Abstract
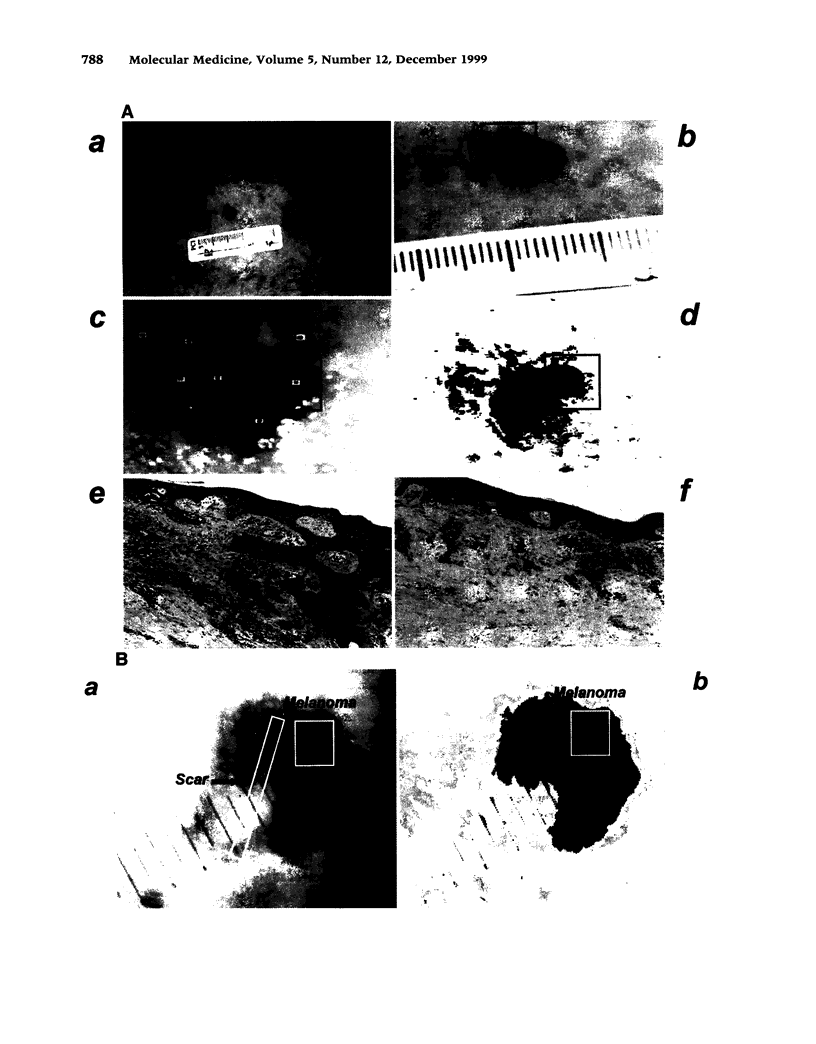
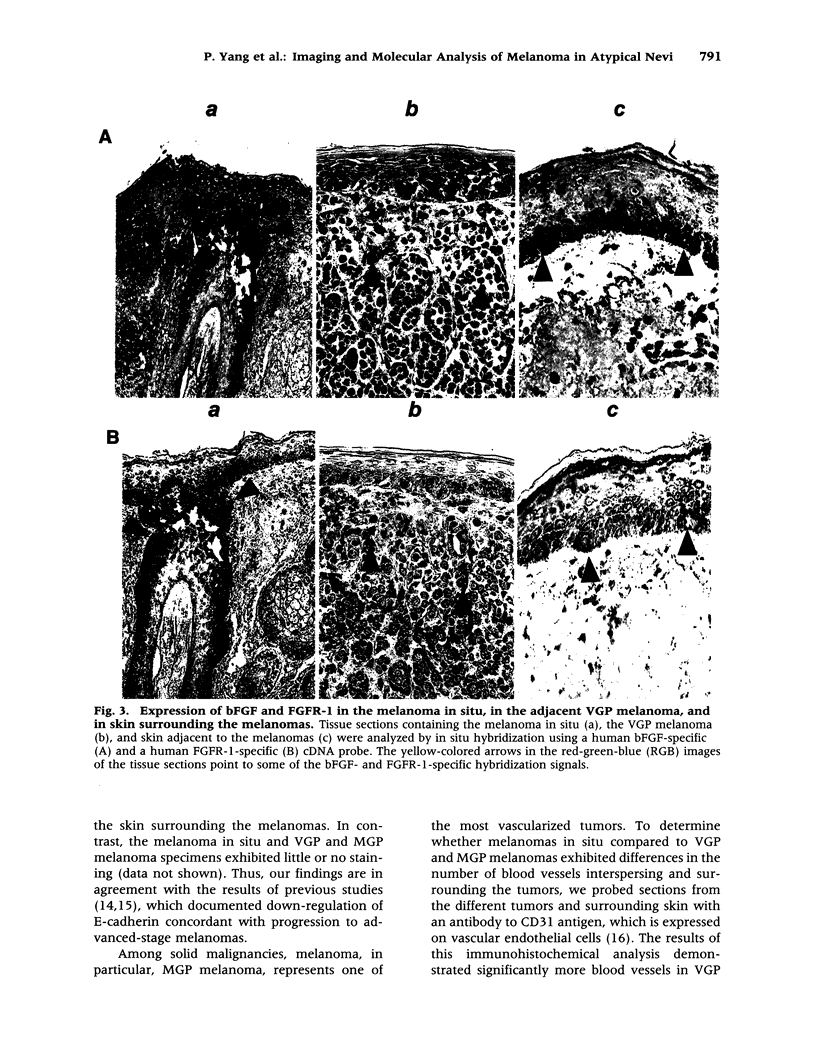
BACKGROUND: The stages of melanocytic progression are defined as atypical (dysplastic) nevus, melanoma in situ, melanoma in the radial growth phase (RGP), melanoma in the vertical growth phase (VGP), and melanoma in the metastatic growth phase (MGP). Melanoma in situ and RGP melanoma often develop in contiguous association with atypical nevi. This frequently poses a problem with respect to their early detection. Furthermore, unlike cells obtained from VGP and MGP melanomas, cells derived from melanoma in situ and RGP melanoma do not proliferate in vitro. Thus, compared to the late stages of the disease, less information is available regarding genes expressed in the early stages. MATERIALS AND METHODS: To determine whether spectral imaging, a recently developed optical imaging technique, can detect melanoma in situ and RGP melanoma arising in melanoma precursor lesions, atypical nevi in patients with a clinical history of melanoma were subjected to noninvasive macroscopic spectral imaging. To determine at what stage in the progression pathway of melanoma genes having important biological functions in VGP and MGP melanomas are activated and expressed, lesions of melanoma in situ were analyzed by immunohistochemistry and in situ hybridization for expression of some of these known molecular and immunologic markers. RESULTS: The present study demonstrates the capability of noninvasive spectral imaging to detect melanoma in situ and RGP melanoma that arise in contiguous association with atypical nevi. Furthermore, the study provides evidence that genes and antigens expressed in VGP and MGP melanoma are also expressed in melanoma in situ. CONCLUSIONS: Because of the dark and variegated pigmentation of atypical nevi, melanoma in situ and RGP melanoma that arise in these melanoma precursor lesions are often difficult to recognize and thus frequently go unnoticed. The application of new optical screening techniques for early detection of melanoma and the identification of genes expressed in the early stages of melanoma development are two important avenues in the pursuit of melanoma prevention. The investigations presented here document that macroscopic spectral imaging has the potential to detect melanoma in its early stage of development and that genes essential for the proliferation and cell adhesion of VGP and MGP melanoma are already expressed in melanoma in situ.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Becker D., Lee P. L., Rodeck U., Herlyn M. Inhibition of the fibroblast growth factor receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene. 1992 Nov;7(11):2303–2313. [PubMed] [Google Scholar]
- Becker D., Meier C. B., Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J. 1989 Dec 1;8(12):3685–3691. doi: 10.1002/j.1460-2075.1989.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boppart S. A., Bouma B. E., Pitris C., Southern J. F., Brezinski M. E., Fujimoto J. G. In vivo cellular optical coherence tomography imaging. Nat Med. 1998 Jul;4(7):861–865. doi: 10.1038/nm0798-861. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Guerry D., 4th, Braitman L. E., Trock B. J., Schultz D., Synnestvedt M., Halpern A. C. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989 Dec 20;81(24):1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- Elder D. E., Clark W. H., Jr, Elenitsas R., Guerry D., 4th, Halpern A. C. The early and intermediate precursor lesions of tumor progression in the melanocytic system: common acquired nevi and atypical (dysplastic) nevi. Semin Diagn Pathol. 1993 Feb;10(1):18–35. [PubMed] [Google Scholar]
- Farkas D. L., Du C., Fisher G. W., Lau C., Niu W., Wachman E. S., Levenson R. M. Non-invasive image acquisition and advanced processing in optical bioimaging. Comput Med Imaging Graph. 1998 Mar-Apr;22(2):89–102. doi: 10.1016/s0895-6111(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Hsu M. Y., Wheelock M. J., Johnson K. R., Herlyn M. Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc. 1996 Apr;1(2):188–194. [PubMed] [Google Scholar]
- Kirkwood J. M., Farkas D. L., Chakraborty A., Dyer K. F., Tweardy D. J., Abernethy J. L., Edington H. D., Donnelly S. S., Becker D. Systemic interferon-alpha (IFN-alpha) treatment leads to Stat3 inactivation in melanoma precursor lesions. Mol Med. 1999 Jan;5(1):11–20. [PMC free article] [PubMed] [Google Scholar]
- Piston D. W., Masters B. R., Webb W. W. Three-dimensionally resolved NAD(P)H cellular metabolic redox imaging of the in situ cornea with two-photon excitation laser scanning microscopy. J Microsc. 1995 Apr;178(Pt 1):20–27. doi: 10.1111/j.1365-2818.1995.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha M., Grossman M., Esterowitz D., Webb R. H., Anderson R. R. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995 Jun;104(6):946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- Tang A., Eller M. S., Hara M., Yaar M., Hirohashi S., Gilchrest B. A. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci. 1994 Apr;107(Pt 4):983–992. doi: 10.1242/jcs.107.4.983. [DOI] [PubMed] [Google Scholar]
- Wachman E. S., Niu W., Farkas D. L. AOTF microscope for imaging with increased speed and spectral versatility. Biophys J. 1997 Sep;73(3):1215–1222. doi: 10.1016/S0006-3495(97)78154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Becker D. Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med. 1997 Aug;3(8):887–893. doi: 10.1038/nm0897-887. [DOI] [PubMed] [Google Scholar]
- Wang Y., Rao U., Mascari R., Richards T. J., Panson A. J., Edington H. D., Shipe-Spotloe J. M., Donnelly S. S., Kirkwood J. M., Becker D. Molecular analysis of melanoma precursor lesions. Cell Growth Differ. 1996 Dec;7(12):1733–1740. [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]