Abstract
Native and recombinant G protein–gated inwardly rectifying potassium (GIRK) channels are directly activated by the βγ subunits of GTP-binding (G) proteins. The presence of phosphatidylinositol-bis-phosphate (PIP2) is required for G protein activation. Formation (via hydrolysis of ATP) of endogenous PIP2 or application of exogenous PIP2 increases the mean open time of GIRK channels and sensitizes them to gating by internal Na+ ions. In the present study, we show that the activity of ATP- or PIP2-modified channels could also be stimulated by intracellular Mg2+ ions. In addition, Mg2+ ions reduced the single-channel conductance of GIRK channels, independently of their gating ability. Both Na+ and Mg2+ ions exert their gating effects independently of each other or of the activation by the Gβγ subunits. At high levels of PIP2, synergistic interactions among Na+, Mg2+, and Gβγ subunits resulted in severalfold stimulated levels of channel activity. Changes in ionic concentrations and/or G protein subunits in the local environment of these K+ channels could provide a rapid amplification mechanism for generation of graded activity, thereby adjusting the level of excitability of the cells.
Keywords: G protein–gated inwardly rectifying potassium channels, phosphatidylinositol-bis-phosphate, Gβγ gating, Mg2+ gating, Na+ gating
INTRODUCTION
In atrial tissue, acetylcholine released by the vagus nerve binds to muscarinic type 2 receptors, activates KACh channels via pertussis toxin–sensitive G proteins, and slows the heart rate. Upon activation, the heterotrimeric G protein dissociates, allowing the Gβγ subunits to directly activate the KACh channel (Logothetis et al. 1987; Krapivinsky et al. 1995b). KACh has been shown to be composed of two types of G protein–gated inwardly rectifying potassiumb channels (GIRK1 and GIRK4),1 associated in a heterotetrameric complex (Krapivinsky et al. 1995a; Silverman et al. 1996; Corey et al. 1998). Recombinant (GIRK) channels expressed in oocytes are also directly activated by G protein βγ subunits (Reuveny et al. 1994). In addition, GIRK channels appear to be activated independently of G proteins. In the absence of agonist, ATP hydrolysis leads to an increase in the mean open time and sensitizes channels to gating by Na+ ions (Sui et al. 1996). Recently, it was shown that the ATP modification of GIRK channels is mediated via phosphatidylinositol phosphates such as phosphatidylinositol-bis-phosphate (PIP2) (Huang et al. 1998; Sui et al. 1998). PIP2 has been implicated in the regulation of the sodium–calcium exchanger (Hilgemann and Ball 1996), the KATP channel (Hilgemann and Ball 1996; Fan and Makielski 1997; Baukrowitz et al. 1998; Shyng and Nichols 1998), the inwardly rectifying ROMK1 and IRK1 channels (Huang et al. 1998) and other Na+-gated nonselective cation channels (Zhainazarov and Ache 1999). Moreover, PIP2 appears to be essential for GIRK channel activation by the G protein βγ subunits (Sui et al. 1998).
Here, using both native and recombinant GIRK channels, we show that Na+ as well as Mg2+ ions gate the ATP- or PIP2-modified channels. While the two ions seem to exert their effects at distinct sites on the channel protein, they showed synergistic effects on gating. In the presence of exogenous PIP2, Gβγ and Na+ and Mg2+ ions showed great synergism in activating the channel. However, in the absence of exogenous PIP2, preactivation by G protein βγ subunits sensitized the channel to gating by Na+ but not Mg2+ ions. These data suggest that the synergism between Mg2+ and Gβγ subunits in gating GIRK channels shows a much greater dependence on PIP2 levels than the synergism between Na+ and Gβγ. The synergism among ions and Gβγ proteins in the gating of GIRK channels implies that variations of the concentrations of these molecules in the local environment of these channels could play an important role in the “fine tuning” of their activity.
MATERIALS AND METHODS
Expression of Recombinant Channels in Xenopus Oocytes
Recombinant channel subunits (GIRK1, GenBank accession No. U39196; GIRK4, GenBank accession No. U39195) were expressed in Xenopus oocytes as described previously (Chan et al. 1996). Channel subunit coexpression was accomplished by coinjection of equal amounts of each cRNA (∼4 ng). The human muscarinic receptor type 2 was coexpressed with the channel subunits (∼1.5 ng injected per oocyte). The β-adrenergic receptor kinase (βARK)–PH construct, altered to incorporate the 15 NH2-terminal residues of Src for membrane targeting, was generously provided by Dr. E. Reuveny (Weizmann Institute of Science, Rehovot, Israel). cRNA concentrations were estimated from two successive dilutions that were electrophoresed on formaldehyde gels in parallel and compared with known concentrations of a RNA marker (GIBCO BRL). Oocytes were isolated and microinjected as described previously (Logothetis et al. 1992). The oocytes were maintained at 18°C, and electrophysiological recordings were performed 2–6 d after injection at room temperature (20–22°C).
Preparation of Chicken Atrial Myocytes
The procedure used for isolating cardiac myocytes from chicken embryos has been described previously (Sui et al. 1996). In brief, atrial tissue was selected using chicken embryos from eggs incubated 14–18 d. Atrial tissue was incubated for 20–30 min at 37°C in 5 ml of Mg2+- and Ca2+-free PBS supplemented with 1–2% trypsin/EDTA solution (10×, GIBCO BRL). Isolated myocytes were collected by triturating the digested tissue in 5 ml of trypsin-free solution and stored in a high potassium (KB) solution (Isenberg and Klöckner 1982) at 4°C for up to 36 h. The cells were allowed to settle on poly-lysine–coated coverslips in the recording chamber before experiments.
Reagents
General chemical reagents, including GTP and ATP, were purchased from Sigma Chemical Co. PIP2 (Boehringer Mannheim) was sonicated on ice for 30 min before application. Purified recombinant G protein subunits dimer β1γ7 was kindly provided by Dr. J. Garrison (University of Virginia, Charlottesville, VA). The stock of β1γ7 (0.86 μg/μl) was dissolved in 20 mM HEPES, 1 mM EDTA, 200 mM NaCl, 0.6% CHAPS, 50 mM MgCl2, 10 mM NaF, 30 μM AlCL3, 3 mM dithiothreitol (DTT), 3 μM GDP, pH 8.0. The final concentration was 20 nM in a solution containing 0.012% CHAPS, and 20 μM DTT. QEHA peptide (Chen et al. 1995) was kindly provided by Dr. R. Iyengar (Mount Sinai School of Medicine) and was used at a final concentration of 50 μM.
Single-Channel Recording and Analysis
Single-channel activity was recorded in the cell-attached or inside-out patch configurations (Hamill et al. 1981) using an Axopatch 200B amplifier (Axon Instruments). All pipettes used in the experiments were pulled using the WPI-K borosilicate glass (World Precision Instruments) and gave resistances of 2–8 MΩ. All experiments were conducted at room temperature (20–22°C). Single-channel recordings were performed at a membrane potential of −80 mV with acetylcholine (ACh, 5 μM) in the pipette, unless otherwise indicated. Single-channel currents were filtered at 1–2 kHz, sampled at 5–10 MHz, and stored directly into the computer's hard disk through the DIGIDATA 1200 interface (Axon Instruments). PCLAMP (v. 6.03; Axon Instruments) was used for data acquisition.
To remove the vitelline membrane, Xenopus oocytes were placed in a hypertonic solution (Stühmer 1992) for 5 min. Shrunk oocytes were transferred into a V-shaped recording chamber and the vitelline membrane was partially removed, exposing just enough plasma membrane for access with a patch pipette (Sui et al. 1996). This procedure increased the success rate of forming gigaseals.
The pipette solution contained 96 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, pH 7.35. The bath solution contained 96 mM KCl, 5 mM EGTA, and 10 mM HEPES, pH 7.35. When high concentrations of Mg2+ ions (>5 mM) were used in the bath solution, the KCl concentration was reduced accordingly to maintain osmolarity. Gadolinium chloride at 100 μM was routinely added to the pipette solution to suppress native stretch channel activity in the oocyte membrane. For chick atrial cells, the experimental solutions were the same as those used with oocyte recordings, except that the KCl concentration was 140 mM without gadolinium chloride.
Free Mg2+ and ATP concentrations were estimated as described previously (Vivaudou et al. 1991). Single-channel records were analyzed using PCLAMP software, complemented with our own analysis routine, as described previously (Sui et al. 1996). Parameters used for single-channel analysis include activity of all channels in the patch (or the total open probability, NP o), the total frequency of opening (NF o), and the mean open time (MT o), and averages over 5-s bins are displayed.
In experiments shown in Fig. 7, where exogenous PIP2 was applied throughout the experiment (i.e., Fig. 7A and Fig. C), occasional applications of the same ion as a function of time in the experiment were used as control to ascertain that the synergistic effects described were not due to a time-dependent accumulation of PIP2 in the membrane patch. Similar precautions were taken in the experiments shown in Fig. 4. Experiments used to generate the data shown in these two figures were never longer than 14 min (usually 10–13 min). Na+ and/or Mg2+ ions were applied for 30 s.
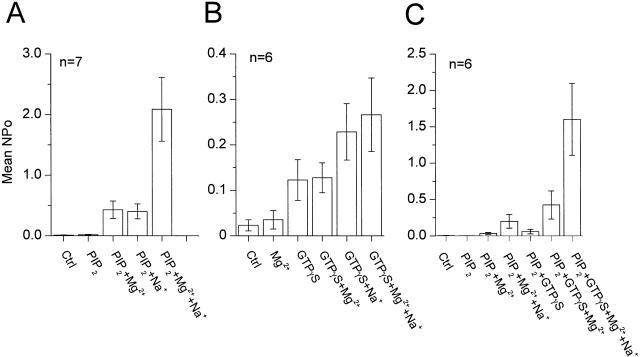
Figure 7.
Synergistic effects of Gβγ, Na+, and Mg2+ ions in activating GIRK channels. (A) The mean NP o for seven patches are plotted for different conditions. The data were obtained from inside-out patches excised from oocytes expressing the recombinant channel GIRK1/GIRK4. The membrane was held at −80 mV and 5 μM acetylcholine was present in the pipette. PIP2 was 2.5 μM, Mg2+ was 10 mM, and Na+ was 10 mM. SEM are indicated by the vertical bars. The mean NP o for the channel activity was 0.011 ± 0.003 in control conditions, and 0.01 ± 0.006 during the application of 2.5 μM PIP2. When 10 mM Mg2+ ions were applied with PIP2, the mean NP o was 0.43 ± 0.14. 10 mM Na+ ions gave a mean NP o of 0.40 ± 0.12. When applied together, in the presence of PIP2, Mg2+ and Na+ ions (10 mM each) yielded a mean NP o of 2.08 ± 0.52. (B) Mean NP o plots for six inside-out patches from oocytes expressing GIRK1/GIRK4. Vm was −80 mV. 5 μM acetylcholine was in the pipette. Mg2+ was 10 mM, Na+ was 10 mM, and GTPγS was 10 μM. The columns GTPγS and GTPγS+x depict the channel activity measured after the GTP analogue was washed out and substance(s) x were added. SEM are indicated by vertical bars. The mean NP o of the channel was 0.023 ± 0.012 in control conditions and 0.035 ± 0.02 in the presence of 10 mM Mg2+ ions. 10 μM GTPγS gave a mean NP o of 0.12 ± 0.045. After GTPγS washout, Mg2+ ions gave a mean NP o of 0.12 ± 0.03. 10 mM Na+ ions gave a mean NP o of 0.23 ± 0.06. Coapplication of Mg2+ and Na+ ions resulted in a mean NP o of 0.26 ± 0.08. (C) Mean NP o plots for six patches. The inside-out patches were excised from oocytes expressing GIRK1/GIRK4. Vm was −80 mV. 5 μM ACh present in the pipette. PIP2 was 2.5 μM, Mg2+ was 10 mM, Na+ was 10 mM, and GTPγS was 10 μM. PIP2+GTPγS refers to the channel activity (at steady state) during the application of the GTP analogue. PIP2+GTPγS+x columns depict the channel activity measured after the washout of the GTP analogue and addition of substance(s) x. In absence of 10 mM Mg2+, all solutions contained 50 μM Mg2+. This low concentration of Mg2+ was necessary to render GTPγS effective. Vertical bars represent SEM. The mean NP o for the channel activity was 0.004 ± 0.002 in control conditions and 0.0007 ± 0.0002 in the presence of 2.5 μM PIP2. When 10 mM Mg2+ ions were applied to the patches in the presence of PIP2, a mean NP o of 0.034 ± 0.013 was obtained. Although this activity appeared small, it was significantly higher than that in PIP2 alone (P < 0.005, paired t test, log scale). 10 mM each of Mg2+ and Na+ ions in combination yielded a mean NP o of 0.2 ± 0.09. GTPγS gave a mean NP o of 0.058 ± 0.03. Again, although this activity appeared relatively small, it was significantly higher than that in PIP2 alone (P < 0.005, paired t test, log scale). When 10 mM Mg2+ ions were applied to the patches after the GTPγS treatment a mean NP o of 0.42 ± 0.19 was obtained. Mg2+ and Na+ ions applied together resulted in NP o of 1.6 ± 0.49.
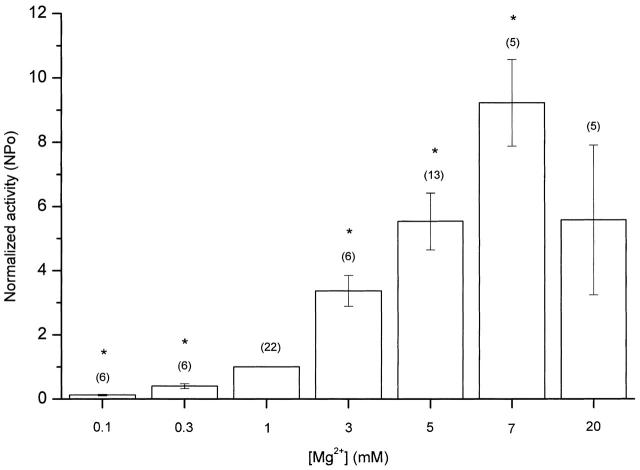
Figure 4.
Concentration dependence of GIRK channel activation on internal Mg2+ ions. Normalized activity (NP o) of GIRK1/GIRK4 channels is plotted for different Mg2+ concentrations. Inside-out patches were exposed to 2.5 μM PIP2 before and during application of Mg2+. Responses were expressed in fold increase above the activity in the presence of PIP2 and were normalized to those recorded in the presence of 1 mM Mg2+. Vertical bars represent SEM. The responses obtained at the low concentrations of Mg2+ tested (<1 mM) were significantly higher than those with PIP2 alone (P < 0.05, paired t test). The holding potential was at −80 mV. 5 μM ACh was present in the pipette. *Significant differences from 1 mM Mg2+ (P < 0.01; paired t test).
RESULTS
MgATP/Na Activation of the KACh Channel Can Proceed Independently of the Involvement of G Proteins
It has been shown previously that Na+ ions can stimulate KACh activity in an ATP-dependent manner in the absence of agonist and internal GTP (Sui et al. 1996). The ATP-dependent modification of the channel is thought to work via the production of membrane phosphoinositide phosphates (e.g., PIP2), which interact directly with members of the inwardly rectifying K+ channel family (Huang et al. 1998; Sui et al. 1998). PIP2 also appears to be essential for G protein regulation of the KACh channel (Sui et al. 1998).
To further test for a dependence of the MgATP/Na+ activation on G protein gating of KAch, we designed experiments where G protein–dependent activation of the channel was impaired. As shown in Fig. 1 A, the KACh channel in an inside-out atrial myocyte patch was activated persistently by 10 μM GTPγS, a nonhydrolyzable analogue of GTP. Activation of the channel by GTPγS was blocked upon perfusion of the QEHA peptide. QEHA is a 27 amino acid long peptide derived from the COOH terminus of the Gβγ-sensitive adenylate cyclase 2 isoform. It has been shown to block Gβγ activation of several different effectors, including the KACh channel (Chen et al. 1995). QEHA (50 μM) application abolished the GTPγS activation of KACh in <2 min (n = 3). After washout, the channel activity remained very low, suggesting the persistence of the QEHA-blocking effect. However, under these conditions, the KACh channel could be activated by MgATP/Na+ (5/20 mM). QEHA coapplication with MgATP/Na+ failed to block channel activation, whereas QEHA did block GTPγS-induced activation in the same oocyte patches (n = 3) (data not shown).
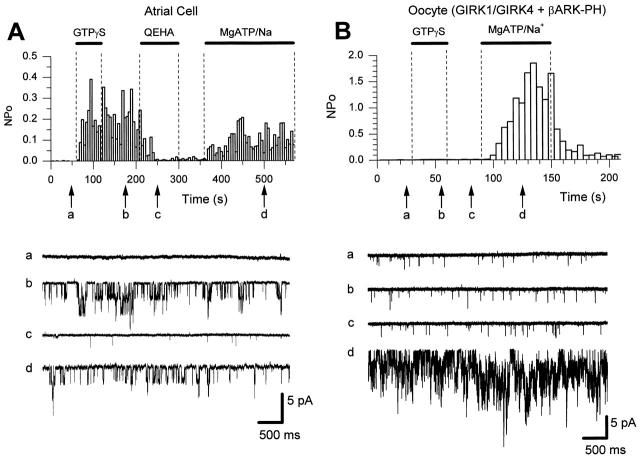
Figure 1.
Impairment of G protein signaling does not affect the activation of KACh by MgATP and Na+. (A) Single channel activity (top; NP o, bin = 5 s) plotted as a function of time. The data were obtained from an inside-out patch excised from an atrial cell. The KACh channel was stimulated by maintaining the membrane at −80 mV and by the presence of 5 μM acetylcholine in the pipette. 10 μM GTPγS, 50 μM QEHA, and 5/20 mM MgATP/Na+ were applied for the duration indicated by the bars. Sample single-channel currents in each condition at the time marked by the arrows are shown under the plot (bottom). (B) NP o plot of KACh channel activity (top, bin = 5 s) in an inside-out patch from an oocyte expressing the human GIRK1/GIRK4 and the construct βARK-PH. The membrane was clamped at −80 mV and 5 μM acetylcholine was present in the pipette. Application of 10 μM GTPγS and 5/20 mM MgATP/Na+ are illustrated by the bars. Labeled arrows correspond to the sample single-channel currents shown under the plot (bottom).
Another way we impaired the G protein regulation of the GIRK channels was by coexpressing them in oocytes with a βγ-binding protein. We used the PH domain of βARK (βARK-PH), which specifically binds the βγ subunits of G proteins, and thus acts as a “βγ sink” (Koch et al. 1993; He et al. 1999). In oocytes coexpressing the recombinant channels GIRK1/GIRK4 and the construct βARK-PH, 10 μM GTPγS did not induce channel activity. This suggests that the βARK-PH protein bound the oocyte endogenous G proteins, such that no βγ subunits were available for channel activation (Fig. 1 B). However, in the same patches, MgATP/Na+ (5/20 mM) caused a >30-fold increase in channel activity. Summary data revealed that channel activities (NP o) before, during, and after GTPγS application were similar, 0.0070 ± 0.0039, 0.0077 ± 0.0037, and 0.0097 ± 0.0045, respectively (mean ± SEM, n = 4). During application of MgATP/Na+, NP o was 0.313 ± 0.221 (n = 4).
In control experiments using inside-out patches from oocytes of the same batch that coexpressed the recombinant channels GIRK1/GIRK4 alone, GTPγS caused great channel activation (n = 3, data not shown). Similar results were obtained in experiments in which we applied Na+ ions with PIP2 rather than MgATP (n = 4, data not shown).
These results suggest that even when G protein regulation is impaired, Na+ ions are still able to activate the channel. Thus, Na+ gating of the channel can indeed proceed independently of Gβγ gating.
Gβγ Subunits Sensitize GIRK Channels to Gating by Na+ Ions
Na+ ions can gate GIRK channels when membrane PIP2 levels are maintained (i.e., via hydrolysis of ATP). We next tested under conditions that did not maintain PIP2 at a constant high level whether Na+ ions could gate these channels after Gβγ activation.
Fig. 2A and Fig. B, show representative and summary data from experiments where Na+ ions gated GIRK1/GIRK4 channels after activation by G proteins. Inside-out patches from oocytes expressing these channels showed no channel activity upon application of 20 mM Na+. This result suggested a low presence of PIP2 in the membrane. However, this PIP2 concentration was sufficient to allow persistent channel activation by a brief exposure to 10 μM GTPγS. Reapplication of Na+ ions produced a more than fourfold increase in the channel activity above the level obtained with GTPγS. It should be noted that the effect of Na+ ions on the basal channel activity was variable from patch to patch, presumably reflecting different levels of endogenous PIP2 at the time of Na+ application.
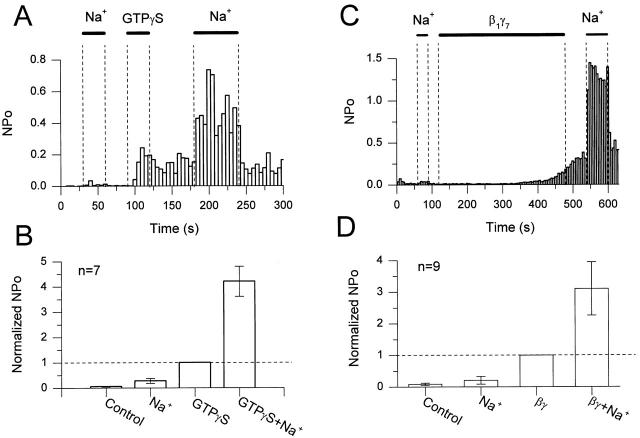
Figure 2.
Na+ ions gate GIRK channels after activation by G protein βγ subunits. (A) Single-channel activity (NP o, bin = 5 s) plotted as a function of time. The data were obtained from an inside-out patch excised from an oocyte expressing the recombinant channel GIRK1/GIRK4. 20 mM Na+ and 10 μM GTPγS were applied as indicated by the bars. The membrane was clamped at −80 mV and 5 μM acetylcholine was in the pipette solution. (B) The mean NP o for seven patches is plotted for different conditions. Steady state channel activity after activation by GTPγS was taken as reference (GTPγS) and NP o were normalized to it. Na+ concentration was 20 mM and GTPγS was 10 μM. GTPγS+Na+ corresponds to the application of 20 mM Na+ after the washout of the GTP analogue. SEM are indicated by the vertical bars. The normalized mean NP o was 0.057 ± 0.018 (mean ± SEM) in control solution, 0.277 ± 0.095 in the presence of 20 mM Na+ ions, 1 after the application of 10 μM GTPγS, and 4.21 ± 0.59 in the presence of 20 mM Na+ ions after channel activation by GTPγS. (C) NP o vs. time plot for the channel activity recorded in an inside-out patch from an oocyte expressing GIRK1/GIRK4. 20 mM Na+ and 20 nM β1γ7 purified subunits were applied via the bath as indicated by the bars. Vm = −80 mV. 5 μM acetylcholine was present in the pipette. (D) The mean NP o for nine patches are plotted for different conditions. Steady state channel activity after β1γ7 activation (after β1γ7 washout) was taken as reference and NP o was normalized to it. Na+ concentration was 20 mM and β1γ7 was 20 nM. βγ+Na+ refers to the application of 20 mM Na+ after the washout of βγ. The vertical bars represent SEM. The normalized mean NP o was 0.084 ± 0.039 (mean ± SEM) in control solution, 0.206 ± 0.12 in the presence of 20 mM Na+ ions, 1 after the application of 20 nM β1γ7, and 3.1 ± 0.84 in the presence of 20 mM Na+ ions after activation of the channel by the G protein subunits.
Na+ ions also gated GIRK channels after stimulation of activity by purified Gβγ subunits. In Fig. 2C and Fig. D, Na+ ions (20 mM) applied on an inside-out patch did not affect significantly the basal activity of the channel. After washout of the Na+ ions, recombinant Gβ1γ7 was applied on the patch at a concentration of 20 nM, causing a slow channel activation. After washout of Gβ1γ7, and as activity stabilized, a second application of Na+ ions produced a more than threefold increase in channel activity, above the level obtained with β1γ7. Combined together, these data suggested that the G protein βγ subunits sensitized GIRK channels to gating by Na+ ions. It has been shown that the mean open time (MT o) increased in the presence of PIP2 that is generated by hydrolysis of ATP or exogenous application (Sui et al. 1996, Sui et al. 1998). In the present experiments, no change in the channel MT o was observed in the different solutions perfusing the patches (data not shown). This suggests that the levels of PIP2 in the membrane were not altered, and thus could not account for the Gβγ-dependent gating effects of Na+ ions.
It has been shown that Li+ ions stimulate GIRK channels modified by ATP to ∼10% the activity level achieved by comparable Na+ ion concentrations (Sui et al. 1996). However, Li+ ions were unable to increase the activity of the channel after activation by GTPγS. In three patches, the mean NP o of the GIRK channel was 0.028 ± 0.013 in control conditions, 0.127 ± 0.035 after application of 10 μM of GTPγS, 0.578 ± 0.15 in the presence of 20 mM Na+ ions, and 0.099 ± 0.045 in the presence of 20 mM Li+ ions (data not shown). When applied together, Li+ ions were also unable to affect the gating of the GIRK channel by Na+ ions. This suggests that the gating effect of Na+ ions on the GIRK channel activated by G protein βγ subunits is specific to Na+ ions.
Mg2+ Ions Gate GIRK Channels After Channel Modification by ATP or PIP2
In certain experiments, 5 mM MgATP increased the activity of the GIRK channels in the absence of Na+ ions (e.g., Sui et al. 1996). 5 mM MgATP in the solution corresponds to a free Mg2+ ion concentration of ∼2.1 mM (Vivaudou et al. 1991). This observation prompted us to test whether Mg2+ ions alone were able to gate the channel that had been modified by ATP. In Fig. 3, in an inside-out patch from an oocyte coexpressing the channel subunits GIRK1/GIRK4, Mg2+ ions (10 mM) had no significant effects on channel activity in the absence of ATP. After washout of Mg2+, the channel was activated by the combination of MgATP (2.5 mM; corresponding to ∼1.1 mM free Mg2+) and Na+ ions (20 mM). MgATP application was maintained and, upon withdrawal of Na+ ions, channel activity became comparable to basal levels. Application of Mg2+ ions (10 mM), in the continuous presence of MgATP (2.5 mM), increased channel activity to levels similar to those obtained with Na+ ions (as confirmed by sequential application of 10 mM of each of the ions at the end of the experiment). Withdrawal of Mg2+ ions caused channel activity to return to basal levels (n = 3). The MT o of the channel activity was increased from ∼1 to ∼2 ms by the application of MgATP, but was not further modified during the gating by Mg2+ or Na+ ions.
Figure 3.
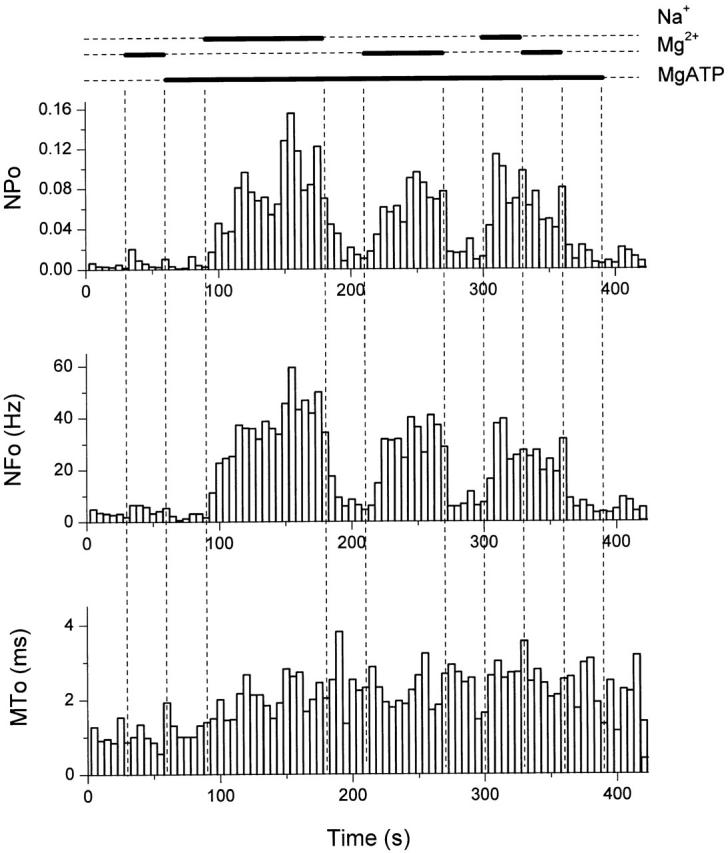
Mg2+ ions gate the ATP-modified GIRK channels. NP o, NF o, and MT o plots (bin = 5 s) of GIRK channel activity in an inside-out patch excised from an oocyte expressing GIRK1/GIRK4. 10 mM Mg2+, 2.5 mM MgATP, and 20 mM Na+ were applied via the bath as indicated by the bars. The membrane was clamped at −80 mV and 5 μM acetylcholine was present in the pipette. NP o, NF o, and MT o in 10 mM Mg2+ were calculated such that the amplitude levels were set to match the reduced amplitude of the channel openings in 10 mM Mg2+.
Using PIP2, we could test the ability of different Mg2+ concentrations to activate the GIRK channels. Fig. 4 represents normalized activity of GIRK channels for different concentrations of Mg2+ ions. The NP o for each concentration was calculated in reference to the NP o measured at 1 mM Mg2+. Mg2+ ions could activate the GIRK channels at concentrations as low as 100–300 μM. Maximal activity could be obtained at a concentration of ∼7 mM Mg2+. At higher concentrations (e.g., 20 mM), Mg2+ ions resulted in a decrease of channel activity relative to lower concentrations (e.g., 7 mM). It has been shown that, at high concentrations, divalent cations can trigger aggregation of PIP2 molecules (Flanagan et al. 1997), a result that could account for the effects of high Mg2+ concentrations on channel activity.
In another set of experiments, we showed that Mg2+, like Na+ gating, can occur independently of G proteins. Patches excised from oocytes coexpressing the βARK-PH domain and GIRK channels were exposed to PIP2 (2.5 μM) and subsequently to Mg2+ ions. In these patches, GTPγS (10 μM) was unable to activate the GIRK channels, giving a NP o of 0.08 ± 0.03, identical to the NP o measured in PIP2 (0.078 ± 0.02). Mg2+ ions (1 mM) could increase the channel activity approximately sixfold (n = 4, data not shown) above the activity measured in PIP2, showing that Mg2+ gating could proceed independently of Gβγ gating.
These results suggest that when modified by ATP or PIP2, GIRK channels become sensitive to either Na+ or Mg2+ ions.
Mg2+ Ion Gating Occurs at a Site Distinct from that of Na+ Action
Recent work has identified an aspartate amino acid residue as the site of action of Na+ ions on GIRK channels, GIRK2 (D228) and GIRK4 (D223) (Ho and Murrell-Lagnado 1999; Zhang et al. 1999). Moreover, it was shown that Na+ sensitivity lies entirely with the heteromeric partners of GIRK1, as this channel possesses an asparagine instead of an aspartate residue at the equivalent position. We used the point mutant GIRK4(S143T) (referred to as GIRK4*) that allows for high levels of activity of homotetrameric GIRK4 channels (Vivaudou et al. 1991) to test for Na+ and Mg2+ sensitivity. GIRK4* channel activity shows high sensitivity to both Na+ and Mg2+. Fig. 5 shows that indeed GIRK4*(D223N) loses its sensitivity to Na+ ions (20 mM). However Mg2+ ion (1 mM) sensitivity was intact (Fig. 5 A). Summary data are shown in Fig. 5 B. These data indicate that Na+ and Mg2+ ions act at distinct sites to activate GIRK channels.
Figure 5.
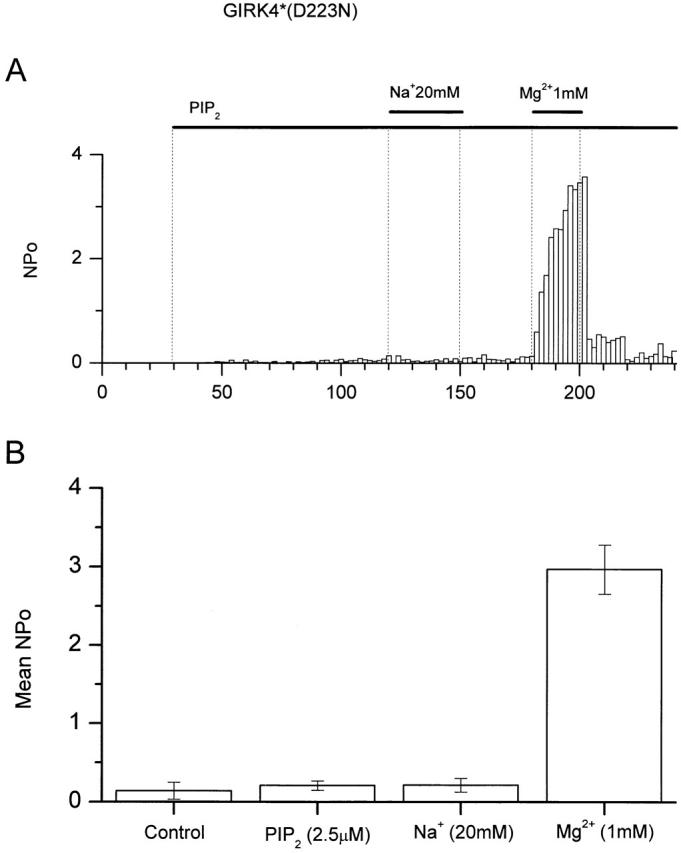
Mg2+ ions act at a site distinct of that used by Na+ ions. (A) Single channel activity (NP o, bin = 5 s) plotted as a function of time. An inside-out patch from an oocyte expressing GIRK4*(D223N) (see text) and human muscarinic receptor type 2 receptor was exposed to 2.5 μM PIP2 and 20 mM Na+ or 1 mM Mg2+ ions, and channel activity was recorded. The membrane potential was kept at −80 mV. The pipette solution contained 5 μM ACh. (B) Summary data plotting mean NP o of four experiments such as that shown in A. The mean NP o values were 0.14 ± 0.1 (mean ± SEM) in control conditions, 0.21 ± 0.06 in the presence of PIP2, 0.21 ± 0.08 in the presence of PIP2 and Na+ ions, and 2.97 ± 0.31 in the presence of PIP2 and Mg2+ ions.
Mg2+ Ions Reduce the Conductance of the GIRK Channels
We observed, particularly at high concentrations (>5 mM), that internal Mg2+ ions reduced the amplitude of single GIRK channel currents. In Fig. 6 A, the activity of the coexpressed channel subunits GIRK1/GIRK4 from an inside-out patch was recorded at −120 mV. After activation by 10 μM GTPγS, channel activity was recorded in a solution containing 1 mM Mg2+ ions, showing an approximate amplitude of −3.2 pA. When the solution applied to the patch was switched to one containing 20 mM Mg2+ ions, the amplitude of the single openings was rapidly reduced to a lower value, approximately −2.5 pA (n = 5). In Fig. 6 B, the activity of native KACh channels in an inside-out patch from an atrial cell was recorded at −90 mV. After exposure to 5 μM PIP2, the patch was perfused with a solution containing 20 mM Mg2+ ions, giving an amplitude of approximately −2.2 pA. When the solution applied to the patch was switched to one containing 20 mM Na+ and 1 mM Mg2+ ions, the channel amplitude immediately increased to a value of approximately −3.5 pA. This amplitude was also obtained in control conditions, where 1 mM Mg2+ ions were present (n = 5). The reduction in the single-channel amplitude was observed at various voltages. Since it was present at negative potentials (i.e., −80, −90, and −120 mV) where no rectification occurs, it is likely to proceed by a mechanism distinct from that of the rectification phenomenon. Mg2+ ions at high concentrations also decreased the amplitude of GIRK single channels when applied together with Na+ ions (data not shown). Thus, regardless of their ability to gate GIRK channels (see Fig. 3 and Fig. 7), Mg2+ ions at high concentrations (>5 mM) show a clear inhibition on single-channel current amplitudes. These data suggest that the inhibitory effect of Mg2+ ions on the single-channel amplitude was not dependent on their ability to gate the channel.
Figure 6.
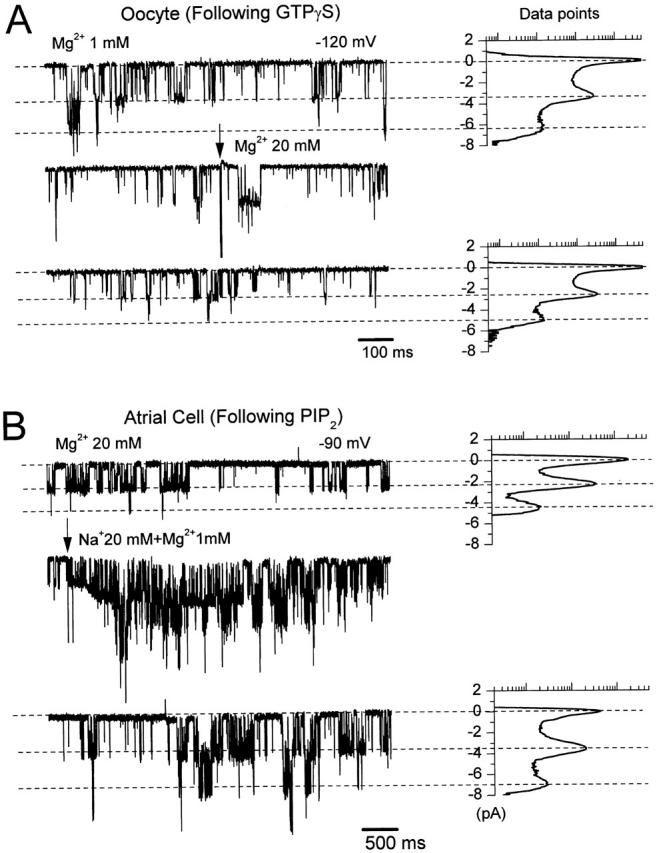
High concentrations of Mg2+ ions reduce the GIRK single-channel currents. (A) Single-channel records of GIRK channels in an inside-out patch excised from an oocyte expressing GIRK1/GIRK4. The membrane was clamped at −120 mV and 5 μM acetylcholine was present in the pipette. The channel was preactivated by 10 μM GTPγS and the current traces shown were recorded after washout of the GTP analogue. The switch between bath solutions containing 1 and 20 mM Mg2+ is visualized by the arrow (and by the corresponding electrical artifact on the record) on top of the second current trace. Associated all-point histogram plots indicate the amplitudes resulting from the various activity levels ranging from closed to multiple open levels and are shown for the first (1 mM Mg2+) and third (20 mM Mg2+) current traces. Data points are shown on a logarithmic scale ranging from 5 to 50,000. (B) KACh channel activity in an inside-out patch from a cardiac cell. The membrane was held at −90 mV and the pipette contained 5 μM acetylcholine. The patch was preincubated with 5 μM PIP2 and the current traces shown were recorded after the washout of PIP2. The arrow on top of the second current trace visualizes the switch between bath solutions containing 20 mM Mg2+ and 20 mM Na+ + 1 mM Mg2+. Associated all-point histogram plots are shown for the first (20 mM Mg2+) and third (20 mM Na+ + 1 mM Mg2+) current traces. Data points are shown on a logarithmic scale ranging from 20 to 50,000.
Synergistic Interactions among Ions and G Protein Subunits in Gating GIRK Channels
ATP modification of GIRK channels (native or recombinant) is likely to proceed through changes in the level of membrane PIP2 in the local environment of the channel (Huang et al. 1998; Sui et al. 1998). In Fig. 7 A, the activity of recombinant GIRK1/GIRK4 channels was not increased by the application of 2.5 μM PIP2 alone. Mg2+ ions (10 mM), applied with PIP2, stimulated activity >40-fold. As shown previously (Sui et al. 1998), Na+ ions (10 mM) were able to gate the channel in the presence of PIP2, resulting in activity equivalent to that obtained with Mg2+ ions. When applied together, in the presence of PIP2, Mg2+ and Na+ ions (10 mM each) showed a synergistic effect stimulating channel activity >200-fold.
We next tested whether Mg2+ ions, like Na+ ions (Fig. 2), can further gate GIRK channels after channel activation by GTPγS, under conditions that do not maintain PIP2 at constant high levels. Fig. 7 B shows that the basal activity of GIRK1/GIRK4 recombinant channels was not affected by Mg2+ ions. 10 μM GTPγS increased the activity of the channel approximately fivefold above basal levels. After GTPγS washout, the channel activity was stable and, when applied to the patches, Mg2+ ions were unable to increase channel activity further. In contrast, Na+ ions (10 mM) increased activity by another twofold above the GTPγS effect. When Mg2+ ions were applied together with Na+ ions, no further increase in channel activity above the levels obtained with Na+ ions was seen. Thus, G protein activation sensitized the GIRK channels to gating by Na+ ions, but not Mg2+ ions.
In Fig. 7 C, we show the effects of Mg2+ and Na+ ions after stimulation of the channel by GTPγS under conditions that kept PIP2 at a constant high level. As shown earlier, in the absence of Mg2+ and Na+ ions, PIP2 was not able to increase the basal activity of the GIRK channels. When Mg2+ ions (10 mM) were applied to the patches in the presence of PIP2, a greater than eightfold increase over control or PIP2 activity levels occurred. Mg2+ and Na+ ions (each 10 mM) in combination could raise channel activity by 50-fold over control levels. We then applied GTPγS and studied the effects of ions on G protein–stimulated channel activity in the continuous presence of PIP2. GTPγS was able to activate the channel >14-fold above control basal levels. After washout of GTPγS, channel activity was stable. When Mg2+ ions (10 mM) were applied to the patches after the GTPγS treatment in the continuous presence of PIP2, they could enhance channel activity to levels >100-fold higher than those obtained under control conditions. Thus, in the continuous presence of PIP2, this high level of activity was greater than that obtained with Mg2+ or GTPγS alone or their sum, suggesting synergistic interactions among the three molecules. Finally, when Mg2+ and Na+ ions were applied together, the channel total activity was increased 400-fold compared with control.
Similar data were obtained when the G protein β1γ7 subunits rather than GTPγS were used. In three cells, the total channel activity measured as the mean NP o was 0.027 ± 0.023 in control conditions, 0.022 ± 0.02 in the presence of 2.5 μM PIP2, 0.12 ± 0.09 in the presence of PIP2 and 10 mM Mg2+ ions, and 1 ± 0.55 in the presence of PIP2 and Mg2+ and Na+ ions. When 20 nM β1γ7 was applied in the presence of PIP2, it gave a steady state activity of the channel corresponding to a mean NP o of 0.25 ± 0.12. In the continuous presence of PIP2 and after stimulation of the channel by βγ subunits, the mean NP o was 1.23 ± 0.23 in the presence of Mg2+ ions and 2.61 ± 0.24 in the presence of Mg2+ and Na+ ions. It should be noted that the differences in channel activity (mean NP o) for the same condition applied to the patches (for example PIP2 + Mg2+ in Fig. 7A and Fig. C) may be related to differences in the level of channel expression between different batches of oocytes. Taken together, these data make four points. (a) Mg2+ ions can gate the channel after modification by PIP2. At a concentration of 10 mM, their gating potency is comparable with that of 10 mM Na+ ions. (b) When applied together, Mg2+ and Na+ ions show synergistic effects, resulting in levels of activity higher than those induced by each of the ions separately or their summed responses. (c) After activation by GTPγS (in the absence of exogenous PIP2 or MgATP), the GIRK channels are not further gated by Mg2+ ions, suggesting an important difference between Mg2+ and Na+ ions in gating these channels. (d) After channel modification by the combination of PIP2 and GTPγS, Mg2+ ions do stimulate the GIRK channel activity to higher levels than those obtained with PIP2 alone, suggesting that PIP2 renders the βγ-activated channels sensitive to gating by Mg2+ ions.
DISCUSSION
In the present study, we have shown that Mg2+ ions at physiological concentrations are additional activators of G protein–gated potassium channels. These K+ channels can be activated independently either by the βγ subunits of GTP-binding proteins (Logothetis et al. 1987) or by intracellular ions, such as Na+ (Sui et al. 1996) or Mg2+ ions. Activation by either G protein subunits or ions shows an absolute dependence on the presence of PIP2 (Sui et al. 1998). Specific combinations of these molecules show synergism and suggest differential dependence on the level of PIP2 for channel activation. This complex dependence of K+ channel activity on G proteins, Mg2+, Na+ ions, and PIP2 could serve to “fine tune” channel activity during physiological and pathophysiological conditions, where changes in the relative concentrations of these molecules might occur.
GIRK Channel Activation by Na+ Ions Can Be Independent of G Protein Subunit Involvement
Previous results from our laboratory showed that intracellular solution containing MgATP/Na+ was able to stimulate K+ channel activity in the absence of acetylcholine in the pipette, suggesting a G protein–independent mechanism of activation (Sui et al. 1996). Subsequently, it was further demonstrated that the ATP dependence of G protein–sensitive K+ channels, as well as of other inwardly rectifying channels, involved phosphoinositide formation, particularly PIP2 (Huang et al. 1998; Sui et al. 1998). In addition, it was reported that G protein activation of the K+ channel showed an absolute dependence on PIP2 (Sui et al. 1998). In the present study, we show that impairment of G protein subunit activation of the channel (by binding and competing away Gβγ from the channel with either QEHA perfusion or βARK-PH coexpression) did not prevent the MgATP/Na+ stimulation of activity (Fig. 1). Thus, we have provided further evidence that Na+ ion gating of the channel modified by ATP (or PIP2) can be independent of G protein subunit activation.
Mg2+ Gating of the G Protein-gated K+ Channel
Mg2+ ions have been shown to play an essential role in the rectification properties of inwardly rectifying K+ channels. Unitary current–voltage relations for G protein–sensitive K+ channels become ohmic if the internal face of the patch is exposed to Mg2+-free solutions. Inward rectification is restored when Mg2+ is reintroduced in the bathing solutions (Matsuda 1991; Kurachi et al. 1992; Nichols and Lopatin 1997).
Mg2+ ions are involved in many other reactions as essential cofactors. Kurachi et al. 1986 showed that G protein activation of the native G protein–sensitive K+ channel was absolutely dependent on Mg2+, possibly due to the requirement of Mg2+ for the binding of GTP to the Gα subunit (also see Logothetis et al. 1987). More recently, it has been appreciated that Mg2+-dependent processes of ATP hydrolysis (likely to be involved in phosphorylation–dephosphorylation of phosphoinositides) regulate channel activity (Sui et al. 1996, Sui et al. 1998; Huang et al. 1998).
Our present data show that Mg2+ ions, in addition to their involvement in the processes mentioned above, are able to activate the ATP- or PIP2-modified G protein–sensitive channel (Fig. 3 and Fig. 7). In the presence of PIP2, similar concentrations of Mg2+ and Na+ ions yielded comparable levels of channel activity, suggesting equivalent gating abilities for both ions. Since PIP2 mimics the MgATP effects on the channel, we have been able to study directly Mg2+ gating effects.
Mutation of the amino acid responsible for Na+-ion activation of GIRK channels did not interfere with Mg2+-ion activation. This result strongly suggests that Mg2+ and Na+ ions act on distinct sites to gate the channel.
Our data also show that Mg2+ ions reduced the conductance of the G protein–gated channels in a manner independent of their stimulatory effect on gating. Since this inhibitory effect of Mg2+ ions on conductance was present at negative potentials (−120, −90, and −80 mV), where no rectification is occurring (Kurachi et al. 1992), it is unlikely that the two processes proceed through a single mechanism. This effect of partial block on channel conductance suggests that Mg2+ ions act at a site located very near the pore. Chuang et al. 1997 described a chronic inhibition of the IRK3 inward rectifier channel by internal Mg2+ ions, which is independent of the rectification process and is voltage independent. However, the on and off rates of this inhibition were slow (in the minute range) and no reduction of the single-channel conductance was reported. Under our conditions, the blocking effect of Mg2+ ions occurred much more rapidly, in the range of seconds (Fig. 6).
Synergism Among G Proteins and Ions in the Gating of GIRK Channels
At higher PIP2 concentrations, the combination of Na+ and Mg2+ ions resulted in a stimulation of channel activity that was greater than the sum of their individual effects, suggesting synergistic interactions of these ions on channel gating (Fig. 7 A).
Na+ ions gate the K+ channel in the presence of hydrolyzable ATP or PIP2 (Sui et al. 1996, Sui et al. 1998; Fig. 3 and Fig. 7 A). In the present study, we show that (shortly after patch excision in solutions that do not replenish or supply PIP2) application of Gβγ subunits, but not of Na+ or Mg2+ ions, results in stimulation of channel activity (Fig. 2 and Fig. 7 B). Our previous study (Sui et al. 1998) showed that in the absence of PIP2 in the membrane (e.g., by its complete hydrolysis by exogenous PLCβ2) no gating molecule (e.g., Gβγ or Na+) could activate the channel. In the present experiment under conditions that we do not expect to have depleted PIP2, Gβγ subunits caused a much greater stimulation of activity than Na+ or Mg2+ ions. This result suggests that the dependence on PIP2 for channel gating is greater for Mg2+ and Na+ than for Gβγ. Under these conditions, we find that Na+ ions do stimulate channel activity after preactivation by GTPγS or by purified Gβγ subunits (Fig. 2 and Fig. 7 B). This result suggests that Gβγ activation sensitizes the K+ channel gating to Na+ ions. Moreover, in such experiments, Gβγ subunits and Na+ ions act synergistically in gating the channel. Interestingly, Mg2+ ions were unable to gate the channel after channel preactivation by GTPγS. These data underscore an interesting difference in the gating of this channel by ions, namely at low PIP2 levels Gβγ subunits synergize with Na+ but not Mg2+ ions to gate the channel.
This difference of the two ions on channel gating is lost at higher PIP2 concentrations (Fig. 7 C). In such experiments, not only were the synergistic effects of the ions shown in Fig. 7 A reproduced, but also Mg2+ as well as Na+ ions cooperated with Gβγ. When applied in combination, all three gating particles showed synergistic effects (Fig. 7 C).
We have previously shown that block of the Na+/K+ pump activates KACh in atrial myocytes with kinetics similar to those seen for Na+ accumulation resulting from the block of the pump (Sui et al. 1996). Thus, it is likely that the effects of cardiac glycosides on cardiac rhythm involve the Na+-sensitive KACh channels. Under physiological conditions, local variations of [Na+]i (e.g., during an action potential) and possibly [Mg2+]i could provide a sensitive and fast control of the GIRK channel gating and activity. The synergism among ions and Gβγ subunits implies that variations in the local levels of these molecules could have a profound impact on the dynamic range of GIRK channel activity under normal or pathophysiologic states.
A Gating Model for GIRK Channels
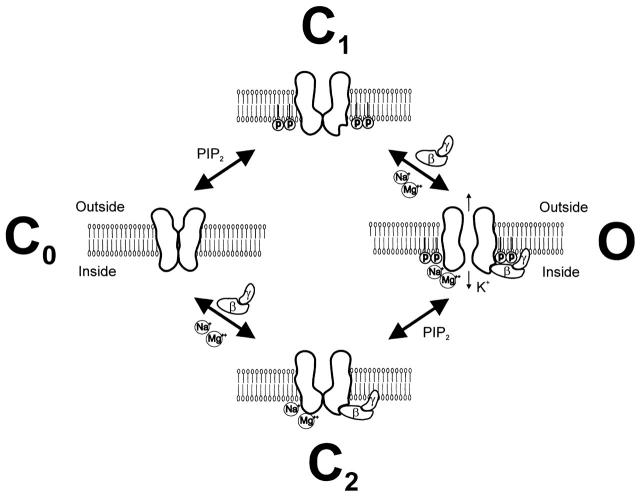
Channel binding sites for PIP2, Gβγ, and Na2+ ions have been identified (Huang et al. 1995; Huang et al. 1997; Kunkel and Peralta 1995; He et al. 1999). We postulate that additional distinct sites exist to completely account for the effects of gating molecules on channel activity. Fig. 8 shows the closed channel state C0 in the absence of PIP2. GIRK channels interact weakly with PIP2, and as a result PIP2 does not directly activate these channels (closed state C1). In the absence of PIP2, gating molecules such as Gβγ, Na+, or Mg2+ are unable to activate the channel (closed state C2). However, in the presence of PIP2, any of the gating molecules can cause channel activation.
Figure 8.
A model depicting gating of GIRK channels by the combination of PIP2 with gating molecules Gβγ and/or Na+ and Mg2+ ions. (Co) Channel closed state, in the absence of PIP2 in the plasma membrane and gating molecules. (C1) Channel closed state, in the presence of PIP2 in the membrane GIRK channels experience weak interactions that in the absence of gating molecules are not of sufficient strength to gate the channel. (C2) Channel closed state, gating molecules can interact with the channel at distinct sites but in the absence of PIP2 they fail to gate the channel. (O) Channel open state, gating molecules in the presence of membrane PIP2 can activate the channel and show synergism.
We envision two possible mechanisms for the synergistic action of gating molecules to activate the channel. Ions and Gβγ subunits maybe exerting their combined effects by synergistic interactions of channel sites with PIP2. Published reports have already suggested a stronger interaction of channel with PIP2 in the presence of either Gβγ subunits or Na+ ions (Huang et al. 1998; Zhang et al. 1999) Alternatively, the gating molecules could be inducing conformational changes, affecting gating directly, independently of PIP2 interactions. Although PIP2 is absolutely required for gating molecules to be effective, we have seen that at low PIP2 concentrations Gβγ, unlike Na+ or Mg2+ ions, can still gate the channels. This result suggests a stronger influence of Gβγ than of Na+ or Mg2+ ions on channel gating, possibly proceeding in a PIP2-independent manner. Further work will be required to distinguish between these possibilities.
Acknowledgments
We are grateful to Lidiya Lontsman and Xixin Yan for technical support and preparation of the oocytes. We thank Drs. James Garrison, Eitan Reuveny, and Ravi Iyengar for their kind gifts of Gβ1γ7 subunits, the βARK-PH construct and QEHA peptide, respectively. We also thank Drs. Vladimir Brezina, Sherman Kupfer, Tooraj Mirshahi, Tibor Rohacs, and Hailin Zhang for critical comments on the manuscript.
This work was supported by grants from the Aaron Diamond Foundation (J.L. Sui), the National Institutes of Health (HL54185), and the American Heart Association, National Center (96011620) (D.E. Logothetis).
Footnotes
1used in this paper: ACh, acetylcholine; βARK-PH, β-adrenergic receptor kinase plekstrin homology domain; GIRK channel, G protein–gated inwardly rectifying potassium channel; PIP2, phosphatidylinositol-bis-phosphate
Dr. Sui's present address is Cambridge Neuroscience, Cambridge, MA 02139.
References
- Baukrowitz T., Schulte U., Oliver D., Herlitze S., Krauter T., Tucker S.J., Ruppersberg J.P., Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Chan K.W., Langan M.N., Sui J., Kozak J.A., Pabon A., Ladias J.A.A., Logothetis D.E. A recombinant inwardly rectifying potassium channel coupled to GTP-binding proteins. J. Gen. Physiol. 1996;107:381–397. doi: 10.1085/jgp.107.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., DeVivo M., Dingus J., Harry A., Li J., Sui J.L., Carty D., Blank J.L., Exton J., Stoffel R.H. A region of adenylyl cyclase 2 critical for regulation by G protein βγ subunits. Science. 1995;268:1166–1169. doi: 10.1126/science.7761832. [DOI] [PubMed] [Google Scholar]
- Chuang H., Jan Y.N., Jan L.Y. Regulation of IRK3 inward rectifier K+ channel by m1 acetylcholine receptor and intracellular magnesium. Cell. 1997;89:1121–1132. doi: 10.1016/s0092-8674(00)80299-8. [DOI] [PubMed] [Google Scholar]
- Corey S., Krapivinsky G., Krapivinsky L., Clapham D.E. Number and stoichiometry of subunits in the native atrial G protein–gated K+ channel, IKACh . J. Biol. Chem. 1998;273:5271–5278. doi: 10.1074/jbc.273.9.5271. [DOI] [PubMed] [Google Scholar]
- Fan Z., Makielski J.C. Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Flanagan L.A., Cunningham C.C., Chen J., Prestwich G.D., Kosik K.S., Janmey P.A. The structure of divalent cation–induced aggregates of PIP2 and their alteration by gelsolin and tau. Biophys. J. 1997;73:1440–1447. doi: 10.1016/S0006-3495(97)78176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- He C., Zhang H., Mirshahi T., Logothetis D.E. Identification of a potassium channel site that interacts with G protein βγ subunits to mediate agonist-induced signaling. J. Biol. Chem. 1999;274:12517–12524. doi: 10.1074/jbc.274.18.12517. [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W., Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2 . Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Ho I.H.M., Murrell-Lagnado R.D. Molecular determinants for sodium-dependent activation of G protein–gated K+ channels. J. Biol. Chem. 1999;274:8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- Huang C., Feng S., Hilgemann D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Huang C.L., Jan Y.N., Jan L.Y. Binding of the G protein βγ subunit to multiple regions of G protein–gated inward-rectifying K+ channels. FEBS. Lett. 1997;405:291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- Huang C.L., Slesinger P.A., Casey P.J., Jan Y.N., Jan L.Y. Evidence that direct binding of Gβγ to the GIRK1 G protein–gated inwardly rectifying K+ channel channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium.”. Pflügers Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Koch W.J., Inglese J., Stone W.C., Lefkowitz R.J. The binding site for the βγ subunits of heterotrimeric G proteins on the β-adrenergic receptor kinase. J. Biol. Chem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- Krapivinsky G., Gordon E.A., Wickman K., Velimirovic B., Krapivinsky L., Clapham D.E. The G protein–gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins Nature. 374 1995. 135 141a [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Krapivinsky L., Wickman K., Clapham D.E. Gβγ binds directly to the G protein–gated K+ channel, IKACh J. Biol. Chem. 270 1995. 29059 29062b [DOI] [PubMed] [Google Scholar]
- Kunkel M.T., Peralta E.G. Identification of domains conferring G protein regulation on inward rectifier potassium channels. Cell. 1995;83:443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Role of intracellular Mg2+ in the activation of muscarinic K+ channel in cardiac atrial cell membrane. Pflügers Arch. 1986;407:572–574. doi: 10.1007/BF00657521. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Tung R.T., Ito H., Nakajima T. G protein activation of cardiac muscarinic K+ channels. Prog. Neurobiol. 1992;39:229–246. doi: 10.1016/0301-0082(92)90017-9. [DOI] [PubMed] [Google Scholar]
- Logothetis D.E., Kurachi Y., Galper J., Neer E.J., Clapham D.E. The βγ subunits of GTP-binding proteins activate the muscarinic K channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Logothetis D.E., Movahedi S., Satler C., Lindpaintner K., Nadal-Ginard B. Incremental reductions of positive charge within the S4 region of a voltage-gated K+ channel result in corresponding decreases in gating charge. Neuron. 1992;8:531–540. doi: 10.1016/0896-6273(92)90281-h. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Magnesium gating of the inwardly rectifying K+ channel. Annu. Rev. Physiol. 1991;53:289–298. doi: 10.1146/annurev.ph.53.030191.001445. [DOI] [PubMed] [Google Scholar]
- Nichols C.G., Lopatin A.N. Inward rectifier potassium channels. Annu. Rev. Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Reuveny E., Slesinger P.A., Inglese J., Morales J.M., Iniguez-Lluhi J.A., Lefkowitz R.J., Bourne H.R., Jan Y.N., Jan L. Y. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Shyng S.L., Nichols C.G. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Silverman S.K., Lester H.A., Dougherty D.A. Subunit stoichiometry of a heteromultimeric G protein–coupled inward-rectifier K+ channel. J. Biol. Chem. 1996;271:30524–30528. doi: 10.1074/jbc.271.48.30524. [DOI] [PubMed] [Google Scholar]
- Stühmer W. Electrophysiological recording from Xenopus oocytes. Methods Enzymol. 1992;207:319–339. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- Sui J.L., Chan K.W., Logothetis D.E. Na+ activation of the muscarinic K+ channel by a G protein–independent mechanism. J. Gen. Physiol. 1996;108:381–391. doi: 10.1085/jgp.108.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J.L., Petit-Jacques J., Logothetis D.E. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. USA. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M.B., Arnoult C., Villaz M. Skeletal muscle ATP-sensitive K+ channels recorded from sarcolemmal blebs of split fibersATP inhibition is reduced by magnesium and ADP. J. Membr. Biol. 1991;122:165–175. doi: 10.1007/BF01872639. [DOI] [PubMed] [Google Scholar]
- Zhainazarov A.B., Ache B.W. Effects of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate on a Na+-gated nonselective cation channel. J. Neurosci. 1999;19:2929–2937. doi: 10.1523/JNEUROSCI.19-08-02929.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., He C., Yan X., Mirshahi T., Logothetis D.E. Specific PIP2 interactions with inwardly rectifying K+ channels determine distinct activation mechanisms. Nat. Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]