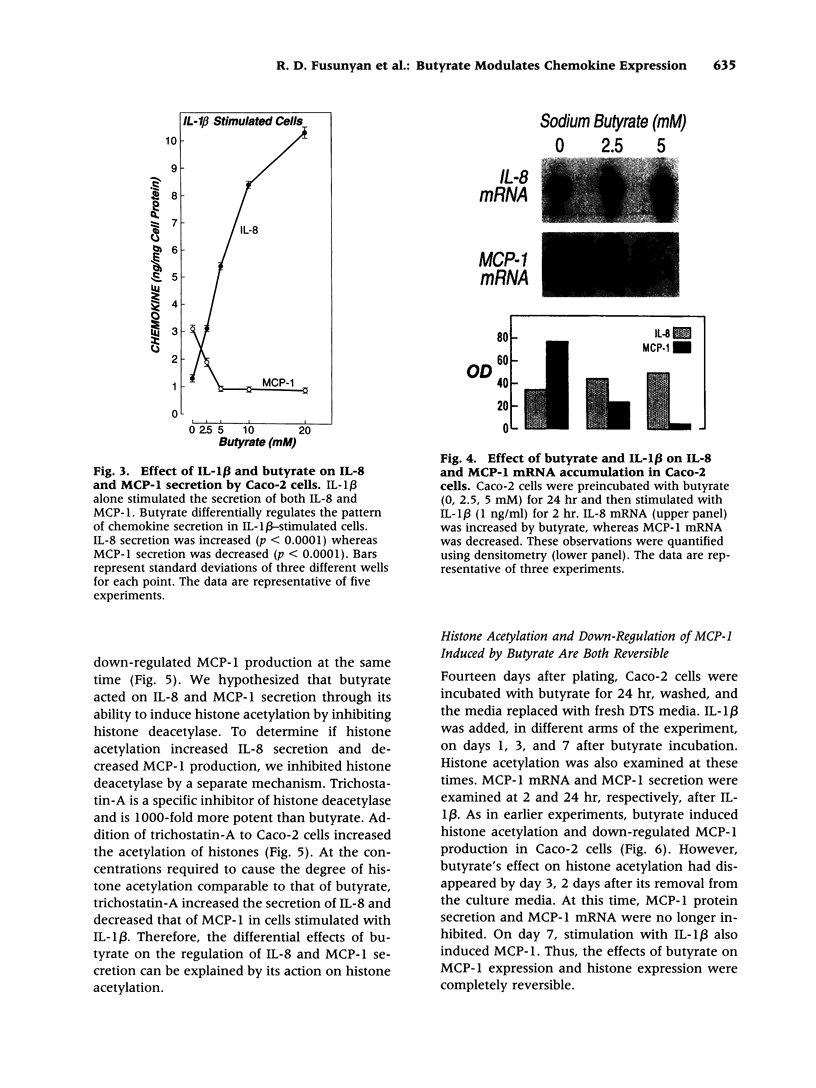
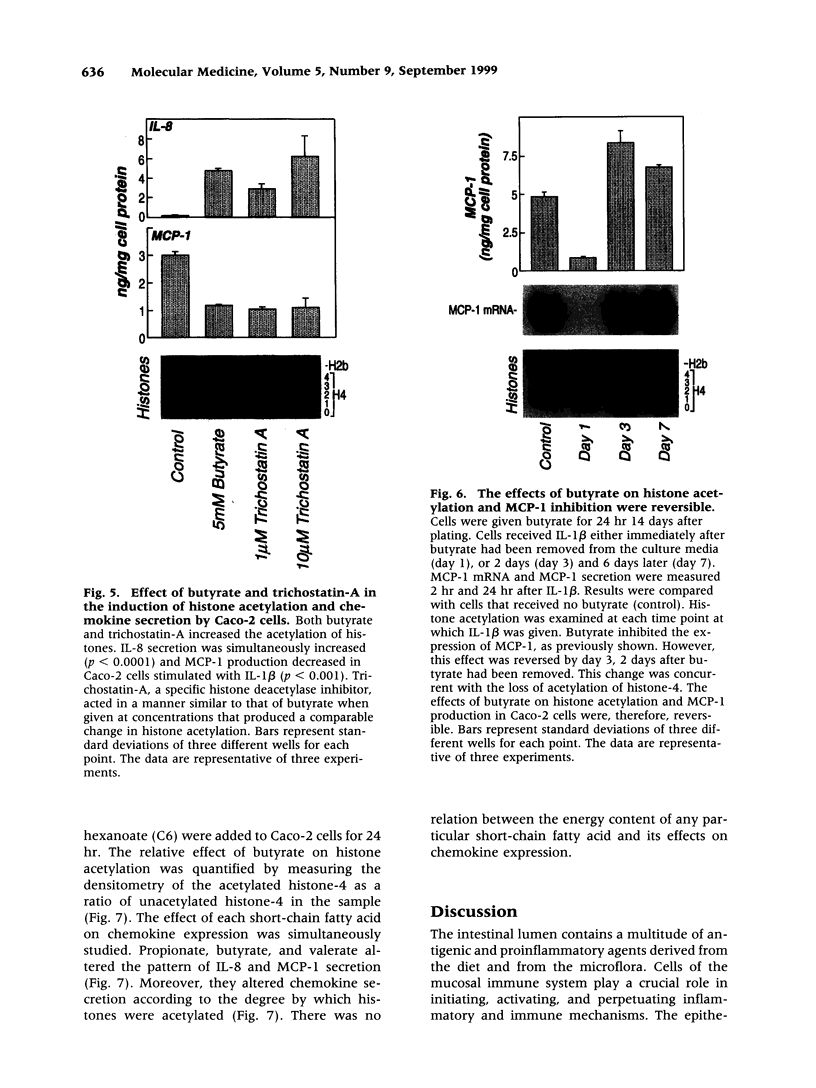
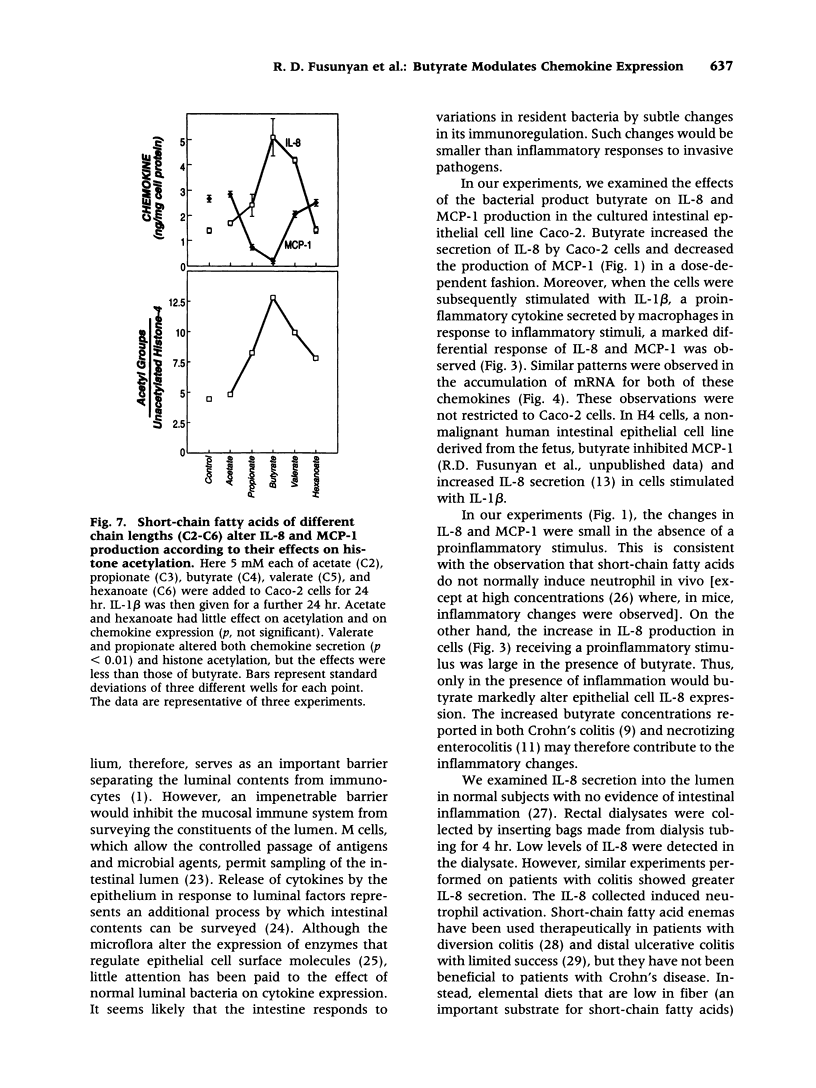
Abstract
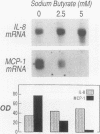
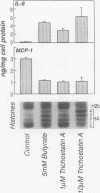
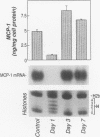
BACKGROUND: Butyrate, a fermentation product of intestinal bacteria, modifies chromatin structure through histone acetylation, thereby altering gene transcription. IL-8 and MCP-1 are chemokines, expressed by intestinal epithelial cells, which attract neutrophils and monocytes, respectively. We hypothesized that butyrate may alter IL-8 and MCP-1 expression by intestinal epithelial cells through histone acetylation. MATERIALS AND METHODS: IL-8 and MCP-1 expression was measured by ELISA and RNA transfer blots. Acetylated histones were separated on acetic acid-urea-triton gels. Butyrate was compared to Trichostatin-A, a specific inhibitor of histone deacetylase and to other short chain fatty acids. RESULTS: Caco-2 cells constitutively secreted MCP-1 but not IL-8. Butyrate reversibly decreased MCP-1 secretion. In contrast, butyrate increased IL-8 production. The effects of butyrate and Trichostatin-A were greater when cells were stimulated with IL-1beta. Butyrate and Trichostatin-A both increased histone acetylation. Trichostatin-A and other short chain fatty acids altered chemokine secretion according to their effect on histone acetylation. CONCLUSIONS: Butyrate reversibly switches chemokine secretion by epithelial cells through histone acetylation. We speculate that butyrate carries information from resident bacteria to epithelial cells. Epithelial cells transduce this signal through histone acetylation, modulating the secretion of chemokines.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R., Hayes J. J., White J. H., Wolffe A. P. Nucleosome structural changes due to acetylation. J Mol Biol. 1994 Feb 25;236(3):685–690. doi: 10.1006/jmbi.1994.1180. [DOI] [PubMed] [Google Scholar]
- Breuer R. I., Soergel K. H., Lashner B. A., Christ M. L., Hanauer S. B., Vanagunas A., Harig J. M., Keshavarzian A., Robinson M., Sellin J. H. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997 Apr;40(4):485–491. doi: 10.1136/gut.40.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens L. S., Alberts B. M. Accessibility of newly synthesized chromatin to histone acetylase. J Biol Chem. 1982 Apr 10;257(7):3945–3949. [PubMed] [Google Scholar]
- Cummings J. H. Short chain fatty acids in the human colon. Gut. 1981 Sep;22(9):763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusunyan R. D., Quinn J. J., Ohno Y., MacDermott R. P., Sanderson I. R. Butyrate enhances interleukin (IL)-8 secretion by intestinal epithelial cells in response to IL-1beta and lipopolysaccharide. Pediatr Res. 1998 Jan;43(1):84–90. doi: 10.1203/00006450-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Harig J. M., Soergel K. H., Komorowski R. A., Wood C. M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989 Jan 5;320(1):23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- Hebbes T. R., Clayton A. L., Thorne A. W., Crane-Robinson C. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 1994 Apr 15;13(8):1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988 May;7(5):1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L., Schroth G. P., Matthews H. R., Yau P., Bradbury E. M. Studies of the DNA binding properties of histone H4 amino terminus. Thermal denaturation studies reveal that acetylation markedly reduces the binding constant of the H4 "tail" to DNA. J Biol Chem. 1993 Jan 5;268(1):305–314. [PubMed] [Google Scholar]
- Janson W., Brandner G., Siegel J. Butyrate modulates DNA-damage-induced p53 response by induction of p53-independent differentiation and apoptosis. Oncogene. 1997 Sep 18;15(12):1395–1406. doi: 10.1038/sj.onc.1201304. [DOI] [PubMed] [Google Scholar]
- Jung H. C., Eckmann L., Yang S. K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M. F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995 Jan;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A., Fusunyan R. D., Jacyno M., Winship D., MacDermott R. P., Sanderson I. R. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999 Mar;94(3):704–712. doi: 10.1111/j.1572-0241.1999.00940.x. [DOI] [PubMed] [Google Scholar]
- Klehr D., Maass K., Bode J. Scaffold-attached regions from the human interferon beta domain can be used to enhance the stable expression of genes under the control of various promoters. Biochemistry. 1991 Feb 5;30(5):1264–1270. doi: 10.1021/bi00219a015. [DOI] [PubMed] [Google Scholar]
- Klehr D., Schlake T., Maass K., Bode J. Scaffold-attached regions (SAR elements) mediate transcriptional effects due to butyrate. Biochemistry. 1992 Mar 31;31(12):3222–3229. doi: 10.1021/bi00127a025. [DOI] [PubMed] [Google Scholar]
- Kripke S. A., Fox A. D., Berman J. M., Settle R. G., Rombeau J. L. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. JPEN J Parenter Enteral Nutr. 1989 Mar-Apr;13(2):109–116. doi: 10.1177/0148607189013002109. [DOI] [PubMed] [Google Scholar]
- Lloyd D. R., Brown J. D., Brown G. A., Booth I. W. Elevated short chain fatty acid concentrations in anaerobic small bowel contamination. Acta Paediatr. 1992 Jan;81(1):51–56. doi: 10.1111/j.1651-2227.1992.tb12078.x. [DOI] [PubMed] [Google Scholar]
- Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997 Sep 18;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Lutter L. C., Judis L., Paretti R. F. Effects of histone acetylation on chromatin topology in vivo. Mol Cell Biol. 1992 Nov;12(11):5004–5014. doi: 10.1128/mcb.12.11.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick B. A., Hofman P. M., Kim J., Carnes D. K., Miller S. I., Madara J. L. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995 Dec;131(6 Pt 1):1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midtvedt A. C., Midtvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr. 1992 Nov;15(4):395–403. doi: 10.1097/00005176-199211000-00005. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Imai B. S., Yau P., Bradbury E. M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989 May 5;57(3):449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- Nowak R. Molecular machines may aid gene expression. Science. 1995 Dec 8;270(5242):1589–1590. doi: 10.1126/science.270.5242.1589. [DOI] [PubMed] [Google Scholar]
- O'Moráin C., Segal A. W., Levi A. J. Elemental diet as primary treatment of acute Crohn's disease: a controlled trial. Br Med J (Clin Res Ed) 1984 Jun 23;288(6434):1859–1862. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi S., Walker W. A., Sanderson I. R. Profile of IGF-binding proteins secreted by intestinal epithelial cells changes with differentiation. Am J Physiol. 1994 Nov;267(5 Pt 1):G843–G850. doi: 10.1152/ajpgi.1994.267.5.G843. [DOI] [PubMed] [Google Scholar]
- Ohno Y., Lee J., Fusunyan R. D., MacDermott R. P., Sanderson I. R. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10279–10284. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., Loh E. Y., Ringler D. J., Mehta A., Rombeau J. L., MacDermott R. P. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995 Jan;108(1):40–50. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Royall D., Wolever T. M., Jeejeebhoy K. N. Clinical significance of colonic fermentation. Am J Gastroenterol. 1990 Oct;85(10):1307–1312. [PubMed] [Google Scholar]
- Sanderson I. R., Udeen S., Davies P. S., Savage M. O., Walker-Smith J. A. Remission induced by an elemental diet in small bowel Crohn's disease. Arch Dis Child. 1987 Feb;62(2):123–127. doi: 10.1136/adc.62.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson I. R., Walker W. A. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update). Gastroenterology. 1993 Feb;104(2):622–639. doi: 10.1016/0016-5085(93)90436-g. [DOI] [PubMed] [Google Scholar]
- Stadnyk A. W. Cytokine production by epithelial cells. FASEB J. 1994 Oct;8(13):1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- Treem W. R., Ahsan N., Shoup M., Hyams J. S. Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1994 Feb;18(2):159–164. doi: 10.1097/00005176-199402000-00007. [DOI] [PubMed] [Google Scholar]
- Vernia P., Cittadini M., Caprilli R., Torsoli A. Topical treatment of refractory distal ulcerative colitis with 5-ASA and sodium butyrate. Dig Dis Sci. 1995 Feb;40(2):305–307. doi: 10.1007/BF02065414. [DOI] [PubMed] [Google Scholar]