Dellabona P. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and gamma-interferon gene or admixed with conventional adjuvants. Cancer Res. 1994 Dec 1;54(23):6022–6026. [PubMed] [Google Scholar]
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Baskar S., Ostrand-Rosenberg S., Nabavi N., Nadler L. M., Freeman G. J., Glimcher L. H. Constitutive expression of B7 restores immunogenicity of tumor cells expressing truncated major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5687–5690. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. C., Dummer R., Hartmann A. A., Burg G., Schmidt R. E. Shedding of ICAM-1 from human melanoma cell lines induced by IFN-gamma and tumor necrosis factor-alpha. Functional consequences on cell-mediated cytotoxicity. J Immunol. 1991 Dec 15;147(12):4398–4401. [PubMed] [Google Scholar]
- Boyer M. W., Orchard P. J., Gorden K. B., Anderson P. M., Mclvor R. S., Blazar B. R. Dependency on intercellular adhesion molecule recognition and local interleukin-2 provision in generation of an in vivo CD8+ T-cell immune response to murine myeloid leukemia. Blood. 1995 May 1;85(9):2498–2506. [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Connor J., Bannerji R., Saito S., Heston W., Fair W., Gilboa E. Regression of bladder tumors in mice treated with interleukin 2 gene-modified tumor cells. J Exp Med. 1993 Apr 1;177(4):1127–1134. doi: 10.1084/jem.177.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Staunton D. E., Springer T. A. Supergene families meet in the immune system. Immunol Today. 1988 Jul-Aug;9(7-8):213–215. doi: 10.1016/0167-5699(88)91216-9. [DOI] [PubMed] [Google Scholar]
- Fady C., Gardner A., Gera J. F., Lichtenstein A. Interferon-gamma-induced increased sensitivity of HER2/neu-overexpressing tumor cells to lymphokine-activated killer cell lysis: importance of ICAM-1 in binding and post-binding events. Cancer Immunol Immunother. 1993 Oct;37(5):329–336. doi: 10.1007/BF01518456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Fong Y., Federoff H. J., Brownlee M., Blumberg D., Blumgart L. H., Brennan M. F. Rapid and efficient gene transfer in Human hepatocytes by herpes viral vectors. Hepatology. 1995 Sep;22(3):723–729. [PubMed] [Google Scholar]
- Geller A. I., Keyomarsi K., Bryan J., Pardee A. B. An efficient deletion mutant packaging system for defective herpes simplex virus vectors: potential applications to human gene therapy and neuronal physiology. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8950–8954. doi: 10.1073/pnas.87.22.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlinger U., Kramm C. M., Aboody-Guterman K. S., Silver J. S., Ikeda K., Johnston K. M., Pechan P. A., Barth R. F., Finkelstein D., Chiocca E. A. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998 Jun;5(6):809–819. doi: 10.1038/sj.gt.3300643. [DOI] [PubMed] [Google Scholar]
- Hock H., Dorsch M., Kunzendorf U., Qin Z., Diamantstein T., Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2774–2778. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanuma Y., Kato K., Yagita H., Okumura K. Induction of tumor-specific cytotoxic T lymphocytes and natural killer cells by tumor cells transfected with the interleukin-2 gene. Cancer Immunol Immunother. 1995 Jan;40(1):17–23. doi: 10.1007/BF01517231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. P. The role of ICAM-1 in tumor development. Chem Immunol. 1991;50:143–163. [PubMed] [Google Scholar]
- Karpoff H. M., D'Angelica M., Blair S., Brownlee M. D., Federoff H., Fong Y. Prevention of hepatic tumor metastases in rats with herpes viral vaccines and gamma-interferon. J Clin Invest. 1997 Feb 15;99(4):799–804. doi: 10.1172/JCI119226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto Y., Monden T., Takeda T., Haba A., Ito Y., Wakasugi E., Wakasugi T., Sekimoto M., Kobayashi T., Shiozaki H. Analysis of cytotoxic activity of the CD4+ T lymphocytes generated by local immunotherapy. Br J Cancer. 1996 Jan;73(1):110–116. doi: 10.1038/bjc.1996.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S. Immunosuppressive effect of shedding intercellular adhesion molecule 1 antigen on cell-mediated cytotoxicity against tumor cells. Jpn J Cancer Res. 1994 Feb;85(2):131–134. doi: 10.1111/j.1349-7006.1994.tb02072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramm C. M., Chase M., Herrlinger U., Jacobs A., Pechan P. A., Rainov N. G., Sena-Esteves M., Aghi M., Barnett F. H., Chiocca E. A. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997 Nov 20;8(17):2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- Lee R. E., Lotze M. T., Skibber J. M., Tucker E., Bonow R. O., Ognibene F. P., Carrasquillo J. A., Shelhamer J. H., Parrillo J. E., Rosenberg S. A. Cardiorespiratory effects of immunotherapy with interleukin-2. J Clin Oncol. 1989 Jan;7(1):7–20. doi: 10.1200/JCO.1989.7.1.7. [DOI] [PubMed] [Google Scholar]
- Levitsky H. I., Lazenby A., Hayashi R. J., Pardoll D. M. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med. 1994 Apr 1;179(4):1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley V., Langlade-Demoyen P., Kourilsky P., Larsson-Sciard E. L. Interleukin 2-dependent activation of tumor-specific cytotoxic T lymphocytes in vivo. Eur J Immunol. 1991 Mar;21(3):851–854. doi: 10.1002/eji.1830210350. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Chang A. E., Seipp C. A., Simpson C., Vetto J. T., Rosenberg S. A. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986 Dec 12;256(22):3117–3124. [PubMed] [Google Scholar]
- Lu B., Gupta S., Federoff H. Ex vivo hepatic gene transfer in mouse using a defective herpes simplex virus-1 vector. Hepatology. 1995 Mar;21(3):752–759. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- Miki I., Ishihara N., Otoshi M., Kase H. Simple colorimetric cell-cell adhesion assay using MTT-stained leukemia cells. J Immunol Methods. 1993 Sep 15;164(2):255–261. doi: 10.1016/0022-1759(93)90318-2. [DOI] [PubMed] [Google Scholar]
- Porgador A., Tzehoval E., Katz A., Vadai E., Revel M., Feldman M., Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992 Jul 1;52(13):3679–3686. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Roth J. A., Cristiano R. J. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997 Jan 1;89(1):21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Sartor W. M., Kyprianou N., Fabian D. F., Lefor A. T. Enhanced expression of ICAM-1 in a murine fibrosarcoma reduces tumor growth rate. J Surg Res. 1995 Jul;59(1):66–74. doi: 10.1006/jsre.1995.1133. [DOI] [PubMed] [Google Scholar]
- Simmons D. L. The role of ICAM expression in immunity and disease. Cancer Surv. 1995;24:141–155. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Tung C., Federoff H. J., Brownlee M., Karpoff H., Weigel T., Brennan M. F., Fong Y. Rapid production of interleukin-2-secreting tumor cells by herpes simplex virus-mediated gene transfer: implications for autologous vaccine production. Hum Gene Ther. 1996 Dec 1;7(18):2217–2224. doi: 10.1089/hum.1996.7.18-2217. [DOI] [PubMed] [Google Scholar]
- Uzendoski K., Kantor J. A., Abrams S. I., Schlom J., Hodge J. W. Construction and characterization of a recombinant vaccinia virus expressing murine intercellular adhesion molecule-1: induction and potentiation of antitumor responses. Hum Gene Ther. 1997 May 1;8(7):851–860. doi: 10.1089/hum.1997.8.7-851. [DOI] [PubMed] [Google Scholar]
- Voraberger G., Schäfer R., Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5'-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991 Oct 15;147(8):2777–2786. [PubMed] [Google Scholar]
- Vánky F., Wang P., Patarroyo M., Klein E. Expression of the adhesion molecule ICAM-1 and major histocompatibility complex class I antigens on human tumor cells is required for their interaction with autologous lymphocytes in vitro. Cancer Immunol Immunother. 1990;31(1):19–27. doi: 10.1007/BF01742491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. S., Mostowski H. S., Gerrard T. L. Cytokine-induced enhancement of ICAM-1 expression results in increased vulnerability of tumor cells to monocyte-mediated lysis. J Immunol. 1991 May 15;146(10):3682–3686. [PubMed] [Google Scholar]
- Wei K., Wilson J. G., Jurgensen C. H., Iannone M. A., Wolberg G., Huber B. E. Xenogeneic ICAM-1 gene transfer suppresses tumorigenicity and generates protective antitumor immunity. Gene Ther. 1996 Jun;3(6):531–541. [PubMed] [Google Scholar]



