Abstract
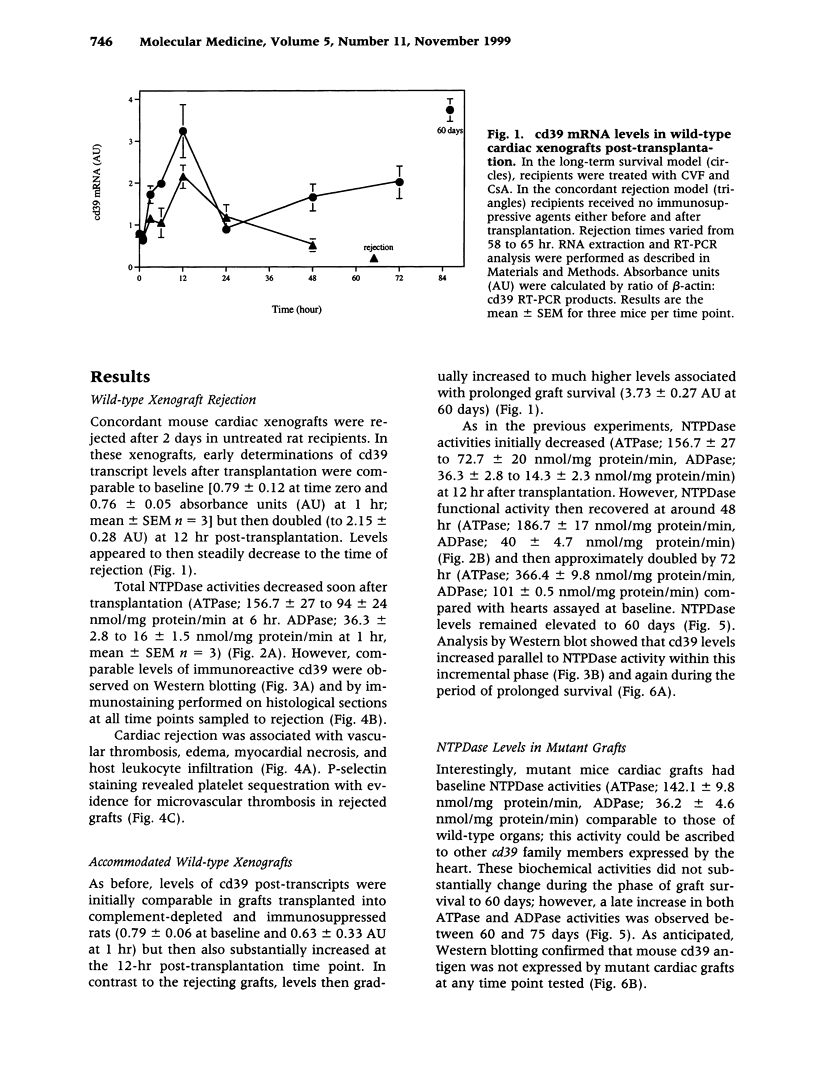
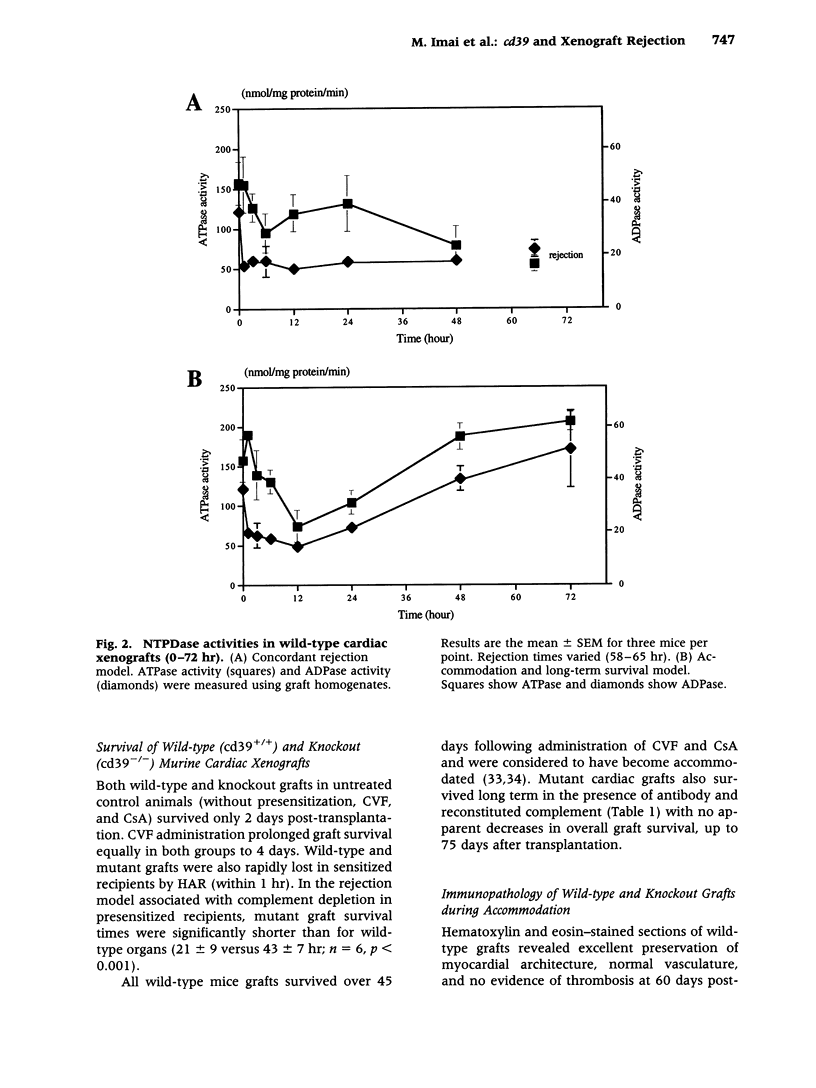
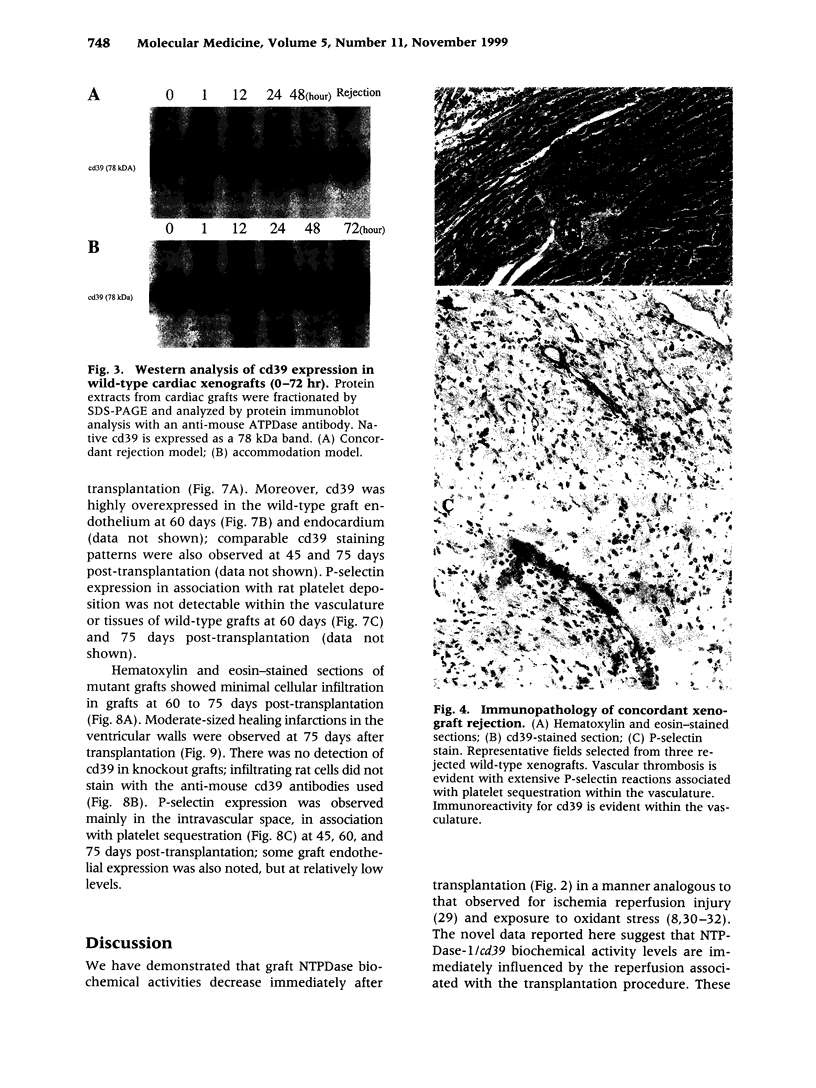
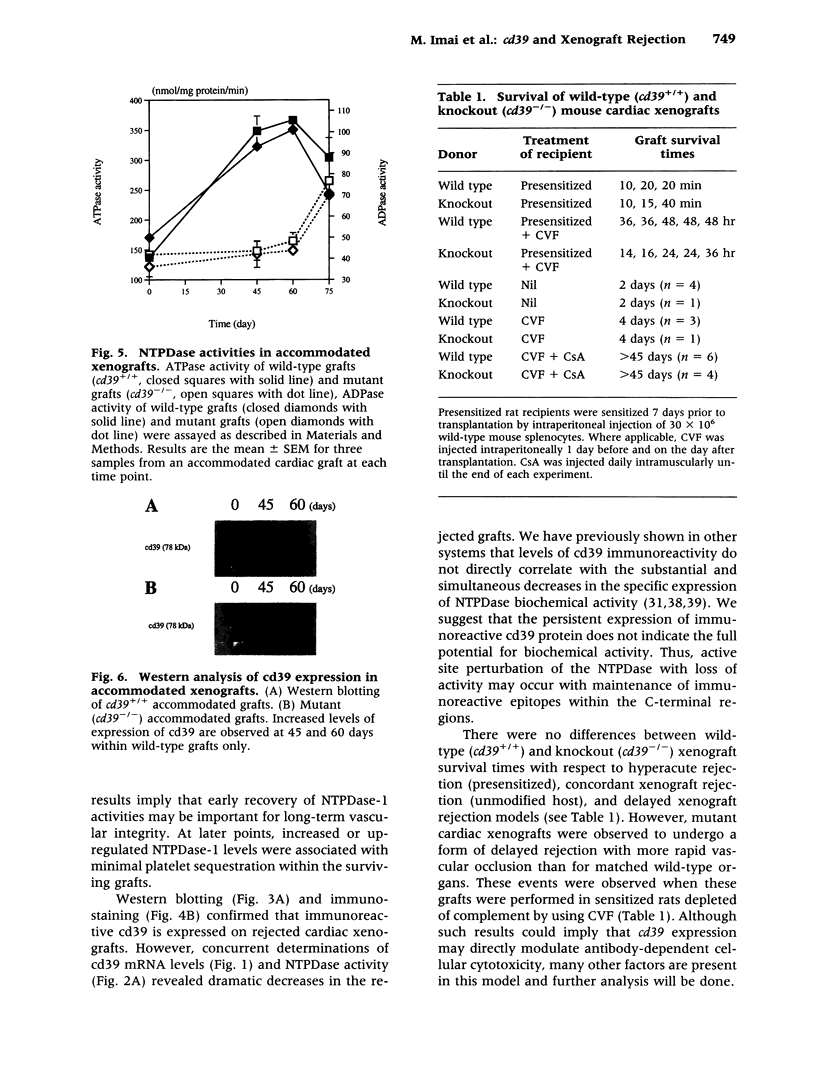
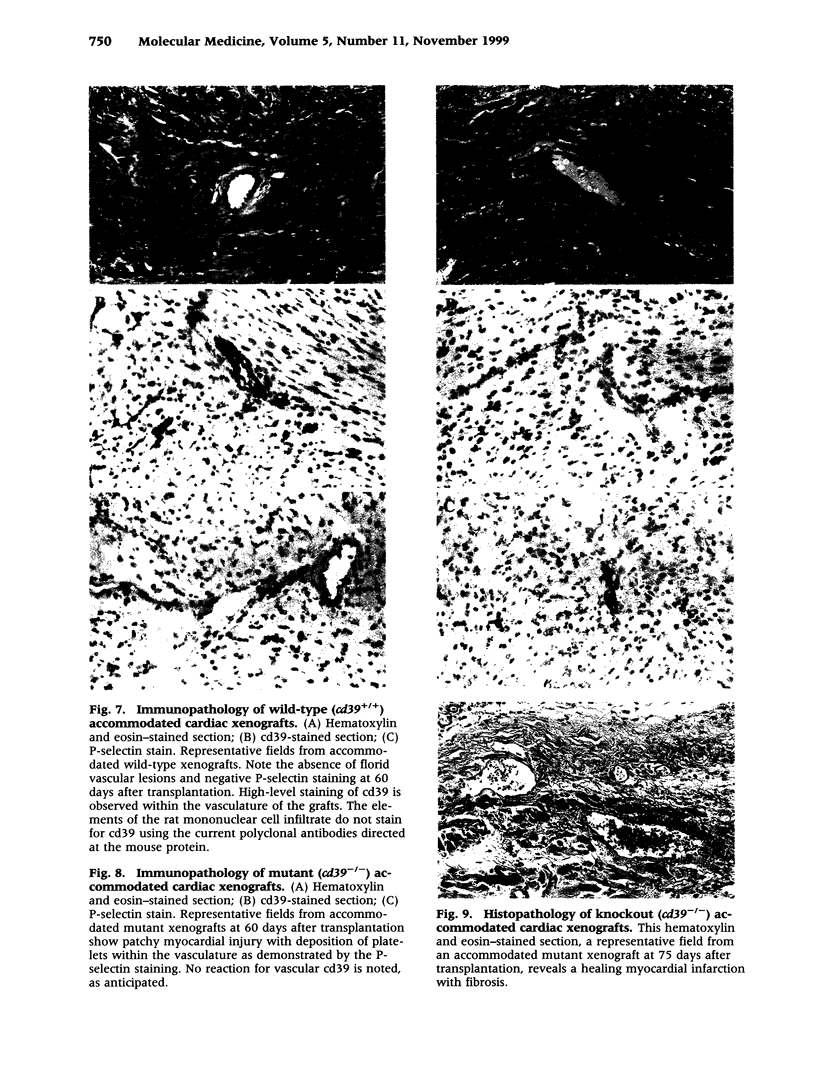
BACKGROUND: There is increasing evidence showing that extracellular nucleosides [corrected] may be important mediators of vascular inflammation. Nucleoside [corrected] triphosphate diphosphohydrolase-1 (NTPDase-1, identical to CD39), the major vascular endothelial ectonucleotidase, is responsible for the hydrolysis of both extracellular ATP and ADP in the blood plasma to AMP. Studies were therefore conducted to evaluate the role of vascular NTPDase-1/cd39 in modulating platelet activation and vascular injury in cardiac xenografts. MATERIALS AND METHODS: Cardiac xenografts from both wild-type and cd39 knockout mice (C57BL/6 x 129 Svj) were transplanted into Lewis rats. Alterations in cd39 mRNA transcripts and NTPDase activity expression were evaluated in wild-type grafts in untreated rats and then following complement depletion and immunosuppression. Rejection responses were studied with both mutant and wild-type grafts in the following models: presensitization with or without complement depletion, complement depletion alone, and with chronic immunosuppression to induce long-term graft survival. RESULTS: NTPDase biochemical activity in wild-type xenografts rapidly decreased after transplantation but soon rebounded with graft survival. Elevated levels of cd39 mRNA with associated increases in NTPDase activity were observed in all long-term surviving wild-type grafts. Hyperacute xenograft rejection times were comparable in wild-type and mutant grafts but cd39-deficient grafts were subject to more rapid rejection and exhibited pronounced vascular injury in complement-depleted, presensitized rats. The cd39-deficient grafts in immunosuppressed recipients were subject to increased intravascular platelet sequestration and fibrin deposition; this resulted in focal myocardial infarction in long-term surviving mutant xenografts. CONCLUSIONS: Augmentation of NTPDase-1 activity may be an important adaptive response for graft survival. Our results suggest that NTPDase-1/cd39 influences pathways of vascular injury in cardiac xenografts.
Full text
PDF




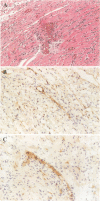
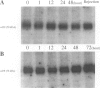
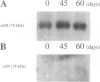
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Ferran C., Hechenleitner P., Mark W., Koyamada N., Miyatake T., Winkler H., Badrichani A., Candinas D., Hancock W. W. Accommodation of vascularized xenografts: expression of "protective genes" by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997 Feb;3(2):196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Robson S. C., Ferran C., Winkler H., Millan M. T., Stuhlmeier K. M., Vanhove B., Blakely M. L., van der Werf W. J., Hofer E. Endothelial cell activation and thromboregulation during xenograft rejection. Immunol Rev. 1994 Oct;141:5–30. doi: 10.1111/j.1600-065x.1994.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Robson S. C., Winkler H., Ferran C., Stuhlmeier K. M., Wrighton C. J., Hancock W. W. Barriers to xenotransplantation. Nat Med. 1995 Sep;1(9):869–873. doi: 10.1038/nm0995-869. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Winkler H., Ferran C., Hancock W. W., Robson S. C. Delayed xenograft rejection. Immunol Today. 1996 Aug;17(8):379–384. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- Bach F. H. Xenotransplantation: problems and prospects. Annu Rev Med. 1998;49:301–310. doi: 10.1146/annurev.med.49.1.301. [DOI] [PubMed] [Google Scholar]
- Baykov A. A., Evtushenko O. A., Avaeva S. M. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988 Jun;171(2):266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- Blakely M. L., Van der Werf W. J., Berndt M. C., Dalmasso A. P., Bach F. H., Hancock W. W. Activation of intragraft endothelial and mononuclear cells during discordant xenograft rejection. Transplantation. 1994 Nov 27;58(10):1059–1066. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Candinas D., Koyamada N., Miyatake T., Siegel J., Hancock W. W., Bach F. H., Robson S. C. Loss of rat glomerular ATP diphosphohydrolase activity during reperfusion injury is associated with oxidative stress reactions. Thromb Haemost. 1996 Nov;76(5):807–812. [PubMed] [Google Scholar]
- Chinellato A., Froldi G., Caparrotta L., Ragazzi E. Pharmacological characterization of endothelial cell nitric oxide synthase inhibitors in isolated rabbit aorta. Life Sci. 1998;62(6):479–490. doi: 10.1016/s0024-3205(97)01144-2. [DOI] [PubMed] [Google Scholar]
- Côté Y. P., Picher M., St-Jean P., Béliveau R., Potier M., Beaudoin A. R. Identification and localization of ATP-diphosphohydrolase (apyrase) in bovine aorta: relevance to vascular tone and platelet aggregation. Biochim Biophys Acta. 1991 Jun 24;1078(2):187–191. doi: 10.1016/0167-4838(91)99008-g. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995 Nov;16(11):524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- Enjyoji K., Sévigny J., Lin Y., Frenette P. S., Christie P. D., Esch J. S., 2nd, Imai M., Edelberg J. M., Rayburn H., Lech M. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999 Sep;5(9):1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- Ferrari D., Chiozzi P., Falzoni S., Dal Susino M., Melchiorri L., Baricordi O. R., Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997 Aug 1;159(3):1451–1458. [PubMed] [Google Scholar]
- Imai M., Kaczmarek E., Koziak K., Sévigny J., Goepfert C., Guckelberger O., Csizmadia E., Schulte Am Esch J., 2nd, Robson S. C. Suppression of ATP diphosphohydrolase/CD39 in human vascular endothelial cells. Biochemistry. 1999 Oct 12;38(41):13473–13479. doi: 10.1021/bi990543p. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E., Koziak K., Sévigny J., Siegel J. B., Anrather J., Beaudoin A. R., Bach F. H., Robson S. C. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996 Dec 20;271(51):33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Koyamada N., Miyatake T., Candinas D., Hechenleitner P., Siegel J., Hancock W. W., Bach F. H., Robson S. C. Apyrase administration prolongs discordant xenograft survival. Transplantation. 1996 Dec 27;62(12):1739–1743. doi: 10.1097/00007890-199612270-00008. [DOI] [PubMed] [Google Scholar]
- Koyamada N., Miyatake T., Candinas D., Mark W., Hechenleitner P., Hancock W. W., Soares M. P., Bach F. H. Transient complement inhibition plus T-cell immunosuppression induces long-term survival of mouse-to-rat cardiac xenografts. Transplantation. 1998 May 15;65(9):1210–1215. doi: 10.1097/00007890-199805150-00012. [DOI] [PubMed] [Google Scholar]
- Lüthje J. Origin, metabolism and function of extracellular adenine nucleotides in the blood. Klin Wochenschr. 1989 Mar 15;67(6):317–327. doi: 10.1007/BF01741386. [DOI] [PubMed] [Google Scholar]
- Maliszewski C. R., Delespesse G. J., Schoenborn M. A., Armitage R. J., Fanslow W. C., Nakajima T., Baker E., Sutherland G. R., Poindexter K., Birks C. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol. 1994 Oct 15;153(8):3574–3583. [PubMed] [Google Scholar]
- Marcus A. J., Broekman M. J., Drosopoulos J. H., Islam N., Alyonycheva T. N., Safier L. B., Hajjar K. A., Posnett D. N., Schoenborn M. A., Schooley K. A. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997 Mar 15;99(6):1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Hajjar K. A., Ullman H. L., Islam N., Broekman M. J., Eiroa A. M. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. Thromboregulation by endothelial cells. J Clin Invest. 1991 Nov;88(5):1690–1696. doi: 10.1172/JCI115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B. Thromboregulation: multicellular modulation of platelet reactivity in hemostasis and thrombosis. FASEB J. 1993 Apr 1;7(6):516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Plesner L. Ecto-ATPases: identities and functions. Int Rev Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- Robson S. C., Candinas D., Hancock W. W., Wrighton C., Winkler H., Bach F. H. Role of endothelial cells in transplantation. Int Arch Allergy Immunol. 1995 Apr;106(4):305–322. doi: 10.1159/000236861. [DOI] [PubMed] [Google Scholar]
- Robson S. C., Candinas D., Siegel J. B., Kopp C., Millan M., Hancock W. W., Bach F. H. Potential mechanism of abnormal thromboregulation in xenograft rejection: loss of ecto-ATPases upon endothelial cell activation. Transplant Proc. 1996 Apr;28(2):536–536. [PubMed] [Google Scholar]
- Robson S. C., Daoud S., Bégin M., Côté Y. P., Siegel J. B., Bach F. H., Beaudoin A. R. Modulation of vascular ATP diphosphohydrolase by fatty acids. Blood Coagul Fibrinolysis. 1997 Jan;8(1):21–27. doi: 10.1097/00001721-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Robson S. C., Kaczmarek E., Siegel J. B., Candinas D., Koziak K., Millan M., Hancock W. W., Bach F. H. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997 Jan 6;185(1):153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson S. C., Schulte am Esch J., 2nd, Bach F. H. Factors in xenograft rejection. Ann N Y Acad Sci. 1999 Jun 18;875:261–276. doi: 10.1111/j.1749-6632.1999.tb08509.x. [DOI] [PubMed] [Google Scholar]
- Robson S. C., Young V. K., Cook N. S., Metternich R., Kasper-Konig W., Lesnikoski B. A., Pierson R. N., 3rd, Hancock W. W., Candinas D., White D. J. Thrombin inhibition in an ex vivo model of porcine heart xenograft hyperacute rejection. Transplantation. 1996 Mar 27;61(6):862–868. doi: 10.1097/00007890-199603270-00003. [DOI] [PubMed] [Google Scholar]
- Rongen G. A., Floras J. S., Lenders J. W., Thien T., Smits P. Cardiovascular pharmacology of purines. Clin Sci (Lond) 1997 Jan;92(1):13–24. doi: 10.1042/cs0920013. [DOI] [PubMed] [Google Scholar]
- Schulte am Esch J., 2nd, Sévigny J., Kaczmarek E., Siegel J. B., Imai M., Koziak K., Beaudoin A. R., Robson S. C. Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry. 1999 Feb 23;38(8):2248–2258. doi: 10.1021/bi982426k. [DOI] [PubMed] [Google Scholar]
- Simonsen U., García-Sacristán A., Prieto D. Involvement of ATP in the non-adrenergic non-cholinergic inhibitory neurotransmission of lamb isolated coronary small arteries. Br J Pharmacol. 1997 Feb;120(3):411–420. doi: 10.1038/sj.bjp.0700918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sévigny J., Levesque F. P., Grondin G., Beaudoin A. R. Purification of the blood vessel ATP diphosphohydrolase, identification and localisation by immunological techniques. Biochim Biophys Acta. 1997 Feb 11;1334(1):73–88. doi: 10.1016/s0304-4165(96)00079-7. [DOI] [PubMed] [Google Scholar]
- Wang T. F., Guidotti G. CD39 is an ecto-(Ca2+,Mg2+)-apyrase. J Biol Chem. 1996 Apr 26;271(17):9898–9901. [PubMed] [Google Scholar]
- Zimmermann H. 5'-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992 Jul 15;285(Pt 2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]