Abstract
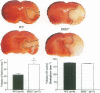
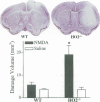
Heme oxygenase (HO) is believed to be a potent antioxidant enzyme in the nervous system; it degrades heme from heme-containing proteins, giving rise to carbon monoxide, iron, and biliverdin, which is rapidly reduced to bilirubin. The first identified isoform of the enzyme, HO1, is an inducible heat-shock protein expressed in high levels in peripheral organs and barely detectable under normal conditions in the brain, whereas HO2 is constitutive and most highly concentrated in the brain. Interestingly, although HO2 is constitutively expressed, its activity can be modulated by phosphorylation. We demonstrated that bilirubin, formed from HO2, is neuroprotectant, as neurotoxicity is augmented in neuronal cultures from mice with targeted deletion of HO2 (HO2(-/-)) and reversed by low concentrations of bilirubin. We now show that neural damage following middle cerebral artery occlusion (MCAO) and reperfusion, a model of focal ischemia of vascular stroke, is substantially worsened in HO2(-/-) animals. By contrast, stroke damage is not significantly altered in HO1(-/-) mice, despite their greater debility. Neural damage following intracranial injections of N-methyl-d-aspartate (NMDA) is also accentuated in HO2(-/-) animals. These findings establish HO2 as an endogenous neuroprotective system in the brain whose pharmacologic manipulation may have therapeutic relevance.
Full text
PDF


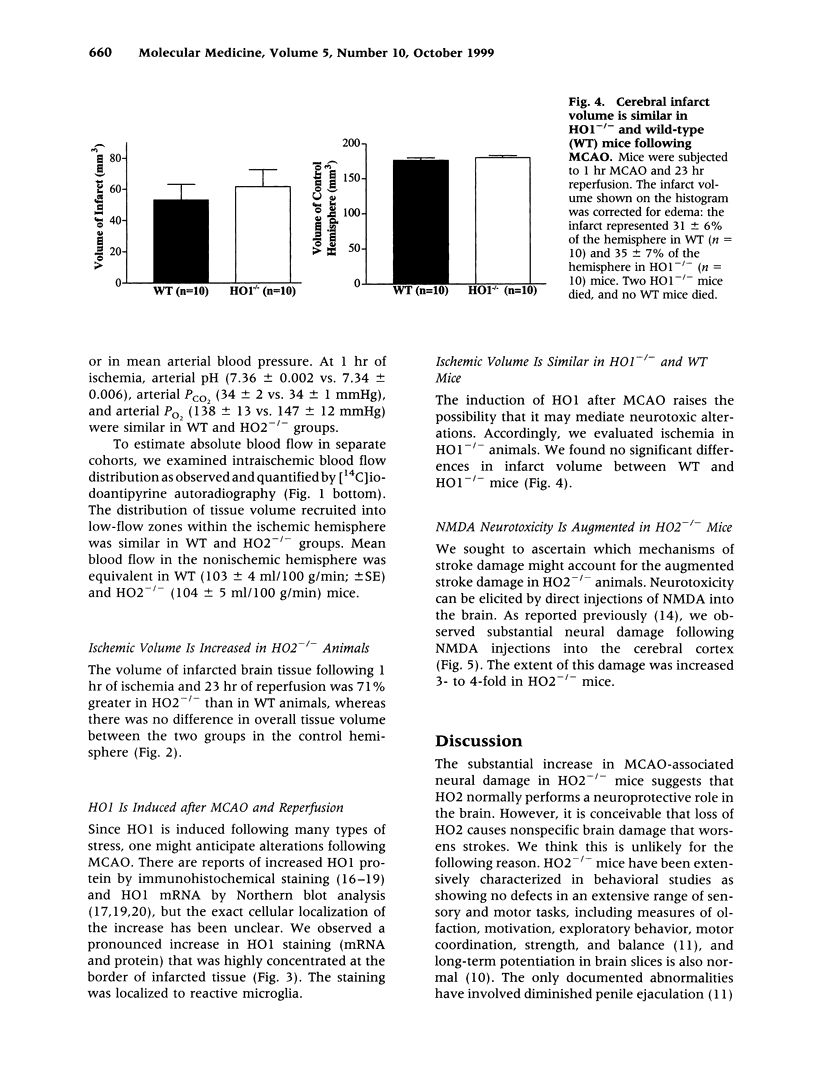



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayata C., Ayata G., Hara H., Matthews R. T., Beal M. F., Ferrante R. J., Endres M., Kim A., Christie R. H., Waeber C. Mechanisms of reduced striatal NMDA excitotoxicity in type I nitric oxide synthase knock-out mice. J Neurosci. 1997 Sep 15;17(18):6908–6917. doi: 10.1523/JNEUROSCI.17-18-06908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett A. L., Johns D. G., Kriegsfeld L. J., Klein S. L., Calvin D. C., Demas G. E., Schramm L. P., Tonegawa S., Nelson R. J., Snyder S. H. Ejaculatory abnormalities in mice with targeted disruption of the gene for heme oxygenase-2. Nat Med. 1998 Jan;4(1):84–87. doi: 10.1038/nm0198-084. [DOI] [PubMed] [Google Scholar]
- Doré S., Takahashi M., Ferris C. D., Zakhary R., Hester L. D., Guastella D., Snyder S. H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson M. J., Sampei K., Mandir A. S., Hurn P. D., Traystman R. J., Bao J., Pieper A., Wang Z. Q., Dawson T. M., Snyder S. H. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997 Oct;3(10):1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Ewing J. F., Haber S. N., Maines M. D. Normal and heat-induced patterns of expression of heme oxygenase-1 (HSP32) in rat brain: hyperthermia causes rapid induction of mRNA and protein. J Neurochem. 1992 Mar;58(3):1140–1149. doi: 10.1111/j.1471-4159.1992.tb09373.x. [DOI] [PubMed] [Google Scholar]
- Ewing J. F., Maines M. D. Histochemical localization of heme oxygenase-2 protein and mRNA expression in rat brain. Brain Res Brain Res Protoc. 1997 May;1(2):165–174. doi: 10.1016/s1385-299x(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Jaffrey S. R., Sawa A., Takahashi M., Brady S. D., Barrow R. K., Tysoe S. A., Wolosker H., Barañano D. E., Doré S. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999 Jul;1(3):152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- Geddes J. W., Pettigrew L. C., Holtz M. L., Craddock S. D., Maines M. D. Permanent focal and transient global cerebral ischemia increase glial and neuronal expression of heme oxygenase-1, but not heme oxygenase-2, protein in rat brain. Neurosci Lett. 1996 Jun 7;210(3):205–208. doi: 10.1016/0304-3940(96)12703-8. [DOI] [PubMed] [Google Scholar]
- Gopinathan V., Miller N. J., Milner A. D., Rice-Evans C. A. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994 Aug 1;349(2):197–200. doi: 10.1016/0014-5793(94)00666-0. [DOI] [PubMed] [Google Scholar]
- Hegyi T., Goldie E., Hiatt M. The protective role of bilirubin in oxygen-radical diseases of the preterm infant. J Perinatol. 1994 Jul-Aug;14(4):296–300. [PubMed] [Google Scholar]
- Heyman E., Ohlsson A., Girschek P. Retinopathy of prematurity and bilirubin. N Engl J Med. 1989 Jan 26;320(4):256–256. doi: 10.1056/NEJM198901263200420. [DOI] [PubMed] [Google Scholar]
- Hopkins P. N., Wu L. L., Hunt S. C., James B. C., Vincent G. M., Williams R. R. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol. 1996 Feb;16(2):250–255. doi: 10.1161/01.atv.16.2.250. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L., Mahoney C. W., Chen K. H. Protein kinase C subtypes and their respective roles. Prog Brain Res. 1991;89:143–155. doi: 10.1016/s0079-6123(08)61720-3. [DOI] [PubMed] [Google Scholar]
- Jay T. M., Lucignani G., Crane A. M., Jehle J., Sokoloff L. Measurement of local cerebral blood flow with [14C]iodoantipyrine in the mouse. J Cereb Blood Flow Metab. 1988 Feb;8(1):121–129. doi: 10.1038/jcbfm.1988.16. [DOI] [PubMed] [Google Scholar]
- Koistinaho J., Miettinen S., Keinänen R., Vartiainen N., Roivainen R., Laitinen J. T. Long-term induction of haem oxygenase-1 (HSP-32) in astrocytes and microglia following transient focal brain ischaemia in the rat. Eur J Neurosci. 1996 Nov;8(11):2265–2272. doi: 10.1111/j.1460-9568.1996.tb01190.x. [DOI] [PubMed] [Google Scholar]
- Maines M. D. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Minetti M., Mallozzi C., Di Stasi A. M., Pietraforte D. Bilirubin is an effective antioxidant of peroxynitrite-mediated protein oxidation in human blood plasma. Arch Biochem Biophys. 1998 Apr 15;352(2):165–174. doi: 10.1006/abbi.1998.0584. [DOI] [PubMed] [Google Scholar]
- Nimura T., Weinstein P. R., Massa S. M., Panter S., Sharp F. R. Heme oxygenase-1 (HO-1) protein induction in rat brain following focal ischemia. Brain Res Mol Brain Res. 1996 Apr;37(1-2):201–208. doi: 10.1016/0169-328x(95)00315-j. [DOI] [PubMed] [Google Scholar]
- Poss K. D., Thomas M. J., Ebralidze A. K., O'Dell T. J., Tonegawa S. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron. 1995 Oct;15(4):867–873. doi: 10.1016/0896-6273(95)90177-9. [DOI] [PubMed] [Google Scholar]
- Poss K. D., Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N., Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993 Dec;100(6):431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schwertner H. A., Jackson W. G., Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994 Jan;40(1):18–23. [PubMed] [Google Scholar]
- Smith A., Alam J., Escriba P. V., Morgan W. T. Regulation of heme oxygenase and metallothionein gene expression by the heme analogs, cobalt-, and tin-protoporphyrin. J Biol Chem. 1993 Apr 5;268(10):7365–7371. [PubMed] [Google Scholar]
- Smith D. R., Striplin C. D., Geller A. M., Mailman R. B., Drago J., Lawler C. P., Gallagher M. Behavioural assessment of mice lacking D1A dopamine receptors. Neuroscience. 1998 Sep;86(1):135–146. doi: 10.1016/s0306-4522(97)00608-8. [DOI] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Takeda A., Onodera H., Sugimoto A., Itoyama Y., Kogure K., Shibahara S. Increased expression of heme oxygenase mRNA in rat brain following transient forebrain ischemia. Brain Res. 1994 Dec 12;666(1):120–124. doi: 10.1016/0006-8993(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Wu T. W., Wu J., Li R. K., Mickle D., Carey D. Albumin-bound bilirubins protect human ventricular myocytes against oxyradical damage. Biochem Cell Biol. 1991 Oct-Nov;69(10-11):683–688. doi: 10.1139/o91-102. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Terakado M., Horio F., Aoki K., Tanaka M., Nakajima H. Role of bilirubin as an antioxidant in an ischemia-reperfusion of rat liver and induction of heme oxygenase. Biochem Biophys Res Commun. 1996 Jun 5;223(1):129–135. doi: 10.1006/bbrc.1996.0857. [DOI] [PubMed] [Google Scholar]
- Zakhary R., Poss K. D., Jaffrey S. R., Ferris C. D., Tonegawa S., Snyder S. H. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]