Abstract
The light-dependent K conductance of hyperpolarizing Pecten photoreceptors exhibits a pronounced outward rectification that is eliminated by removal of extracellular divalent cations. The voltage-dependent block by Ca2+ and Mg2+ that underlies such nonlinearity was investigated. Both divalents reduce the photocurrent amplitude, the potency being significantly higher for Ca2+ than Mg2+ (K 1/2 ≈ 16 and 61 mM, respectively, at Vm = −30 mV). Neither cation is measurably permeant. Manipulating the concentration of permeant K ions affects the blockade, suggesting that the mechanism entails occlusion of the permeation pathway. The voltage dependency of Ca2+ block is consistent with a single binding site located at an electrical distance of δ ≈ 0.6 from the outside. Resolution of light-dependent single-channel currents under physiological conditions indicates that blockade must be slow, which prompted the use of perturbation/relaxation methods to analyze its kinetics. Voltage steps during illumination produce a distinct relaxation in the photocurrent (τ = 5–20 ms) that disappears on removal of Ca2+ and Mg2+ and thus reflects enhancement or relief of blockade, depending on the polarity of the stimulus. The equilibration kinetics are significantly faster with Ca2+ than with Mg2+, suggesting that the process is dominated by the “on” rate, perhaps because of a step requiring dehydration of the blocking ion to access the binding site. Complementary strategies were adopted to investigate the interaction between blockade and channel gating: the photocurrent decay accelerates with hyperpolarization, but the effect requires extracellular divalents. Moreover, conditioning voltage steps terminated immediately before light stimulation failed to affect the photocurrent. These observations suggest that equilibration of block at different voltages requires an open pore. Inducing channels to close during a conditioning hyperpolarization resulted in a slight delay in the rising phase of a subsequent light response; this effect can be interpreted as closure of the channel with a divalent ion trapped inside.
Keywords: photoreceptors, K+ channels, channel block, rectification
INTRODUCTION
Many ion channels found in a variety of cell types exhibit nonlinear conduction properties that arise from voltage-dependent block by extracellular divalent cations. Examples include nicotinic acetylcholine-receptor channels (Ifune and Steinbach 1991), L-type Ca2+ channels (Lux et al. 1990; Kuo and Hess 1993), N-methyl-d-arginine–receptor channels (Nowak et al. 1984), histamine-operated cationic channels (Yamamoto et al. 1992), and cAMP-activated channels (Zufall and Firestein 1993). In vertebrate photoreceptors, extracellular calcium and magnesium also exert a voltage-dependent blocking effect on the cGMP-gated conductance that underlies visual transduction (Yau et al. 1986; Haynes 1995). This phenomenon confers to the current–voltage (I–V)1 relation of the light-sensitive current a pronounced outward rectification under physiological conditions (Bader et al. 1979). A number of detailed investigations have appeared, addressing the mechanisms of blockage of the cGMP-dependent channels in excised membrane patches of vertebrate photoreceptors, and a complex picture has emerged that includes interactions of divalent effects with the cyclic nucleotide concentration and the gating machinery (Colamartino et al. 1991; Karpen et al. 1993) and differences between rods and cones (Picones and Korenbrot 1995). The binding and unbinding of divalents to the cGMP-activated channels of rods is extremely fast, causing the current through individual open channels to fluctuate at high frequency (Haynes et al. 1986). Because rapid transitions are filtered out by the membrane impedance, the net result is a reduction of the effective single-channel conductance (Bodoia and Detwiler 1984; Gray and Attwell 1985), which has been suggested to serve the purpose of lowering the dark noise and, consequently, to enhance the detection of faint stimuli (Yau and Baylor 1989). By the same token, the rapidity of the phenomenon makes it challenging to characterize its kinetics.
The light-sensitive potassium conductance (gL) of hyperpolarizing (ciliary) photoreceptors in the retina of the scallop, Pecten irradians, shares significant similarities with that of vertebrate photoreceptors, including activation by cGMP (Gomez and Nasi 1995) and susceptibility to blockade by l-cis-diltiazem and 3,4-dichlorobenzamil (Gomez and Nasi 1997a). As in rods, the I–V relation also rectifies in the outward direction because of voltage-dependent blockade by extracellular divalent cations (Gomez and Nasi 1994a). However, light-activated single-channel currents can still be measured in the presence of Ca2+ and Mg2+ (Gomez and Nasi 1994a) without a reduction in their apparent amplitude, indicating a slow block/unblock interaction on the order of milliseconds. In the present report, we have taken advantage of this favorable situation to resolve the blocking and unblocking kinetics of the whole-cell photocurrent, and examine interactions between blocking by divalents and the gating process. These functional observations suggest some constraints on the topological organization of the channel pore and gate.
MATERIALS AND METHODS
Specimens of Pecten irradians were obtained from the Aquatic Resources Division of the Marine Biological Laboratory and used immediately. Retinas were dissected and enzymatically dissociated, as previously described, to yield solitary ciliary photoreceptors (Nasi and Gomez 1992; Gomez and Nasi 1994a). Cells were plated in a low-volume recording chamber (≈200 μl) continuously perfused with a solution that could be exchanged via a system of reservoirs and manifolds. The composition of artificial sea water (ASW) and of the other extracellular solutions is listed in Table . Junction potential changes were measured and compensated. All experiments were conducted at room temperature (≈22°C). Patch electrodes used for whole-cell recording were fabricated with thin-wall (1.5-mm o.d., 1.1-mm i.d.) borosilicate capillary tubing (7052; Garner Glass) pulled to a 2–3-μm outer tip diameter and fire-polished immediately before use. The “intracellular” filling solution contained 100 mM KCl, 200 mM K-aspartate or K-glutamate, 22 mM NaCl, 5 mM Mg ATP, 10 mM HEPES, 1 mM EGTA, 100 μM GTP, and 300 mM sucrose, pH 7.3. Electrode resistance, measured in ASW, was 2–4 MΩ. In all recordings series resistance was compensated via a positive feedback circuit in the amplifier (maximal residual error typically <2 mV). Whole-cell currents were low-pass filtered with a Bessel four-pole filter, using a cutoff frequency of 500–2,000 Hz, and digitized online at 2–5 kHz sampling rate by a 12-bit resolution analogue/digital interface board (2821; Data Translation). For single-channel recordings, finer electrodes (tip diameter < 2 μm, 8–12 MΩ resistance) were fabricated with thick-wall glass (1.5-mm o.d., 0.75-mm i.d.) and filled with either normal ASW or high-K ASW; the cutoff frequency and the sampling rate used for unitary current measurements were 5 and 10 kHz, respectively. Voltage and light stimuli were applied by a microprocessor-controlled programmable stimulator (Stim 6; Ionoptix).
Table 1.
Bath Solutions
| Solution | NaCl | KCl | CaCl2 | MgCl2 | HEPES |
|---|---|---|---|---|---|
| ASW | 480 | 10 | 10 | 49 | 10 |
| 0-Divalent ASW | 570 | 10 | — | — | 10 |
| 2 Ca ASW | 567 | 10 | 2 | — | 10 |
| 10 Ca ASW | 555 | 10 | 10 | — | 10 |
| 30 Ca ASW | 525 | 10 | 30 | — | 10 |
| 60 Ca ASW | 480 | 10 | 60 | — | 10 |
| 60 Mg ASW | 480 | 10 | — | 60 | 10 |
| High K ASW | 440 | 50 | 10 | 49 | 10 |
| High K/ 0-divalent | 530 | 50 | — | — | 10 |
| High K/2 Ca | 527 | 50 | 2 | — | 10 |
| High K/10 Ca | 515 | 50 | 10 | — | 10 |
| High K/60 Ca | 440 | 50 | 60 | — | 10 |
| High K/60 Mg | 440 | 50 | — | 60 | 10 |
Flashes and steps of broad-band light (515–650 nm) were provided by a 100-W tungsten-halogen optical stimulator whose output beam was combined with that of the microscope illuminator via a beam splitter prism placed above the condenser, as previously described (Nasi 1991a,Nasi 1991b; Nasi and Gomez 1992). Light intensity is expressed in terms of equivalent photon flux at 500 nm, as determined by an in vivo calibration (Gomez and Nasi 1994a, Gomez and Nasi 1995) in which responses to broad-band illumination were matched to photocurrents elicited by monochromatic stimuli (500-nm peak, 10-nm half width; Ditric Optics); the intensity of monochromatic light was measured with a radiometer (370; UDT). Calibrated neutral-density filters (Melles Griot) provided controlled light attenuation. During experimental manipulations, the cells were viewed with the aid of a Newvicon TV camera (WV-1550; Panasonic) using a near-IR long-pass filter for illumination (λ > 780 nm; Andover Corp.). The infrared illuminator was turned off for several minutes before testing light responses.
RESULTS
Divalent Cations Block gL with Negligible Permeation
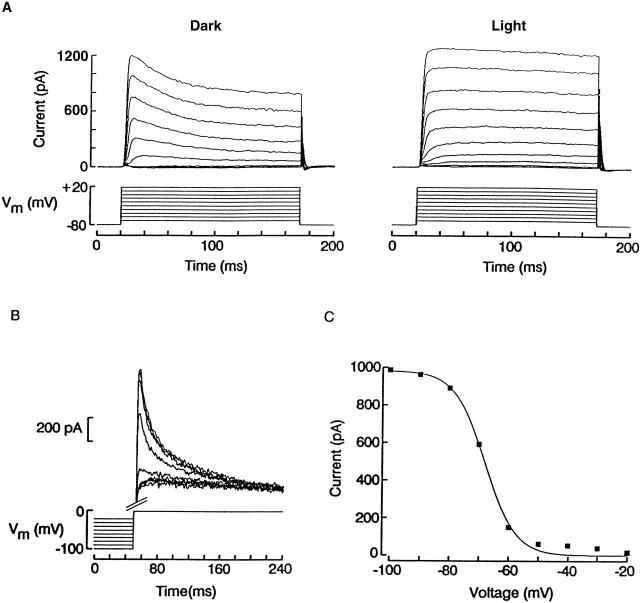
In vertebrate photoreceptors, the light-sensitive conductance is poorly selective among cations, and exhibits a substantial permeability to calcium ions (Capovilla et al. 1983; Hodgkin et al. 1984), which carry a significant fraction of the dark current under physiological conditions (Yau and Nakatani 1985; Nakatani and Yau 1988; Perry and McNaughton 1991; Haynes 1995). “Blockade” by Ca2+ reflects the prolonged dwell times in the permeation pathway (presumed to be single file) due to tight binding. In ciliary invertebrate photoreceptors, by contrast, the contribution of divalents to the photocurrent is expected to be negligible because the reversal potential is near EK and changes with a near-perfectly Nernstian dependency upon manipulating [K]o, even in the presence of normal concentrations of extracellular Ca2+ and Mg2+ (Gomez and Nasi 1994a). The experiments illustrated in Fig. 1 were designed to assess in a direct way the permeation of Ca2+ and Mg2+ through light-activated channels. In Fig. 1 A, the membrane potential of a photoreceptor was stepped in 10-mV increments for several seconds before stimulating with a flash of light of fixed intensity; Vm was returned to −30 mV between trials. In normal ASW, the peak amplitude of the photocurrent decreased in a markedly nonlinear way with hyperpolarization, and at −100 mV a barely detectable inward photocurrent was elicited (left). After switching to a solution lacking both calcium and magnesium (middle), the amplitude of the light responses significantly increased, especially at hyperpolarized voltages. In particular, below the reversal potential, a sizable inward photocurrent could be clearly observed. This effect was fully reversible (right). The I–V relation for the three phases of the experiment, plotted in Fig. 1 B, shows that removal of divalents virtually eliminated the rectification, without any obvious concomitant displacement of the reversal potential, which remained near −80 mV (n = 3). To provide a more sensitive test, additional measurements were conducted in the presence of elevated [K]o (50 mM), as shown in Fig. 1 C. These conditions were designed to displace Vrev to a range in which gL is larger so that the greater slope of the I–V curve improves the signal-to-noise ratio (S/N); in addition, the accuracy of the measurements was increased by changing the holding potential in 2-mV increments to detect any small change in the reversal voltage. As shown in Fig. 1 D, Vrev was not significantly affected by the presence or absence of divalent cations (mean shift 0.8 ± 0.3 mV, n = 3), confirming that the permeability of Ca2+ and Mg2+ must be negligible.
Figure 1.
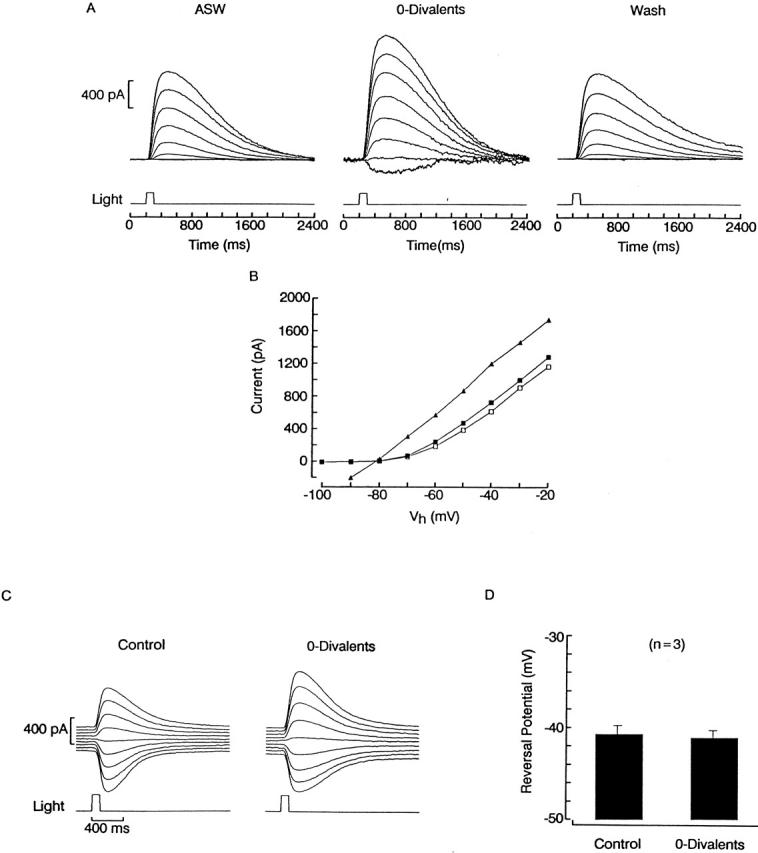
Divalents act like negligibly permeant blockers of the light-dependent conductance. (A) A ciliary photoreceptor was voltage clamped and stimulated with 100-ms flashes of light (9.5 × 1014 photons s−1 cm−2) spaced 1-min apart. On successive trials, the holding potential was stepped in 10-mV increments ≈5 s before the flash. The procedure was carried out in ASW (left), then in divalent-free solution (middle), and back to control (right). At the more negative voltages, light-evoked currents were minute in ASW, but became conspicuous after removal of extracellular Ca2+ and Mg2+. (B) Peak amplitude of the photocurrent plotted as a function of membrane voltage in ASW (▪), 0-divalent solution (▴), and wash (□). Omission of Ca2+ and Mg2+ removed the outward rectification, making the I–V relation essentially linear. (C) Reversal of the photocurrent in high-K solution, in the presence and absence of divalents. Membrane voltage was varied in 2-mV increments between −32 and −48 mV; light intensity was 2.4 × 1014 photons s−1 cm−2. (D) Lack of shift in Vrev upon removing extracellular Ca2+ and Mg2+. Data from three photoreceptor cells tested as in C were pooled; error bars indicate standard deviation.
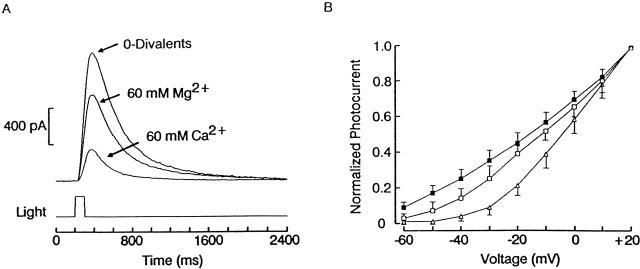
To determine whether both Ca2+ and Mg2+ are capable of blocking the channel, we tested each divalent cation individually. Fig. 2 A shows photocurrents elicited at −30 mV by repetitive flashes delivered initially in 0-divalents extracellular solution, and subsequently after introducing either 60 mM Ca2+ or Mg2+. The amplitude of the light response was reduced in both cases, but the effect was significantly greater for calcium (82% ± 3%, n = 5) than for magnesium (31% ± 6%, n = 3). The average normalized I–V curves measured between −60 and +20 mV in the three conditions is shown in Fig. 2 B (n = 5 per group). Each test was preceded and followed by a control flash at −30 mV; cells that failed to satisfy the criterion of <5% change between the pre- and post-test responses were discarded. It is readily apparent that, although Mg2+ induced a significant curvature in the I–V relation (especially evident at Vm < −40 mV), the outward rectification induced by Ca2+ was far more pronounced. The greater relative potency of Ca2+ vs. Mg2+ was also corroborated by the observation that, when the solution is changed from normal to 0-Ca ASW (by substituting Ca2+ with Mg2+ on an equimolar basis), the photocurrent amplitude is increased and the outward rectification is substantially attenuated (n = 3; data not shown); conversely, a switch from normal to 0-Mg ASW (60 Ca2+) leads to a reduction in the photoresponse (n = 4).
Figure 2.
Comparison of the blockade of the photocurrent by Ca2+ and by Mg2+. A photoreceptor was voltage clamped at −30 mV and stimulated repetitively with 100-ms flashes (2.4 × 1014 photons s−1 cm−2) delivered every minute while superfusing with 0-divalents ASW, or in the presence of 60 mM Mg2+ or 60 mM Ca2+. Both divalents reduced the light response amplitude, but the effect was substantially greater for calcium. (B) Rectification of the photocurrent induced by Ca2+ or by Mg2+. The I–V current for the light-evoked current was measured in 10-mV increments in 0-divalents ASW (▪), 60 mM Mg2+ (□), or 60 mM Ca 2+ (▵). Each point represents the average of five cells: peak current amplitudes for each cell were normalized with respect to the value measured at +20 mV; error bars represent standard deviation. The same light intensity as in A was used throughout.
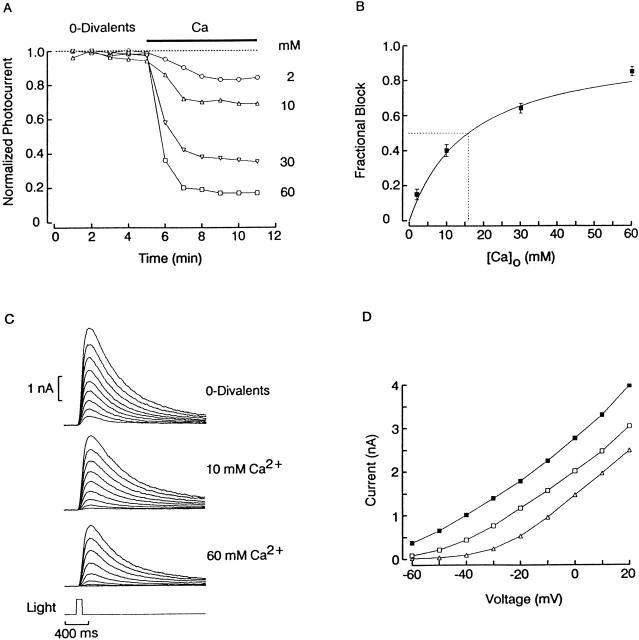
The apparent affinity of Ca2+for the channel was determined as illustrated in Fig. 3. Photoreceptors were voltage clamped at −30 mV and stimulated with repetitive flashes of constant intensity, initially in the absence of all divalents, and then after introducing either 2, 10, 30, or 60 mM Ca2+ (0-Mg). For comparison purposes, responses were normalized with respect to the maximum amplitude attained during the control trials in 0-divalents. Calcium depressed the light-evoked current in a concentration-dependent way. To quantify the dose dependency of the blockade, data from 12 cells tested with the same light intensity were pooled and plotted in Fig. 3 B. The smooth curve represents a Langmuir function fitted to the average data points by the method of least squares; half-maximal block was attained at ≈16 mM. A Hill function provided no better fit, and the resulting Hill coefficient was not significantly different from 1 (0.91), suggesting that a single calcium ion blocks the channel. Similar measurements were conducted at 0 mV, and the obtained K 1/2 was ≈61 mM for calcium. The corresponding estimate for Mg2+ would be difficult to obtain because of its lower affinity: 60 mM Mg2+ only reduced the photocurrent by 12.5% (n = 2), so that an extrapolated figure would be >>100 mM. The remaining parts of Fig. 3 illustrate the concentration-dependent induction of outward rectification in the photocurrent by extracellular Ca2+. In Fig. 3 C, families of light-evoked currents were measured as the holding potential was stepped from −60 to +20 mV in 10-mV increments; the experiment was conducted initially in divalent-free solution, and subsequently repeated in the presence of 10 and 60 mM Ca2+ (in the same cell). As [Ca2+]o was raised, the size of the currents evoked at the more negative voltages became compressed. The peak amplitude of the responses, plotted in D, clearly illustrates the progressively increasing curvature in the I–V relation. Comparable dose-dependent effects were observed in another photoreceptor tested with 0, 2, and 10 mM Ca2+, and 10 additional cells in which the 0-divalent solution was compared with one fixed calcium concentration, in the range of 2–60 mM.
Figure 3.
Concentration dependence of the photocurrent blockade by extracellular Ca2+. (A) Normalized peak amplitude of the light response measured at −30 mV in different photoreceptors. After four flashes, the solution was switched from 0-divalents to the indicated concentration of calcium. (B) Average blockade of the photocurrent obtained from 12 cells, each tested in 0-divalents and in one concentration of Ca2+. A Langmuir function was fitted to the data point by the method of least squares; the half-maximal blockade was attained at 16 mM Ca2+. (C) Graded outward rectification induced by two different Ca2+ concentrations in the same cell. Photocurrents were recorded at potentials varying from −60 to +20 mV in 10-mV increments in each of the solutions indicated. (D) plots for the data shown in C, illustrating the progressively greater outward rectification as Ca2+ concentration was increased. Light intensity: 2.4 × 1014 photons s−1 cm−2.
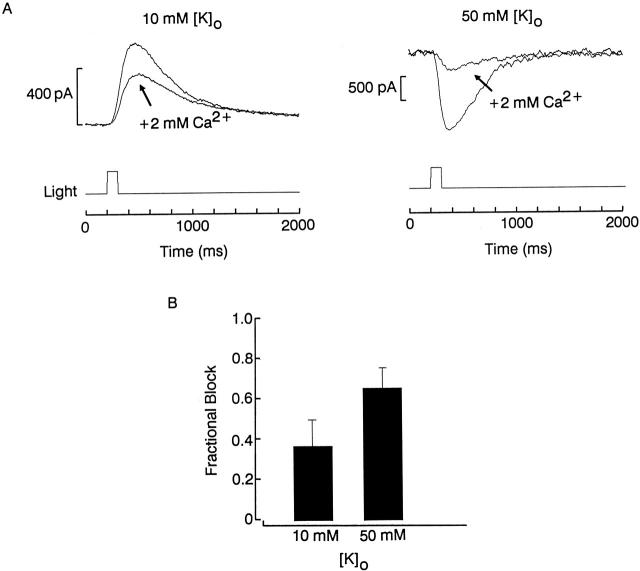
To help clarify the site of action of calcium ions, we ascertained possible interactions between blockade and potassium permeation; for example, occlusion of the pore may be alleviated by a knock-off mechanism (Armstrong 1971; Yellen 1984). We addressed this possibility by determining the degree of photocurrent reduction induced by a fixed [Ca2+]o at a steady membrane potential, as the concentration of extracellular potassium was manipulated. Fig. 4 A illustrates a comparison between the responses elicited by a flash of constant intensity at −60 mV, in 0-divalents extracellular solution and after introducing 2 mM Ca2+; the left traces were obtained in the presence of normal [K]o (10 mM), whereas those on the right were recorded with elevated [K]o (50 mM), which made the photocurrent inwardly directed. The extent of blockade was noticeably greater when measured in high-K solution. The histogram in Fig. 4 B shows the average blockade for cells tested under the two conditions (n = 3 and 2). This result suggests a site of block by divalent cations that is located within the permeation pathway of the light-dependent channels.
Figure 4.
Interaction between calcium blockade and potassium permeation in the light-sensitive conductance. (A) A cell was voltage clamped at −60 mV and stimulated with a 100-ms flash while superfused either with divalent-free solution or after introducing 2 mM Ca2+. (Left) Both extracellular solutions contained the normal potassium concentration (10 mM), whereas (right) the test was performed with elevated [K]o (50 mM), hence the inwardly directed photocurrents. Calcium induced a more pronounced reduction of the light response when it was added to the high-potassium solution. Light intensity 6.1 × 1013 photons s−1 cm−2 (left) and 6.9 × 1014 photons s−1 cm−2 (right). (B) The histogram shows the average blockade for cells tested under the two conditions (n = 3 and 2, respectively).
Single-Channel Recordings Suggest Slow Blockade
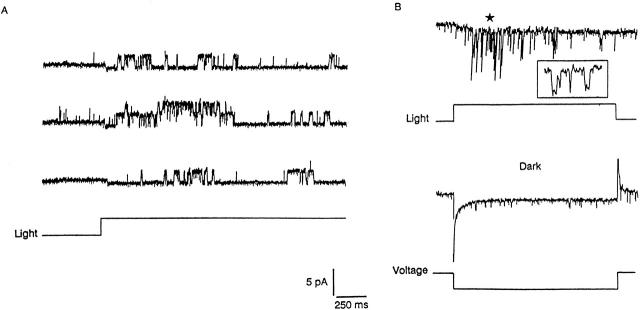
Unlike in vertebrate rods, in Pecten ciliary photoreceptors, light-dependent single-channel currents can be resolved in the presence of normal concentrations of extracellular Ca2+ and Mg2+ (Gomez and Nasi 1994a). Fig. 5 A illustrates an example obtained in a cell-attached patch from a ciliary appendage, with the pipette filled with normal ASW and depolarized by 90 mV to produce a suitable driving force. Steps of light lasting for 2 s repeatedly administered at 1-min intervals elicited distinct outward single-channel currents. Because the intensity of the light was saturating, the receptor potential may have approached −80 mV at the peak of the response (Gorman and McReynolds 1978), bringing the absolute voltage across the patch somewhat positive of 0 mV. At that membrane potential, the effect of divalents is inevitably greatly reduced (see Fig. 1 A and 3 D); therefore, additional measurements were performed at more negative voltages where blockade is far more substantial. To that end, a pipette solution containing elevated potassium (490 mM, replacing Na on an equimolar basis) was used; the resulting estimated value of E K is near +12 mV, which ensures a sizable inwardly directed driving force on K ions over the voltage range of interest. Fig. 5 B shows that with Vp set at −30 mV, light-activated single-channel currents could still be recorded. Application of a hyperpolarizing voltage step in the dark to mimic the receptor potential failed to evoke any openings, confirming that channel activation was not a consequence of the change in Vm. Two additional patches tested under the same conditions yielded similar results; when light stimulation was delivered at Vp = 0 mV (i.e., with the physiological potential across the patch), unitary currents were again clearly resolved.
Figure 5.
Light-dependent single-channel currents are resolvable in the presence of divalent cations. (A) Example of cell-attached recording in the ciliary appendages of a hyperpolarizing photoreceptor cell, under normal ionic conditions. The pipette contained ASW and was depolarized by −90 mV. A step of light (3 × 1016 photons s−1 cm−2) repeatedly applied for 2 s at 1-min intervals elicited outward channel openings; no activity was observed in the dark. (B) Light-evoked unitary currents can also be resolved at negative potentials, provided that conditions are arranged to ensure a suitable driving force. The recording was obtained at −30 mV electrode potential, with a filling solution containing 490 mM KCl (in addition to the normal concentration of divalent cations). A step of light (4.4 × 1014 photons s−1 cm−2) evoked distinct channel openings in the inward direction (top). (Inset) A 40-ms stretch of recording, marked by the star, is shown on an expanded time scale to illustrate the lack of rapid flickering. A 50-mV hyperpolarizing voltage-step applied in the dark to mimic the receptor potential produced no channel events (bottom).
The following considerations suggest that in Pecten divalent blockade of light-dependent channels must be sluggish: at negative voltages, where blockade is strongest, the unitary conductance (28.6 ± 1.2 pS, n = 3) was not lower than the value previously reported for a more depolarized range (27 pS; Gomez and Nasi 1994a). The implication is that the block–unblock interactions between channels and divalent cations must be slower than the recording bandwidth, otherwise the apparent amplitude of the open-channel current would have decreased as the blockade increased. A direct comparison in the presence vs. complete absence of divalents could not be carried out because in the latter case seals were invariably poor, and the resulting noise level precluded accurate resolution of single-channel currents; it was possible, nevertheless, to do so with just 1 mM Mg2+ in the electrode, and a similar conductance value was again obtained (29 ± 1.2 pS, n = 3). Finally, although the obtained data were insufficient for a quantitative analysis of open times distributions (partly because of the presence of multiple channels with frequent overlapping events), it is clear that at negative potentials channel events were briefer (i.e., reduced P o; compare Fig. 5A and Fig. B), most likely a reflection of the enhancement of divalent block; however, the currents did not exhibit rapid flickering (see Fig. 5 B, inset). This indicates that the kinetics of block/unblock must be at least on a time scale comparable with that of channel gating (several milliseconds).
In summary, these results point to the conclusion that the slowness of the blockade (milliseconds) should be sufficient to resolve it temporally and to directly examine possible influences of the conformational state of the channels. A detailed analysis using single-channel recording, however, is not feasible for several reasons. (a) Patch clamping onto the light-sensitive ciliary appendages is very challenging owing to their minute size (≈1 μm). (b) Seal resistance rarely exceeds 2 GΩ, so that S/N is often inadequate for high-resolution measurements; furthermore, most patches contain multiple channels, which greatly complicates the analysis. (c) The need to manipulate the concentration of external divalents would pose the additional difficulty of perfusing the recording patch pipette. (d) Unless a second electrode is used to voltage clamp the photoreceptor under study, the membrane potential across the patch will not only be unknown, but will also change during light stimulation, thus affecting the voltage-dependent block by divalents.
Kinetics of Blockade by Extracellular Ca2+ and Mg2+
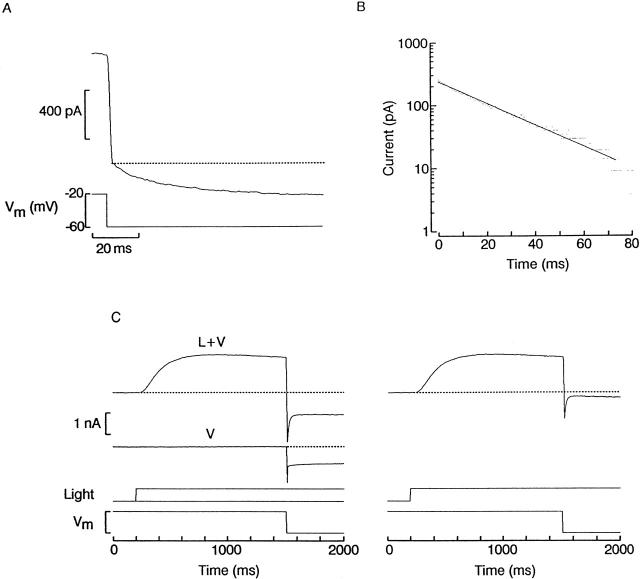
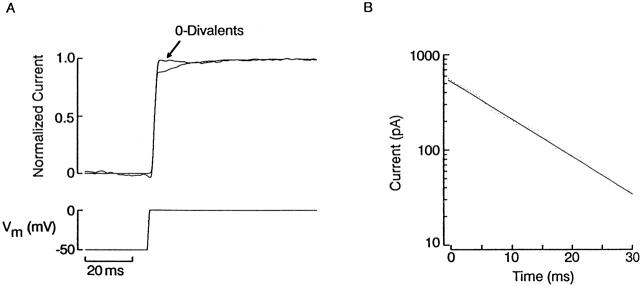
Our experimental approach to characterizing the kinetics of the interaction between divalent cations and the light-dependent conductance consisted of recording whole-cell currents under voltage clamp and applying perturbations to the command voltage during sustained activation of the light-dependent conductance: an abrupt change in Vm will alter the blockade by divalents, and the resulting reequilibration should manifest itself as a resolvable relaxation, provided its kinetics are sufficiently sluggish. The basic phenomenon is shown in Fig. 6 A: a ciliary photoreceptor was voltage clamped at −20 mV in ASW under continuous illumination and the voltage was stepped to −60 mV, causing an abrupt current jump, as one would expect from the sudden reduction in driving force on K+; additionally, however, a slower further decrease in current is clearly visible. In Fig. 6 B, a semi-logarithmic plot of the tail shows that this relaxation obeyed a single-exponential time course with a time constant of 19 ms.
Figure 6.
Relaxation of the membrane current after abrupt hyperpolarization. (A) A photoreceptor was voltage clamped at −20 mV and continuously illuminated (1.5 × 1014 photons s−1 cm−2) to activate a steady photocurrent. The voltage was stepped to −60 mV, causing a sudden current jump, due to the reduction in driving force on potassium ions, followed by a slower further decrease in current amplitude. (B) Semi-logarithmic plot of the relaxation phase, showing that its time course followed an exponential time course. (C) Protocol to analyze voltage-induced time-dependent changes in the photocurrent. (Left) A cell was superfused with high-potassium (50 mM) ASW, and the membrane potential was maintained at 0 mV. A sustained step of light was applied (1.5 × 1014 photons s−1 cm−2) and, when the photoresponse was nearly steady, the holding voltage was stepped to −70 mV (L+V). To assess the contribution of non–light-dependent ionic currents, a similar voltage-step was subsequently administered in the dark (V). This current record was subtracted from the previous one to remove leakage, possible voltage-dependent current, and residual uncompensated capacitative currents. (Right) The result is shown, illustrating the time course of the photocurrent alone in response to the voltage jump. The initial inward transient rapidly decays to a reduced plateau level.
The first issue to be established is whether the observed relaxation is indeed due to time-dependent changes in the current through light-dependent channels. To this end, experiments were designed to remove other possible confounding factors and optimize the resolution of current transients after perturbations of Vm. Under normal ionic conditions, the range of voltages where blockade by external divalents is most pronounced is not far from Vrev and the current is necessarily small. To improve the signal-to-noise ratio, we resorted to increasing [K]o fivefold to 50 mM; because the light-sensitive conductance in these cells behaves like a near-perfect K electrode, this manipulation shifts the reversal potential by ≈40 mV in the positive direction (Gomez and Nasi 1994a). As a result, a significant inwardly directed driving force will exist in the voltage range of interest.
Fig. 6 C illustrates the protocol used to examine in isolation the changes in light-dependent current after a voltage perturbation. A ciliary photoreceptor was voltage clamped at 0 mV in high-potassium ASW and stimulated with a sustained step of light, which elicited an approximately half-saturating outward photocurrent. When the response was nearly stable, the holding voltage was stepped to −70 mV, causing a rapid downward peak followed by a plateau (Fig. 6 C, L+V). This may reflect, in addition to the changes in the current through the light-activated conductance, contributions by leakage and other non–light-dependent ionic currents, as well as residual capacitative transients. To remove these extraneous factors, a similar voltage step was administered in the dark, and the resulting record (V) subtracted from the previous one. The corrected trace, shown in Fig. 6, right, reveals the time course of the photocurrent alone. The hyperpolarizing step induced an inward current that is initially quite large, but rapidly relaxed by ≈1 nA to a small-amplitude plateau (n = 11). Capacitative and non–light-dependent ionic currents were subtracted from all records presented below.
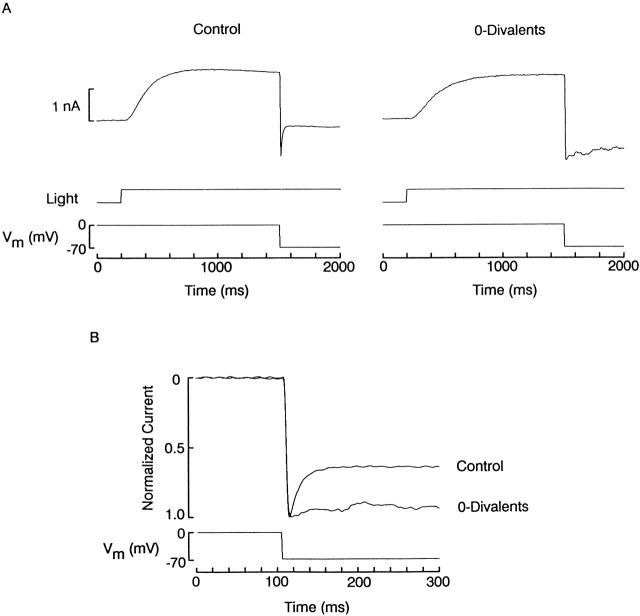
The next step was to determine the relationship between these relaxations and blockade by divalents. To this end, the effects of voltage perturbations applied in the presence vs. absence of extracellular Ca2+ and Mg2+ were compared. Fig. 7 A demonstrates that after removal of divalent cations, a hyperpolarizing step from 0 to −70 mV elicited a downward shift in membrane current that totally lacked the rapid relaxation. The different time course obtained in the two cases is highlighted in Fig. 7 B, which shows the two normalized superimposed traces in an expanded time scale (n = 5).
Figure 7.
Removal of divalent cations eliminates the photocurrent relaxations induced by voltage perturbations. (A) The effects of voltage steps on the current through the light-sensitive channels was compared in the presence (left) and absence (right) of extracellular Ca2+ and Mg2+. After removal of divalent cations, a hyperpolarizing step from 0 to −70 mV elicited a downward shift in membrane current, of comparable amplitude as the control trace, but lacking the rapid relaxation. (B) Normalized, superimposed recordings shown in an expanded time scale, to underscore the disappearance of the relaxation in divalent-free solution. All the current records were obtained in the presence of 50 mM extracellular potassium and were corrected for capacitative and non-light-dependent ionic currents. Light intensity 1.5 × 1014 photons s−1 cm−2.
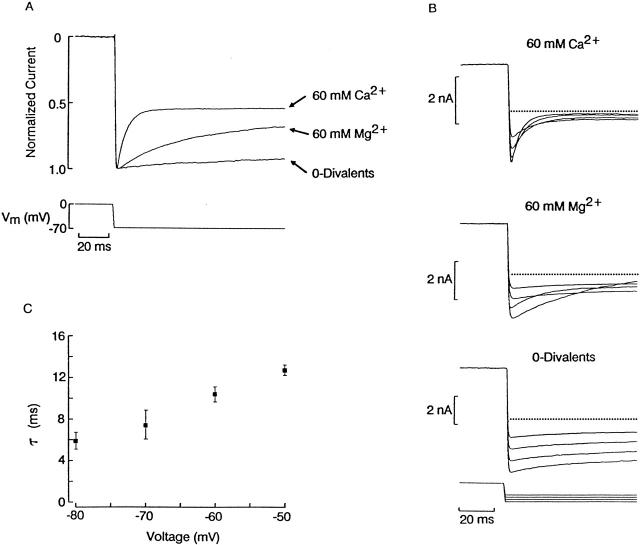
Considering the disparate potency of voltage-dependent block by Ca2+ and Mg2+ at steady holding potentials (Fig. 2), some mechanistic insight can be gained by comparing their respective kinetics by perturbation/relaxation analysis. Fig. 8 A shows the normalized currents evoked by a hyperpolarizing step from 0 to −70 mV during illumination, in a cell that was successively superfused with extracellular solution containing elevated K (50 mM), either devoid of divalents or in the presence of 60 mM Ca2+ or 60 mM Mg2+. As before, in 0-divalents, the photocurrent remained stationary after the voltage perturbation, whereas a conspicuous relaxation occurred in the presence of either divalent cation; the most striking difference, however, is that the time course with calcium (τ = 7 ms) was much faster than with magnesium (τ = 29 ms). In both ionic conditions, the relaxations accelerated as a function of the membrane hyperpolarization: in Fig. 8 B, the voltage was stepped in 10-mV increments between −50 and −80 mV, from a holding potential of 0 mV. It is clear that both the amplitude and the speed of the relaxation are graded with the size of the voltage stimulus, although with Mg2+ these transients remained significantly smaller and slower. By contrast, in 0-divalents, the currents after each step retained a nearly flat time course (except for some slow creep), and their amplitude changed linearly with voltage. These effects were confirmed in a total of five cells (two tested with a shortened protocol). The range of voltages examined could not be extended further, because the applied stimulus had to be significantly more negative than approximately −40 mV to insure a reasonable driving force, but not too large, otherwise membrane breakdown occurs (Gomez and Nasi 1994a), a situation that is exacerbated by removal of divalents (notice the noisy traces in Fig. 1 A and 7 B). The progressive shortening of the relaxation time constant with membrane hyperpolarization in the presence of Ca2+ is illustrated in Fig. 8 C; each point in the plot represents an average value (n = 3). The corresponding data for magnesium (not shown) were more complex in that the sum of two exponential functions was often required to satisfactorily fit the data, with the relative contribution of the two components changing significantly as a function of voltage.
Figure 8.
Comparison of the effects of Ca2+ and Mg2+ on photocurrent changes after hyperpolarizing steps. (A) Superimposed normalized currents evoked by steps of voltage from 0 to −70 mV in 0-divalents, 60 mM Ca2+ or 60 mM Mg2; in each case a 2-s light (2.4 × 1014 photons s−1 cm−2) was turned on 1.3 s before the change in Vm. (B) Effect of varying the size of the voltage step. A different photoreceptor was stimulated with a similar protocol, except that the voltage was stepped to −50, −60, −70, and −80 mV on succeeding trials. In divalent-free solution, the current change after the voltage step had a nearly rectangular time course and an amplitude proportional to the voltage stimulus. In the presence of Ca2+, the current swiftly decayed to a small-amplitude plateau level that was graded with Vm. A qualitatively similar effect occurred with Mg2+, but the effect was much reduced, both in terms of relaxation amplitude and speed. The dotted lines mark the 0-current level. (C) Plot of the time constant obtained by fitting single exponential functions to the relaxations measured in 60 mM Ca2+, in response to steps to different voltages from a holding potential of 0 mV. Each point is the average of three cells; error bars represent standard deviations.
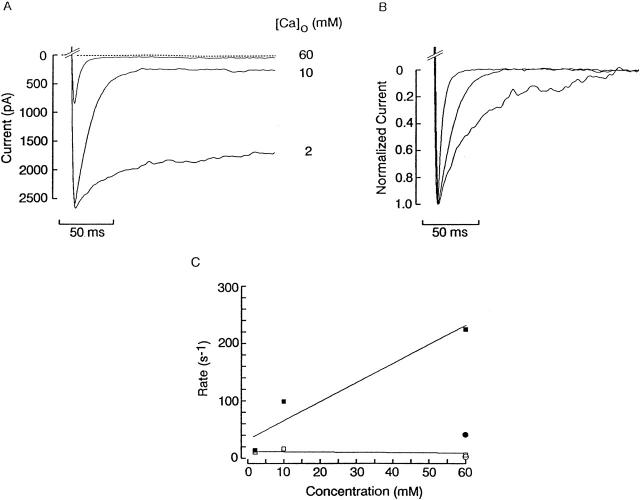
The dependency of the relaxation parameters on the concentration of the blocking ion is illustrated in Fig. 9 A. A photoreceptor was voltage clamped at 0 mV and stimulated with a step to −80 mV; the test was repeated using different concentrations of Ca2+ (2, 10, and 60 mM) as the sole extracellular divalent cation. Increasing calcium had two clear effects: (a) it resulted in a smaller steady state current, and (b) it accelerated the time course of the relaxation. The latter result is highlighted by Fig. 9 B, in which the relaxations measured at different [Ca2+]o were normalized and superimposed. Similar observations were made in three cells. Notice that the initial peak amplitude of the transient was also inversely related to the Ca2+ concentration, owing to the fact that, at the holding voltage of 0 mV, a significant degree of blockade is already present (e.g., ≈50% for 60 mM Ca2+; see Fig. 3 C). In Fig. 9 C, the kinetic rates were estimated after pooling the data obtained across different photoreceptors; assuming one-to-one interaction, these can be derived from the standard relations: τ = 1/(α × [D] + β) and K d = β/α, where τ is the measured time constant of the relaxation, [D] is the divalent concentration, and the apparent K d was determined from the reduction of photocurrent amplitude upon introducing the divalents at a steady Vm (−80 mV). α are β are the forward and reverse rate constants, respectively. As expected, the “off” rate (□) was independent of [Ca2+]o (≈11 s−1), whereas the apparent association rate (▪) increased as a function of the concentration of the blocking ion. The plot also includes the corresponding values obtained with 60 mM Mg2+ (○; average of n = 3). It is noteworthy that the intrinsic “on” rate constant for Mg2+ (≈6.8 × 102 M−1 s−1) was substantially lower than that for Ca2+ (≈3.7 × 103 M−1 s−1 from the fitted line).
Figure 9.
Effect of divalent concentration on the voltage-induced photocurrent transients. (A) Relaxations measured with hyperpolarizing voltage steps from 0 to −80 mV in a photoreceptor cell bathed in high-K solution containing 2, 10, or 60 mM calcium (no Mg2+). The speed and amplitude of the relaxation increased in a manner graded with [Ca2+]o. (B) Normalized traces from A, underscoring the progressive acceleration of the enhancement of blockade induced by perturbations of Vm, as extracellular Ca2+ was raised. Light intensity 3.5 × 1014 photons s−1 cm−2. (C) Apparent association (▪) and dissociation (□) rates for calcium, calculated from the average relaxation time constants and steady state blockade in the presence of the three Ca2+ concentrations (n = 3). The circles represent the corresponding parameters for magnesium, estimated at 60 mM.
To demonstrate the relief of block, symmetrical experiments were conducted in which depolarizing steps were applied from a negative holding voltage, with and without illumination. This procedure, however, requires some caution for the following reason: whereas membrane hyperpolarization elicits no active currents, depolarization can trigger several voltage-dependent mechanisms, including Ca2+ and K channels (Cornwall and Gorman 1979). In principle, none of these would be expected to pose a problem in that the current subtraction protocol should cancel out any contribution by non–light-dependent processes. However, a recent report in distal photoreceptors of a related species of scallop (Patinopecten yessoensis) demonstrated the presence of a transient K current (IA) whose decay kinetics are modulated by light (Shimatani and Katagiri 1995). Fig. 10 A shows that a similar phenomenon also occurs in Pecten irradians: an isolated photoreceptor was voltage clamped at −80 mV and stimulated with depolarizing pulses of increasing amplitude in 10-mV increments. In the dark, voltage steps more positive than −30 mV elicited an outward current consisting of a transient and a sustained component (left); the inactivating current is carried by potassium and blocked by 4-aminopyridine (4-AP, data not shown). When the protocol was repeated in the presence of steady light (right), the time course of the outward current changed markedly, becoming sustained (n = 8). This phenomenon can undermine the validity of subtracting currents elicited by depolarizing pulses in the dark and light because spurious relaxations could be artifactually introduced. Blockade of IA by 4-AP is not a viable option, as we have previously shown that the light-dependent K conductance in these photoreceptor cells is also extraordinarily susceptible to blockade by this drug (Gomez and Nasi 1994b). An alternative strategy is to exploit the steady-state inactivation properties of IA to minimize its contribution: Fig. 9 B shows recordings obtained in the dark in a distal photoreceptor subjected to a 500-ms conditioning prestep to various voltages between −10 and −100 mV, followed by a depolarization to 0 mV. As the conditioning voltage was made more negative, a distinct transient outward current was evoked by the step to 0 mV. In Fig. 9 C, the steady state inactivation data were fitted by a Boltzmann function; the average voltage for half-maximal inactivation was −67 ± 8 mV (n = 4).
Figure 10.
(A) Modulation of the kinetics of the depolarization-activated outward current by light. A photoreceptor was voltage clamped at a holding potential of −80 mV and stimulated with depolarizing steps in 10-mV increments. In the dark (left), part of the outward current is contributed by IA, which confers the characteristic decaying kinetics. In the presence of steady light (2.4 × 1014 photons s−1 cm−2), the time course became sustained (right). (B) Steady state inactivation of the transient component of the outward current in the dark. A ciliary photoreceptor was stimulated by a depolarizing voltage step to 0 mV, preceded by a conditioning step between −10 and −100 mV, lasting for 500 ms (only the last 50 ms are shown). A distinct transient outward current was elicited when the conditioning voltage was substantially negative, but inactivated at more positive voltages. (C) Steady state inactivation curve. The peak amplitude of the outward current at 0 mV from B is plotted as a function of the voltage of the preceding conditioning step. The data points were fitted by a Boltzmann function; the half-maximum inactivation voltage was approximately −67 mV. Recordings were performed in standard ASW.
To examine the kinetics of unblock of the photocurrent, we used cells screened for a particularly small IA, and restricted the holding potential to −50 mV so that contributions by IA were minimized; unfortunately, at that voltage blockade by divalents is also relatively modest and, therefore, the sensitivity of this test is necessarily reduced. Fig. 11 A shows the results of abruptly depolarizing the membrane to 0 mV during sustained activation of the photocurrent; the procedure was conducted first in normal ASW, and then after removal of Ca2+ and Mg2+. In the presence of divalent cations, the voltage jump produced a rapid step increase in the current, reflecting the increase in driving force on K ions, followed by an outward relaxation. As shown by the semi-logarithmic plot in Fig. 11 B, the relaxation had an exponential time course, with a time constant of 8.5 ms. After removal of Ca2+ and Mg2+, the current became rectangular, directly jumping to the asymptotic amplitude. These observations suggest that the relaxation arises from relief of block by divalent cations (n = 6).
Figure 11.
Voltage-induced relief of photocurrent blockade. (A) A photoreceptor stimulated with light (1.5 × 1014 photons s−1 cm−2) was held at −50 mV (where IA is largely inactivated) and Vm was stepped to 0 mV. In normal ASW, the current was biphasic: an abrupt jump, reflecting the increased driving force on potassium, followed by an outward relaxation. After removal of divalents, the relaxation disappeared and the current abruptly attained its steady state amplitude. Traces were normalized. (B) Semi-log plot of the relaxation phase of the current in ASW; the time course was exponential, with a time constant of 8.5 ms.
Blockade Requires an Open Channel
The time-resolved reequilibration of blockade of the light-sensitive conductance by divalent cations, demonstrated in the preceding section, raises the question of whether occupancy of the blocking site(s) is linked to the gating process.
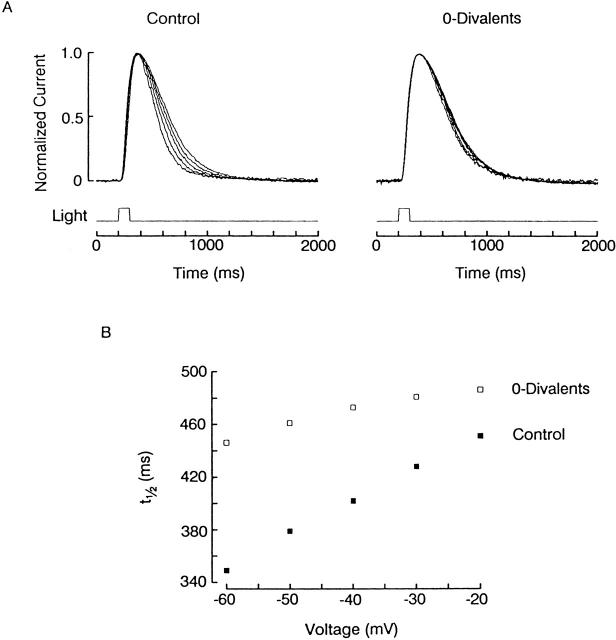
One possibility is that the binding sites become available to external divalents only when the light-dependent channels are in the open conformation. A straightforward prediction from this conjecture is that the kinetics of the light response should be affected by the membrane potential imposed at the time of photostimulation. The rationale is that equilibration will only begin as the light-dependent channels gradually open, so that the extent of blockade at different voltages would become fully manifest in the late phase of the photocurrent: the more negative the Vm, the faster the apparent decay of the response. This prediction is borne out by the data shown in Fig. 12. On the left side of Fig. 12 A, photoresponses to a fixed flash were measured in control conditions (ASW), at holding voltages that varied between −60 and −20 mV in 10-mV increments; between trials, the membrane potential was returned to −30 mV. The records were normalized with respect to their peak amplitude. The light response decayed progressively more rapidly as the holding potential was made more negative. To rule out the possibility that the phenomenon may simply be due to a direct effect of voltage on the gating of the light-sensitive channels, the procedure was repeated after superfusing the same cell with divalent-free solution: under these conditions, the flash responses remained virtually superimposable (Fig. 12 A, right), with a slow time course resembling that of the photocurrent in ASW at a depolarized Vm. Shifting the range of voltages tested in ASW by 20 mV in the depolarizing direction, to check for possible effects of surface charge screening, did not alter the differences across the two ionic conditions (not shown). As a simple measure of time course, the response half-width (i.e., the time elapsed between the two crossings of the half-maximal amplitude level) is plotted for the two conditions in Fig. 12 B. In normal ASW, the half-width of the light responses increased progressively with depolarization, approaching the value obtained in 0-divalents, which remained relatively constant. The near invariance of response kinetics in 0-divalents was corroborated in a total of eight cells; the pronounced acceleration of the time course with hyperpolarization in ASW was observed numerous times (n > 20).
Figure 12.
Voltage-dependent effects of divalents on the photocurrent kinetics. (A, left) Normalized light responses (100-ms flashes, 9.5 × 1014 photons s−1 cm−2), recorded at different holding voltages between −60 and −20 mV, in 10-mV increments. The time course became more rapid with membrane hyperpolarization. (Right) Photocurrents recorded in the same cell after removal of external Ca2+ and Mg2+: the time course became independent of membrane potential and had a kinetics similar to that obtained at the most depolarized voltages in control conditions. (B) Plot of the photoresponse half-width as a function of membrane potential. The change in the presence of Ca2+ and Mg2+ (▪) is substantially more pronounced than after their removal (□). The two tend to converge at the more depolarized range of values.
An alternative approach to testing whether the blockade by divalents interacts with the state of the channel entails applying conditioning voltage steps that are terminated just before the delivery of a light stimulus. If the occupancy of the blocking site by divalents can only change when the channel is in the open conformation (i.e., after photostimulation), then the voltage pre-pulses should have no effect whatsoever. However, if the blocking site is also accessible in the dark (i.e., with the channels closed), then the prepulse should either enhance or depress blockade, depending on the polarity of the stimulus. Such an effect would be expected to linger, owing to the relatively sluggish blocking/unblocking kinetics; as a result, the rising phase of the photocurrent activated immediately after should be affected. Fig. 13 shows the results of an experiment in which a photoreceptor was voltage clamped at a holding potential of 0 mV. The cell was stimulated with a 200-ms voltage step to −70 mV, which terminated immediately before the delivery of a light flash (L+V). A similar voltage step without the flash was also applied (V) to subtract residual leak and capacitative currents. The corrected record was compared with a photocurrent evoked by an identical flash not preceded by the conditioning voltage step (L), as shown in Fig. 13 B: the time course of the two traces is indistinguishable, irrespective of prepulse (n = 5). It should be pointed out that contamination of the rising phase of the light response by the subtraction procedure (owing to possible light-induced changes of IA time course) is negligible here for two reasons. (a) The prestep voltage was chosen to lie near the midpoint of the h∞ curve and its short duration (although >>τ of blockade equilibration) only allows a fraction of the recovery from inactivation that can be attained at that Vm (≈60% of the asymptotic level; data not shown). As a result, IA is reduced by ≈65%. (b) The brief flash followed the voltage transition, precluding the development of any significant modulatory effect on the kinetics of IA.
Figure 13.
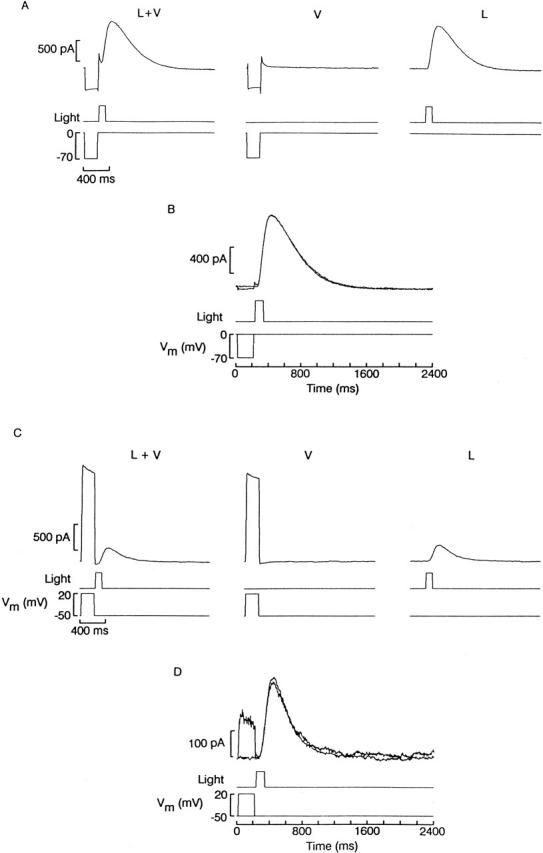
Ineffectiveness of voltage perturbations immediately before photostimulation. (A, left) Membrane current measured in a photoreceptor held at 0 mV and stepped for 200 ms to −70 mV. Just after the termination of the voltage pulse, a flash of light was delivered (100 ms, 5.8 × 1014 photons s−1 cm−2). (Center) Effect of the conditioning voltage pulse alone. (Right) Photocurrent in the absence of the prestep. (B) Comparison of the photocurrent elicited with or without a conditioning depolarizing step. The trace with voltage stimulation was corrected for capacitative and leak current by subtracting the record V from L+V. The two photocurrents follow an identical time course. (C) Similar experiment conducted with depolarizing prepulses. In this case, the photoreceptor was voltage clamped at −50 mV and Vm was briefly stepped to +20 mV. (D) Comparison of the leak-corrected photocurrent with or without the conditioning voltage prestep revealed no significant differences in kinetics.
The converse experiment was also performed, as shown in Fig. 13 C. In this case, the cell membrane was clamped at a more negative holding potential (−50 mV) and a depolarizing step to +20 mV was applied to determine whether blockade could be relieved before the presentation of the light. Again, as shown in Fig. 13 D, the rising phase of the current recorded when the light was present either alone or preceded by the conditioning voltage step were superimposable (n = 7). The results of these experiments complement those presented in Fig. 12 and corroborate the notion that the blocking site is only accessible when stimulation induces a conformational change of the light-dependent channels to the open state.
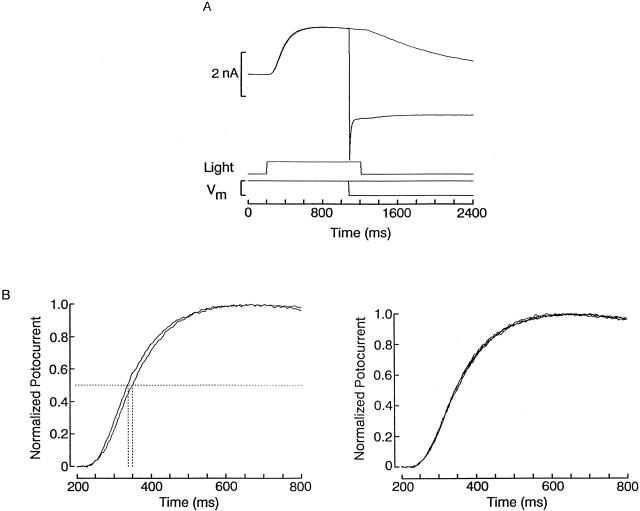
A final question concerns the fate of a blocking ion upon cessation of photostimulation. Either the gate has to wait for the divalent to vacate the site before closing (owing to some steric hindrance) or, alternatively, the channel could close with the divalent bound within the pore. In the latter case, the fact that calcium and magnesium are not measurably permeant precludes the possibility of any significant fluxing to the cytosol, and so the ion would remain trapped. Appropriate tests to reveal either phenomenon are conceptually straightforward, but achieving the necessary sensitivity with a low-affinity blocker can be arduous. For the “foot in the door” case, one would expect the blocker to slow down the falling phase of the photocurrent; however, because this time constant is already on the order of hundreds of milliseconds, the unblock kinetics would be unlikely to make any significant contribution. In case trapping occurs, if one induced the channels to close during strong blockade, the response to a subsequent light delivered under conditions of reduced block would be expected to have a delayed onset, as the blocker would have to leave its site before current can flow. Because in Pecten ciliary photoreceptors the rising phase of the photocurrent elicited by a bright stimulus is swift and highly reproducible, the possibility exists, in principle, that this effect may be detectable. The results of such an experiment are shown in Fig. 14. A photoreceptor was voltage clamped at 0 mV and stimulated every 30 s with a light step lasting 1 s. On alternating trials, the voltage was abruptly stepped to −70 mV during presentation of the light in order to greatly enhance blockade by divalents; the negative Vm was maintained for ≈5 s after light termination, before being gradually returned to the holding level of 0 mV. The subsequent light may thus activate channels while still in a blocked state, and, upon opening, blockade would take milliseconds to reequilibrate at 0 mV. Alternating stimuli either not preceded by the trapping hyperpolarization or in which the hyperpolarizing step ended before the light termination provided a suitable control. Two superimposed traces obtained with this protocol are shown in Fig. 14 A: the photocurrent that had been preceded by a trapping voltage stimulus during the previous light stimulus exhibited a slight temporal lag with respect to the control record. This difference is more clearly visible in B, where the rising phase of the response is shown in an expanded time scale; the phenomenon could be reproduced with successive repetitions of the protocol. In the same cell, control trials in which the light was presented alone gave rise to photocurrents with virtually identical kinetics (C). The effect was observed in six of nine cells tested, and the average temporal lag, measured at half-maximal response amplitude, was 3.6 ± 2.2 ms.
Figure 14.
Demonstration of trapping of blocking divalents within light-sensitive channels. (A) Light steps lasting 1 s were delivered 30-s apart to a photoreceptor cell voltage clamped at 0 mV. During presentation of the first stimulus, the voltage was abruptly stepped to −70 mV until ≈5 s after light termination, after which it was returned to the holding level of 0 mV. The response to the next light exhibits a noticeable delay in its activation. This effect could be replicated by alternating trials with and without the conditioning hyperpolarizing voltage step. (B) Expanded view of the rising phase of the photocurrent, to highlight the temporal lag between the two normalized traces. (C) Superimposed control photocurrents obtained with four repetitions of the light stimulus, without conditioning voltage steps. Light intensity 3.5 × 1014 photons s−1 cm−2.
DISCUSSION
In the present report, we examined the interaction between the light-dependent K conductance of Pecten ciliary photoreceptors and extracellular divalent cations. Ca2+ and Mg2+ confer to the I–V relation of the photocurrent its characteristic outward rectification (Gomez and Nasi 1994a) via a voltage-dependent block mechanism, much like it occurs in vertebrate rods. This causes the light-dependent conductance to increase sigmoidally with membrane depolarization (see Figure 5 D of Gomez and Nasi 1994a). In the present case, the analysis of the blockade was greatly simplified by two fortunate circumstances. (a) Divalents are negligibly permeant, unlike in other cyclic nucleotide–gated channels (CNGC), as demonstrated by the lack of Vrev shift when Ca2+ and Mg2+ were removed from the extracellular solution. To estimate the possible extent of any residual permeation by calcium, we used the constant-field equation and gauged how low P Ca/P K would have to be so that the shift in Vrev upon removal of calcium will fall below the detection limit of our measurements. Considering the minute incremental changes applied to the holding voltage and the very high signal-to-noise ratio and reproducibility of the recordings obtained (Fig. 1C and Fig. D), the reversal voltage could be measured with submillivolt precision. A permeability ratio of 1:10 would predict a shift of 8.4 mV; for a ratio of 1:100, the value would still be in excess of 1 mV. For a shift in Vrev to go undetected under our experimental conditions, Ca ions would have to be on the order of 200-fold less permeable than potassium. Furthermore, in normal ASW, the I–V relation remained flat at voltages as negative as −100 mV, implying that even large hyperpolarizations fail to relieve blockade by pushing the occluding divalent cations through the pore. This significantly reduces complexities, as one only needs to consider the reversible binding from outside, which is determined essentially by two rate constants, and their voltage dependence. (b) The interaction with the channels appears to be substantially slower than in rods, making it possible to apply certain direct approaches to study block kinetics, such as perturbation/relaxation analysis.
We must point out that possible confounding effects of changes in extracellular divalents on the transduction cascade are ruled out, not only because recent measurements with fluorescent indicators confirmed the invariance of [Ca]i in these cells (Gomez and Nasi 1999), but also because the light response was previously shown to be unaffected by direct manipulations of cytosolic Ca2+, which included intracellular application of high concentrations of buffered calcium, as well as superfusion with calcium-free solution and internal dialysis with 1,2-bis-(o-aminophenoxy) ethane-N,N,N ′,N ′-tetraacetic acid (BAPTA; Gomez and Nasi 1995, Gomez and Nasi 1997b).
Concentration and Voltage Dependency of Steady State Block
By introducing either divalent cation individually in the extracellular medium and examining both the reduction of the flash response amplitude at a fixed holding voltage and the curvature imparted on the I–V relation, it was found that Ca2+ blocks the light-sensitive conductance with substantially greater potency than Mg2+. Dose-response analysis showed that at a holding potential of −30 mV half-maximal blockade by Ca2+ is attained in the vicinity of 16 mM, whereas 60 mM Mg2+ only blocked ≈32% of the current at the same potential. Thus, the affinity is quantitatively much lower that in the case of mammalian rod CNGC heterologously expressed in Xenopus oocytes (where Ca2+ and Mg2+ have an effective K 1/2 of ≈1.4 and ≈46 μM at −25 mV, respectively; Root and MacKinnon 1993), and those of amphibian olfactory neurons (≈300 μM for Mg2+ and ≈10 μM for Ca2+ at −50 mV; Zufall and Firestein 1993); however, the relative blocking potency of these two ions is preserved. The dependency of blockade on calcium concentration (Hill coefficient ≈1) is suggestive of a single ion interacting with a channel. The more substantial photocurrent reduction and outward rectification in 10 mM Ca2+, as compared with 60 mM Mg2+ (Fig. 2 A and 3, A and B), indicates that under physiological conditions calcium ions contribute the larger share of the voltage-dependent block of the light-sensitive conductance.
The steady state blockade increased dramatically with hyperpolarization; a Woodhull model was applied to analyze this voltage dependency for the case of calcium, using the equation: I X/I = 1/(1 + [X]exp(zδFV/RT)/K X (Woodhull 1973), where I X and I are the photocurrent amplitude in the presence and absence of blocking ion X, respectively, [X] is the blocker concentration, K X is its apparent affinity at 0 mV, V is the membrane voltage, δ is the fractional electrical distance, and F, R, and T have their usual meanings. The analysis suggests that the blocking site is located at δ ≈ 0.57 through the membrane field, measured from the extracellular side. A requirement to justify such an approach is that the voltage-dependent block of the light-activated conductance by divalent cations not be due to an allosteric effect, such as voltage-induced conformational changes of the channel that may alter the accessibility of the binding site. This contention is supported by the following observations: in the first place, in the absence of extracellular Ca2+ and Mg2+, the I–V relation of the photocurrent is essentially linear (Fig. 1 B); as a consequence, any intrinsic voltage dependency of the gating of these channels is marginal at best. Furthermore, the data shown in Fig. 4 demonstrate that the extent of blockade by calcium is affected by the permeating potassium ions in a manner suggestive of a knock-off phenomenon (Yellen 1984). This strengthens the notion that blockade is likely to involve occlusion of the channel pore.
Resolving Blockade Kinetics
The kinetics of the block were deduced from the effects of rectangular perturbations of the command potential during activation of the light-dependent channels: after the abrupt jump at the onset of the stimulus, a distinct exponential relaxation of the membrane current was observed, provided that Ca2+ or Mg2+ were present in the extracellular medium. For hyperpolarizing pulses, the amplitude of the relaxations increased with the size of the applied voltage stimulus, and correlated with the divalent-induced reduction of the peak photocurrent measured with Vm steadily clamped at the same potentials. A quantitative correspondence was also observed between the relative size of the relaxation in the presence of calcium vs. magnesium, and the degree of suppression of the light response induced by either divalent cation at that fixed membrane voltage. These observations lend support to the notion that the relaxations indeed reflect the time course of enhancement of voltage-dependent block in response to membrane hyperpolarization. For depolarizing pulses, only a cursory analysis could be carried out, because of possible contamination of the photocurrent by a light-modulated fast-inactivating K current over most of the voltage range of interest. Pharmacological separation was not feasible because 4-AP, the antagonist of choice to suppress IA, is also an extremely effective blocker of light-dependent channels in these cells (Gomez and Nasi 1994b). Nevertheless, using a holding potential at which IA is largely inactivated, it could be demonstrated that step depolarization causes the photocurrent to relax in a manner consistent with a relief from blockade. Upon removal of divalent cations, these relaxations disappeared and the current time course became steplike.
A striking difference was observed in the kinetics of the relaxations in presence of Ca2+ vs. Mg2+: with Ca2+ in the bath the time course was substantially faster (average τ ≈ 4.3 ms at −80 mV) than with Mg2+ (τ ≈ 22 ms). For a simple bimolecular interaction in which the on rate is essentially diffusion limited, one would not expect a faster equilibration with the higher affinity blocker. However, the estimated association rates fall greatly below the diffusion limit, suggesting the presence of additional factors. Considering that the binding site appears to lie deep in the channel, the phenomenon may be explained if the steps leading to access to the site are rate limiting, so that the relaxation time constant becomes dominated by the association rate. One possible contributing factor is that the blocking ion may be required to shed its hydration shell; taking into account the higher hydration energy of Mg2+, one may then predict that this ion would equilibrate more slowly. Consistently with this conjecture, estimated on rates were over fivefold greater for calcium than for magnesium.
The slow kinetics of the block by divalents deduced from the analysis of the whole-cell photocurrent are compatible with the observation that in these photoreceptors light-activated single channels can be resolved in the presence of normal concentrations of Ca2+ and Mg2+ (Fig. 5) and their I–V relation appears to be linear (Gomez and Nasi 1994a). If blockade occurred on a faster time scale than that of channel opening or than the recording bandwidth, one would expect either rapid flickering fluctuations or a decrease in the apparent unitary conductance with hyperpolarization (as reported, for example, for the blockade of K channels from the SR channels by Cs; Coronado and Miller 1979). The present results, therefore, contrast with the stronger and faster blockade observed for the CNG conductance of other sensory cells of ciliary origin: both in vertebrate photoreceptors and olfactory neurons omission of Ca2+ and Mg2+ is required to measure single-channel openings (Haynes et al. 1986; Zimmerman and Baylor 1986; Zufall and Firestein 1993). In fact, experiments on rod cGMP-gated channels heterologously expressed in Xenopus oocytes and native cAMP-activated channels from olfactory cells have shown that even after lowering extracellular divalent concentration to micromolar levels the fluctuations are too rapid to resolve, unless large depolarizations are imposed to relieve the voltage-dependent block (Root and MacKinnon 1993; Zufall and Firestein 1993).
Interactions between Blockade and Gating: Implications for Channel Topology
The slowness of the block/unblock process in ciliary photoreceptors afforded the possibility of directly examining the interactions between blockade by divalent cations and gating of the light-dependent conductance. The results of experiments using two complementary strategies suggest that changes in occupancy of the blocking site require the channels to be in the open conformation, which poses a constraint on the location of the gate with respect to the blocking site. In rod excised patches, Karpen et al. 1993 concluded that Ca2+ and Mg2+ may bind similarly to open and closed channels, but other blocking divalents did behave as though their binding site was located more intracellularly than some gating structures. In the present case, the deep estimated location of the divalent cation binding site would conceivably allow for the gating machinery to occupy a more external region of the pore. This, however, diverges from the picture that has emerged for other K+ channels that are activated by voltage (to which CNGCs are presumably related; Guy et al. 1991; Kaupp 1991; Yau 1994), where the gate appears to be located near the intracellular side of the membrane (Armstrong 1971; Liu et al. 1997; Holmgren et al. 1997). The notion that in CNGCs permeation may be controlled by a more externally located structure has been proposed by Sun et al. 1996. Furthermore, recent data show that rod channels expressed in Xenopus oocytes are blocked by tetracaine from the intracellular side preferentially when they are in the closed conformation, and that the binding site is approximately half way through the membrane field (Fodor et al. 1997a,Fodor et al. 1997b), again suggesting a different topology of the gate.
In Pecten ciliary photoreceptors, the proposition that the divalent-binding site lies more deeply than the gate, together with the demonstration that Ca2+ and Mg2+ do not appear to permeate the light-sensitive conductance, raises the possibility that the channels may close with the blocking ion trapped inside. This situation differs with respect to that of rods, where Ca2+ and Mg2+ can flux through the pore into the cytosol, and thereby would not remain trapped upon closure of the gate (see Karpen et al. 1993). Trapping was first proposed by Armstrong 1971 in a classic study of squid K+ channel block by quaternary ammonium compounds. Subsequently, Miller 1987 elegantly demonstrated the phenomenon in single Ca2+-activated K+ channels from t tubules reconstituted into planar bilayers, with Ba2+ serving as an open-pore blocker. Several other instances have been reported, such as N-methyl-d-arginine–receptor channels blocked by various organic antagonists (Huettner and Bean 1988; Blanpied et al. 1997). In the present case, the low affinity blockade posed taxing requirements on the temporal resolution necessary to demonstrate trapping; the situation is exacerbated by spontaneous channel openings in the dark, which, although infrequent, over the lengthy interval interposed between trials (tens of seconds, necessary for dark adaptation) can lead to a gradual loss of divalents remaining in the pore, thus diluting any trapping effect. It is nevertheless noteworthy that a significant fraction of the cells tested exhibited a small but reproducible lag in the rising phase of the light response whenever the preceding photostimulation was accompanied by a hyperpolarizing voltage step designed to promote maximum occupancy of the blocking site. A parsimonious interpretation of this phenomenon is that a blocking divalent cation had remained trapped in the pore.
Functional Significance
It is unlikely that in these cells blockade by divalent cations may serve the purpose of boosting S/N, as has been proposed for rods: in Pecten photoreceptors, like in other invertebrates, light-sensitive channels are closed in the dark (and background noise is therefore already at a minimum), so that detectability of faint stimuli would not benefit from a reduction of the effective single-channel conductance. Furthermore, because the kinetics of block is not rapid, noise variance of Vm during the light response may not be decreased by this mechanism. On the other hand, it is noteworthy that the outward rectification of the photocurrent develops most prominently over the normal operating range of voltages for these cells (McReynolds and Gorman 1970) and may thus be of physiological significance, perhaps as a mechanism for regulating the amplitude of the light response: near rest the block is reduced and the response to dim lights would be optimized, whereas, with the hyperpolarization caused by stronger illumination, partial blockade may avoid excessively large sustained currents.
Irrespective of commonalties of purpose across different cells, voltage-dependent divalent block can be an important molecular mechanism that modulates channel function and has recently been described in another K+-selective channel, TOK1, which was cloned from Saccharomyces serevisiae (Ketchum et al. 1995). In view of the functional similarities between the light-dependent channels of Pecten ciliary photoreceptors and those of rods (including activation by cGMP and blockade by l-cis-diltiazem and 3′,4′-dichlorobenzamil; see Gomez and Nasi 1995, Gomez and Nasi 1997a), the mechanisms that underlie the observed nonlinear conduction properties may be of relevance to other cyclic nucleotide–gated channels.
Acknowledgments
We thank Drs. Denis Baylor and Miguel Holmgren for critically reading the manuscript.
Supported by National Institutes of Health grant RO1 EY-07559.
Footnotes
1used in this paper: 4-AP, 4-aminopyridine; ASW, artificial sea water; CNGC, cyclic nucleotide–gated channels; gL, light-sensitive potassium conductance; I–V, current–voltage; S/N, signal-to-noise ratio
Portions of this work have been previously published in abstract form (Nasi, E., and M. Gomez. 1996. Biophys. J. 70:A137).
References
- Armstrong C.M. Interactions of tetraethylammonium ion derivatives with the potassium channel of giant axons. J. Gen. Physiol. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C.R., MacLeish P.R., Schwartz E.A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J. Physiol. 1979;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied T.A., Boeckman F.A., Aizenman E., Johnson J.W. Trapping channel block NMDA-activated responses by amantadine and memantine. J. Neurophysiol. 1997;77:309–323. doi: 10.1152/jn.1997.77.1.309. [DOI] [PubMed] [Google Scholar]
- Bodoia R.D., Detwiler P.B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J. Physiol. 1984;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Caretta A., Cervetto L., Torre V. Ionic movement through light-sensitive channels of toad rods. J. Physiol. 1983;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamartino G., Menini A., Torre V. Blockage and permeation of divalent cations through the cyclic GMP–activated channel from tiger salamander retinal rods. J. Physiol. 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Gorman A.L.F. Contribution of calcium and potassium permeability changes to the off response of scallop hyperpolarizing photoreceptors. J. Physiol. 1979;291:207–232. doi: 10.1113/jphysiol.1979.sp012808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Miller C. Voltage-dependent caesium blockade of a cation channel from fragmented sarcoplasmic reticulum. Nature. 1979;280:807–810. [Google Scholar]
- Fodor A.A., Gordon S.E., Zagotta W.N. Mechanism of tetracaine block of cyclic nucleotide-gated channels J. Gen. Physiol 109 1997. 3 14a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A.A., Black K.D., Zagotta W.N. Tetracaine reports a conformational change in the pore of cyclic nucleotide–gated channels J. Gen. Physiol. 110 1997. 591 600b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M., Nasi E. The light-sensitive conductance of hyperpolarizing invertebrate photoreceptorsa patch-clamp study J. Gen. Physiol 103 1994. 939 956a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M., Nasi E. Blockage of the light-sensitive conductance in hyperpolarizing photoreceptors of the scallop. Effects of tetraethylammonium and 4-aminopyridine J. Gen. Physiol 104 1994. 487 505b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M., Nasi E. Activation of light-dependent potassium channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca cascade. Neuron. 1995;15:607–618. doi: 10.1016/0896-6273(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Gomez M., Nasi E. Antagonists of the cGMP-gated conductance of vertebrate rods block the photocurrent in scallop ciliary photoreceptors J. Physiol. 500 1997. 367 378a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M., Nasi E. Light adaptation in Pecten hyperpolarizing photoreceptorsinsensitivity to Ca manipulations J. Gen. Physiol 109 1997. 371 384b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M., Nasi E. Calcium-independent, cGMP-mediated desensitization of the light response in Pecten ciliary photoreceptors. Biophys. J. 1999;76:A243. [Google Scholar]
- Gorman A.L.F., McReynolds J.S. Ionic effects on the membrane potential of the hyperpolarizing photoreceptors in scallop retina. J. Physiol. 1978;275:345–355. doi: 10.1113/jphysiol.1978.sp012193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc. R. Soc. Lond. B Biol. Sci. 1985;223:379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Guy H.R., Durell S.R., Warmke J., Drysdale R., Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide–-gated channels. Science. 1991;254:730. doi: 10.1126/science.1658932. [DOI] [PubMed] [Google Scholar]
- Haynes L.W. Permeation and block by internal and external divalent cations of the catfish cone photoreceptor cGMP-gated channel. J. Gen. Physiol. 1995;106:507–523. doi: 10.1085/jgp.106.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L.W., Kay A.R., Yau K.-W. Single cGMP–activated channel activity in excised patches of rod outer segment membrane. Nature. 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A.L., McNaughton P.A., Nunn B.J., Yau K.-W. Effect of ions on retinal rods from Bufo marinus . J. Physiol. 1984;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M., Smith P.L., Yellen G. Trapping of organic blockers by closing of voltage dependent K+ channels. Evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 1997;109:527–535. doi: 10.1085/jgp.109.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J.E., Bean B.P. Block N-methyl-d-aspartate–activated current by the anticonvulsant MK-801selective binding to open channels. Proc. Natl. Acad. Sci. USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifune C.K., Steinbach J.H. Voltage-dependent block by magnesium of neuronal nicotinic acetylcholine receptor channels in rat phaeochromocytoma cells. J. Physiol. 1991;443:683–701. doi: 10.1113/jphysiol.1991.sp018858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J.W., Brown R.L., Stryer L., Baylor D.A. Interactions between divalent cations and the gating machinery of cyclic GMP–activated channels in salamander retinal rods. J. Gen. Physiol. 1993;101:1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp B.U. The cyclic nucleotide–gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991;14:150–157. doi: 10.1016/0166-2236(91)90087-b. [DOI] [PubMed] [Google Scholar]
- Ketchum K.A., Joiner W.J., Sellers A.J., Kaczmarec L.K., Goldstein S.A.N. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- Kuo C.-C., Hess P. Block of the L-type channel pore by external and internal Mg2+ in rat phaeochromocytoma cells. J. Physiol. 1993;466:683–706. [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Holmgren M., Jurman M.E., Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Lux H.D., Carbone E., Zucker H. Na+ currents through low-voltage–activated Ca2+ channels of chick sensory neuronesblock by external Ca2+ and Mg2+ . J. Physiol. 1990;430:159–188. doi: 10.1113/jphysiol.1990.sp018287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.S., Gorman A.L.F. Photoreceptor potentials of opposite polarity in the eye of the scallop, Pecten irradians . J. Gen. Physiol. 1970;56:375–391. doi: 10.1085/jgp.56.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Trapping single ions inside single ion channels. Biopys. J. 1987;52:123–126. doi: 10.1016/S0006-3495(87)83196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K.-W. Calcium and magnesium fluxes across the plasma membrane of the tad rod outer segment. J. Physiol. 1988;395:695–725. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi E. Electrophysiological properties of isolated photoreceptors from the eye of Lima scabra J. Gen. Physiol 97 1991. 17 34a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi E. Two light-dependent conductances in the membrane of Lima photoreceptor cells J. Gen. Physiol 97 1991. 55 72b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi E., Gomez M. Light-activated ion channels in solitary photoreceptors from the eye of the scallop Pecten irradians . J. Gen. Physiol. 1992;99:747–769. doi: 10.1085/jgp.99.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perry R.J., McNaughton P.A. Response properties of cones from the retina of the tiger salamander. J. Physiol. 1991;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A., Korenbrot J.I. Permeability and interaction of Ca2+ with cGMP-gated ion channels differ in retinal rods and cone photoreceptors. Biophys. J. 1995;69:120–127. doi: 10.1016/S0006-3495(95)79881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M.J., MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993;11:459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- Shimatani Y., Katagiri Y. Light removes inactivation of the A-type potassium channels in scallop hyperpolarizing photoreceptors. J. Neurosci. 1995;15:6489–6497. doi: 10.1523/JNEUROSCI.15-10-06489.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.-P., Akabas M.H., Goulding E.H., Karlin A., Siegelbaum S.A. Exposure of residues in the cyclic nucleotide–gated channel poreP region structure and function in gating. Neuron. 1996;16:141–149. doi: 10.1016/s0896-6273(00)80031-8. [DOI] [PubMed] [Google Scholar]
- Woodhull A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Chen G., Miwa K., Suzuki H. Permeability and Mg2+ blockade of histamine-operated cation channel in endothelial cells of rat intrapulmonary artery. J. Physiol. 1992;450:395–408. doi: 10.1113/jphysiol.1992.sp019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K.-W. Cyclic-nucleotide–gated channelsan expanding new family of ion channels. Proc. Natl. Acad. Sci. USA. 1994;91:3481–3483. doi: 10.1073/pnas.91.9.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K.-W., Baylor D.A. Cyclic GMP–activated conductance of retinal photoreceptor cells. Annu. Rev. Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K.-W., Haynes L.W., Nakatani K. Roles of calcium and cyclic GMP in visual transduction. In: Lüttgau H.C., editor. Membrane Control. Gustav Fischer Verlag; Stuttgart, Germany: 1986. pp. 343–366. [Google Scholar]
- Yau K.-W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985;313:579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+ channels by external cations. J. Gen. Physiol. 1984;84:187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A.L., Baylor D.A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986;321:70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zufall F., Firestein S. Divalent cations block the cyclic nucleotide–gated channels from olfactory receptor neurons. J. Neurophysiol. 1993;69:1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]