Abstract
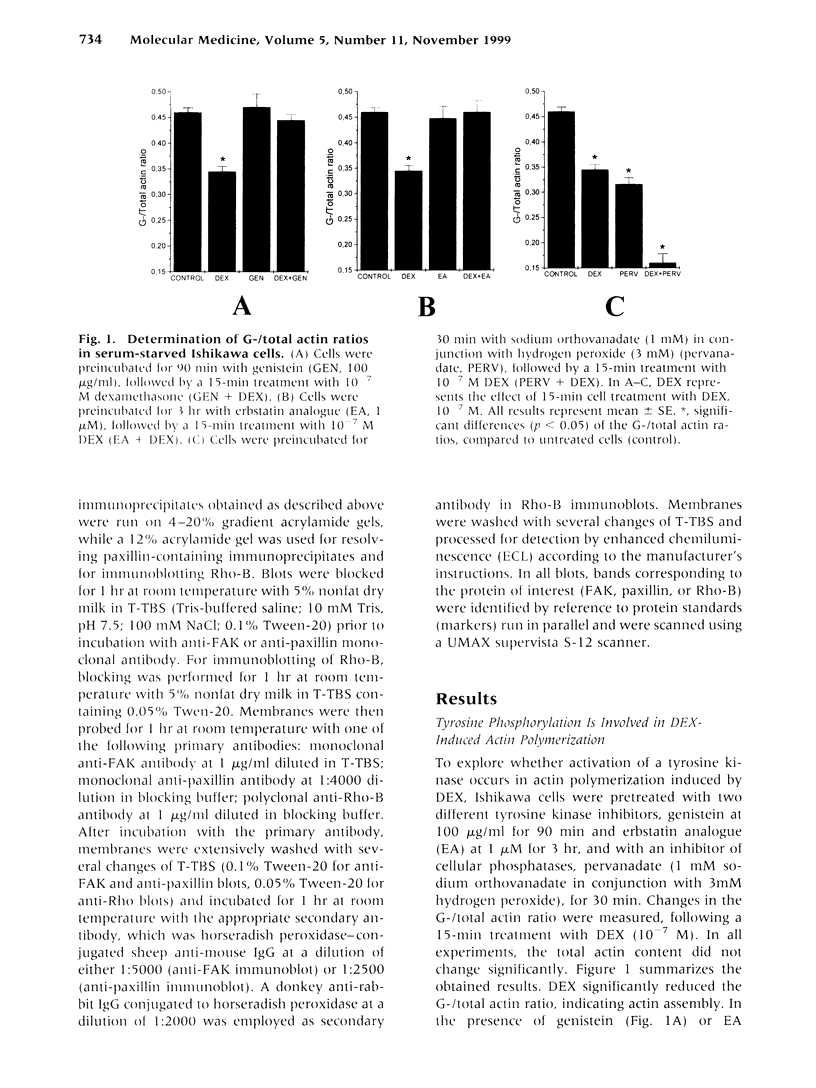
We have previously shown that dexamethasone (DEX) stimulates rapid polymerization of actin and stabilization of microfilaments in human endometrial adenocarcinoma cells. As the content of total cellular actin and the concentration of the actin transcript did not change, we concluded that polymerization of actin by glucocorticoids involves nongenomic mechanisms. However, the signaling events by which the latter is achieved remain unknown. In the present study we evaluated whether tyrosine phosphorylation is required for the rapid, nongenomic DEX effect on actin assembly. In cells preincubated with the tyrosine kinase inhibitors, genistein or erbstatin analogue (EA), before adding DEX the G-/total actin ratio remained unchanged, whereas DEX in the absence of both inhibitors reduced the ratio by 25%. In addition, when cells were preincubated with the protein tyrosine phosphatase inhibitor pervanadate and subsequently incubated with DEX, the G-/total actin ratio was dramatically reduced by 65%. Furthermore, DEX increased transiently the levels of tyrosine phosphorylation of focal adhesion kinase (FAK) and paxillin within 2 to 15 min, without a change in their expression levels. Pervanadate mimicked this effect of DEX and enhanced tyrosine phosphorylation of both proteins. In addition, when cells were exposed to the anticytoskeletal agent cytochalasin B, the basal levels of tyrosine phosphorylation of both proteins were reduced. This effect was reversed by DEX, indicating that actin cytoskeleton integrity is required for the effect of DEX on tyrosine phosphorylation of FAK and paxillin. Finally, we documented enhanced expression of the Ras-related GTP-binding protein Rho-B after long-term (12- and 24-hr) treatment with DEX, whereas Rho-B levels remained unchanged after short-term (3- and 6-hr) treatment. Our observations demonstrate a novel mechanism through which the rapid nongenomic effect of DEX on actin assembly requires tyrosine phosphorylation of the cytoskeleton-associated proteins FAK and paxillin. We also propose that the DEX-induced actin polymerization may constitute a mechanism for transduction of signals resulting in tyrosine phosphorylation of FAK and paxillin. Moreover, the enhanced Rho-B levels observed after long-term treatment with DEX imply a mechanism for the well-described, long-term effects of glucocorticoids on actin cytoskeleton.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Bellis S. L., Miller J. T., Turner C. E. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995 Jul 21;270(29):17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Petrin J., Wang L., Ramesh U., Doll R. J. Inhibition of protein kinase C by the tyrosine kinase inhibitor erbstatin. Biochem Pharmacol. 1990 Nov 1;40(9):2129–2135. doi: 10.1016/0006-2952(90)90245-g. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F., Heuser J., Marchetti S., Bruno B., Luini A. Glucocorticoid stabilization of actin filaments: a possible mechanism for inhibition of corticotropin release. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3775–3779. doi: 10.1073/pnas.89.9.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Kinch M. S., Lin T. H., Burridge K., Juliano R. L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994 Oct 28;269(43):26602–26605. [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994 Dec;107(Pt 12):3643–3654. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Integrins and signal transduction pathways: the road taken. Science. 1995 Apr 14;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Faulstich H., Merkler I., Blackholm H., Stournaras C. Nucleotide in monomeric actin regulates the reactivity of the thiol groups. Biochemistry. 1984 Apr 10;23(8):1608–1612. doi: 10.1021/bi00303a004. [DOI] [PubMed] [Google Scholar]
- Flinn H. M., Ridley A. J. Rho stimulates tyrosine phosphorylation of focal adhesion kinase, p130 and paxillin. J Cell Sci. 1996 May;109(Pt 5):1133–1141. doi: 10.1242/jcs.109.5.1133. [DOI] [PubMed] [Google Scholar]
- Gravanis A., Gurpide E. Effects of estradiol on deoxyribonucleic acid polymerase alpha activity in the Ishikawa human endometrial adenocarcinoma cell line. J Clin Endocrinol Metab. 1986 Aug;63(2):356–359. doi: 10.1210/jcem-63-2-356. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Polte T. R. Signaling through focal adhesion kinase. Bioessays. 1997 Feb;19(2):137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- Jaken S., Leach K., Klauck T. Association of type 3 protein kinase C with focal contacts in rat embryo fibroblasts. J Cell Biol. 1989 Aug;109(2):697–704. doi: 10.1083/jcb.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsantonis J., Tosca A., Koukouritaki S. B., Theodoropoulos P. A., Gravanis A., Stournaras C. Differences in the G/total actin ratio and microfilament stability between normal and malignant human keratinocytes. Cell Biochem Funct. 1994 Dec;12(4):267–274. doi: 10.1002/cbf.290120407. [DOI] [PubMed] [Google Scholar]
- Kiley S. C., Parker P. J., Fabbro D., Jaken S. Hormone- and phorbol ester-activated protein kinase C isozymes mediate a reorganization of the actin cytoskeleton associated with prolactin secretion in GH4C1 cells. Mol Endocrinol. 1992 Jan;6(1):120–131. doi: 10.1210/mend.6.1.1738365. [DOI] [PubMed] [Google Scholar]
- Kim B., Feldman E. L. Differential regulation of focal adhesion kinase and mitogen-activated protein kinase tyrosine phosphorylation during insulin-like growth factor-I-mediated cytoskeletal reorganization. J Neurochem. 1998 Sep;71(3):1333–1336. doi: 10.1046/j.1471-4159.1998.71031333.x. [DOI] [PubMed] [Google Scholar]
- Koukouritaki S. B., Margioris A. N., Gravanis A., Hartig R., Stournaras C. Dexamethasone induces rapid actin assembly in human endometrial cells without affecting its synthesis. J Cell Biochem. 1997 Jun 15;65(4):492–500. doi: 10.1002/(sici)1097-4644(19970615)65:4<492::aid-jcb5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Koukouritaki S. B., Theodoropoulos P. A., Margioris A. N., Gravanis A., Stournaras C. Dexamethasone alters rapidly actin polymerization dynamics in human endometrial cells: evidence for nongenomic actions involving cAMP turnover. J Cell Biochem. 1996 Aug;62(2):251–261. doi: 10.1002/(SICI)1097-4644(199608)62:2%3C251::AID-JCB13%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Koukouritaki S. B., Vardaki E. A., Papakonstanti E. A., Lianos E., Stournaras C., Emmanouel D. S. TNF-alpha induces actin cytoskeleton reorganization in glomerular epithelial cells involving tyrosine phosphorylation of paxillin and focal adhesion kinase. Mol Med. 1999 Jun;5(6):382–392. [PMC free article] [PubMed] [Google Scholar]
- Maher P. A. Activation of phosphotyrosine phosphatase activity by reduction of cell-substrate adhesion. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11177–11181. doi: 10.1073/pnas.90.23.11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed I., Downey G. P., Roifman C. M. Tyrosine phosphorylation is essential for microfilament assembly in B lymphocytes. Biochem Biophys Res Commun. 1991 May 15;176(3):1424–1429. doi: 10.1016/0006-291x(91)90445-d. [DOI] [PubMed] [Google Scholar]
- Melamed I., Turner C. E., Aktories K., Kaplan D. R., Gelfand E. W. Nerve growth factor triggers microfilament assembly and paxillin phosphorylation in human B lymphocytes. J Exp Med. 1995 Mar 1;181(3):1071–1079. doi: 10.1084/jem.181.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A., Stournaras C. Regulation of actin organisation by TGF-beta in H-ras-transformed fibroblasts. J Cell Sci. 1999 Apr;112(Pt 8):1169–1179. doi: 10.1242/jcs.112.8.1169. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Theodoropoulos P. A., Gravanis A., Häussinger D., Stournaras C. The cytoskeleton in cell volume regulation. Contrib Nephrol. 1998;123:121–134. doi: 10.1159/000059925. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- O'Brien E. T., Kinch M., Harding T. W., Epstein D. L. A mechanism for trabecular meshwork cell retraction: ethacrynic acid initiates the dephosphorylation of focal adhesion proteins. Exp Eye Res. 1997 Oct;65(4):471–483. doi: 10.1006/exer.1997.0357. [DOI] [PubMed] [Google Scholar]
- Pumiglia K. M., Lau L. F., Huang C. K., Burroughs S., Feinstein M. B. Activation of signal transduction in platelets by the tyrosine phosphatase inhibitor pervanadate (vanadyl hydroperoxide). Biochem J. 1992 Sep 1;286(Pt 2):441–449. doi: 10.1042/bj2860441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S., Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. Bell-shaped dose response and cross-talk with bombesin. J Biol Chem. 1994 Jan 7;269(1):704–710. [PubMed] [Google Scholar]
- Ridley A. J., Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994 Jun 1;13(11):2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fernández J. L., Rozengurt E. Bombesin, vasopressin, lysophosphatidic acid, and sphingosylphosphorylcholine induce focal adhesion kinase activation in intact Swiss 3T3 cells. J Biol Chem. 1998 Jul 24;273(30):19321–19328. doi: 10.1074/jbc.273.30.19321. [DOI] [PubMed] [Google Scholar]
- Rosales C., O'Brien V., Kornberg L., Juliano R. Signal transduction by cell adhesion receptors. Biochim Biophys Acta. 1995 Jul 28;1242(1):77–98. doi: 10.1016/0304-419x(95)00005-z. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Convergent signalling in the action of integrins, neuropeptides, growth factors and oncogenes. Cancer Surv. 1995;24:81–96. [PubMed] [Google Scholar]
- Rozengurt E. V. Gastrointestinal peptide signaling through tyrosine phosphorylation of focal adhesion proteins. Am J Physiol. 1998 Aug;275(2 Pt 1):G177–G182. doi: 10.1152/ajpgi.1998.275.2.G177. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Parsons J. T. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994 Oct;6(5):705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Seufferlein T., Rozengurt E. Sphingosylphosphorylcholine rapidly induces tyrosine phosphorylation of p125FAK and paxillin, rearrangement of the actin cytoskeleton and focal contact assembly. Requirement of p21rho in the signaling pathway. J Biol Chem. 1995 Oct 13;270(41):24343–24351. doi: 10.1074/jbc.270.41.24343. [DOI] [PubMed] [Google Scholar]
- Symons M. Rho family GTPases: the cytoskeleton and beyond. Trends Biochem Sci. 1996 May;21(5):178–181. [PubMed] [Google Scholar]
- Tachibana K., Sato T., D'Avirro N., Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J Exp Med. 1995 Oct 1;182(4):1089–1099. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N., Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997 Feb;9(1):86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Turner C. E., Glenney J. R., Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990 Sep;111(3):1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. E., Schaller M. D., Parsons J. T. Tyrosine phosphorylation of the focal adhesion kinase pp125FAK during development: relation to paxillin. J Cell Sci. 1993 Jul;105(Pt 3):637–645. doi: 10.1242/jcs.105.3.637. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Turner C. E., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation of the focal adhesion-associated protein paxillin in Swiss 3T3 cells. J Biol Chem. 1993 Oct 15;268(29):22060–22065. [PubMed] [Google Scholar]








