Abstract
The plant parasitic nematode Heterodera schachtii induces specific syncytial feeding sites in the roots of Arabidopsis thaliana from where it withdraws all required nutrients. Therefore, syncytia have to be well supplied with assimilates and generate strong sinks in the host plant's transport system. Import mechanisms and consequent accumulation of sucrose in syncytia were described recently. In this work, we studied the starch metabolism of syncytia. Using high-performance liquid chromatography and microscopic analyses, we demonstrated that syncytia store carbohydrates by starch accumulation. Further, we monitored the expression of genes involved in the starch metabolic pathway by gene chip analysis and quantitative reverse transcription-PCR. Finally, we provide functional proof of the importance of starch synthesis for nematode development using T-DNA insertion lines. We conclude that syncytia accumulate starch as a carbohydrate buffer to compensate for changing solute uptake by the nematode and as long-term storage during juvenile development.
The beet cyst nematode Heterodera schachtii induces syncytial feeding sites in the roots of its hosts. Infective second-stage juveniles (J2) migrate toward plant roots and penetrate intracellularly toward the vascular cylinder where they pierce a single cell with their stylet to release secretions (Golinowski et al., 1996). In the following hours, the affected plant cells start to hypertrophy. The central vacuoles disintegrate into several small vesicles, nuclei enlarge, and the number of organelles, such as plastids, mitochondria, and structures of the endoplasmic reticulum, increase (Golinowski et al., 1996). Cell walls are dissolved locally along plasmodesmata, thus giving rise to the formation of a syncytium. About 2 weeks after infection, nematodes reach maturity and are associated with a syncytium that is typically composed of several hundred cells (Golinowski et al., 1996; Grundler et al., 1998). During the sedentary phase of the second developmental stage, the unisex J2s differentiate into females or males. Hitherto, only little is known about the factors influencing sex determination, but nutrient availability and composition appear to be essential factors (Betka et al., 1991; Grundler et al., 1991).
Successfully established syncytia serve as the sole nutrient source for obligate parasitic cyst nematodes. Nematodes withdraw solutes in repeated feeding cycles, each consisting of three phases (Wyss, 1992). First, the stylet is carefully inserted into the syncytium and the nematode releases secretions that lead to the formation of a feeding tube. Second, nutrients are withdrawn; and third, the stylet is retracted and the feeding tube is removed from the stylet orifice. One feeding cycle lasts for about 1.5 h during which about 65% of the time is spent withdrawing nutrients. Nematode feeding is interrupted by molting events that last for about 20 h (Wyss, 1992).
The formation of feeding sites is accompanied by a massive solute import into syncytia, leading to highly elevated Suc levels and, thus, to high osmotic pressure (Böckenhoff, 1995; Hofmann et al., 2007). In general, plant cells cope with excessive amounts of sugars by the formation of starch that accumulates in plastids as water-insoluble granules. Starch molecules elongate by linkage of ADP-Glc to the nonreducing end of an α-glucan, resulting in linear amylose chains. However, the major component of starch is amylopectin, which is synthesized by branching enzymes forming α-1,6 O-glycosidic bonds. As soon as sugars are required by the cell, starch is degraded by several enzymes, such as glucan-water dikinases (GWDs), debranching enzymes, starch phosphorylases, amylases, and glucosidases (Dennis and Blakeley, 2000). Starch is the major form of carbohydrate storage in plants. It is synthesized mainly during photosynthesis in the light period buffering CO2 fixation or in storage organs like tubers, bulbs, or seeds (Dennis and Blakeley, 2000). In recent years, numerous studies have been performed to increase knowledge of starch metabolism, including in the model plant Arabidopsis (Arabidopsis thaliana; Zeeman et al., 2002, 2004; Siedlecka et al., 2003; Smith et al., 2004; Delvallé et al., 2005; Lloyd et al., 2005; Dumez et al., 2006). In most of these studies, plant leaf material of the wild type and mutant lines was used to investigate starch metabolism under different light regimes or altered sugar concentrations. The results indicate that starch synthesis and degradation are regulated by sugar levels rather than by the photoperiod.
Although there are some studies on sugar import into nematode-induced syncytia, hardly anything is known about sugar processing and sugar metabolism. Starch granules have been observed in early ultrastructural studies in syncytia of Nacobbus sp. and surrounding cells in sugar beet (Beta vulgaris) and tomato (Solanum lycopersicum) roots (Schuster et al., 1964; Jones and Payne, 1977), but this has never been investigated in detail in these or other nematodes that induce feeding sites. In this study, we monitored the occurrence of starch in nematode-induced syncytia and analyzed the expression of genes involved in starch metabolism. Our results give a detailed description of this part of carbohydrate metabolism in syncytia. They support the concept of syncytia being formed as new heterotrophic plant structures that use starch as intermediate carbohydrate storage to compensate fluctuating sugar levels occurring during nematode feeding and development.
RESULTS
Starch Accumulates in Nematode-Induced Syncytia
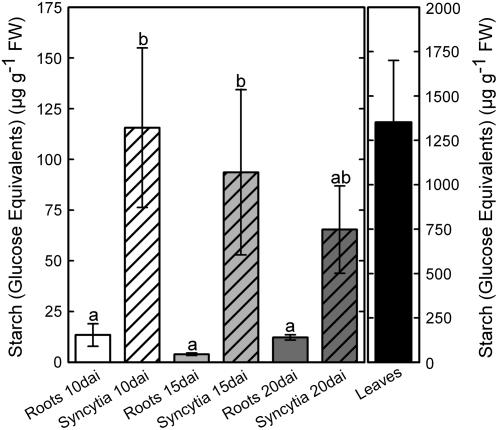
As a first step, we analyzed the starch content in syncytia and noninfected control roots. Therefore, Arabidopsis plants grown on sand/soil culture were used to avoid exposing roots to light and culturing in sugar-enriched medium. HPLC analysis showed massive starch accumulation in syncytia, whereas levels of starch in control roots were low at all tested time points (Fig. 1). The most dramatic increase of starch content occurred in 10-d-old syncytia showing levels of starch 10 times higher than in noninfected roots. Later, the levels decreased steadily until 20 d after inoculation (dai). To validate our data, we measured starch levels in Arabidopsis leaves (Fig. 1) and found that they were similar to previously published data (Smith et al., 2004).
Figure 1.
Arabidopsis wild-type Columbia-0 plants were grown in sand/soil culture. Nematode-induced syncytia and noninfected control roots were harvested at 10, 15, and 20 dai and starch content was measured as Glc equivalents. Values are means ± se, n = 3. Different letters indicate significant variations (P < 0.05).
Starch Granules Are Formed in Syncytial Plastids
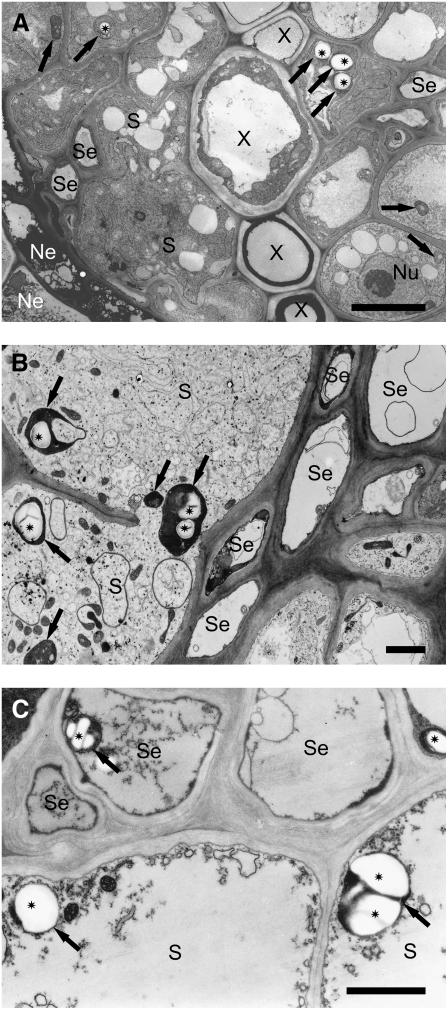
Ultrastructural investigations revealed few small starch granules randomly occurring in plastids present in cells of different tissues of the vascular cylinder in the elongation zone of noninfected roots. Syncytial plastids were not altered structurally and contained starch granules only very rarely during the J2 developmental stage, which lasts until about the seventh day after inoculation (Fig. 2A). In syncytia associated with third- and fourth-stage juveniles, plastids were morphologically strongly modified and acquired cup-like shapes, thus appearing ring-shaped on sections. Within such plastids, large starch granules could be detected (Fig. 2B). Starch granules were found abundantly in syncytia abandoned by males or associated with prematurely degenerated females (Fig. 2C). In these cases, the lack of sugar withdrawal by the nematode and the subsequent increase of sugar levels in the cells were apparently compensated for the formation of starch until the syncytia eventually degraded. In cells around syncytia, no conspicuous accumulation of starch granules occurred.
Figure 2.
Transmission electron microscope picture of syncytia induced in roots of Arabidopsis by H. schachtii. A, Cross section of syncytium associated with J2 18 h after syncytium induction. B, Cross section of syncytium associated with female J4 juvenile. C, Cross section of a degraded syncytium associated with a degenerated female J4. Bars = 5 μm (A); 2 μm (B and C). Ne, Necroses; Nu, nucleus; S, syncytium; Se, sieve tube; X, xylem vessel; arrow, plastid; asterisk, starch granule.
Expression of Genes Involved in Starch Metabolism Is Strongly Influenced by Nematode Infection
Encouraged by the high accumulation of starch in nematode-induced syncytia, we studied the expression of genes involved in the starch metabolic pathway by a transcriptome approach. RNA isolated from micro-aspirated syncytial protoplasts was hybridized onto Affymetrix gene chips. For this purpose, we selected an early developmental stage (5 dai), as well as fully developed (15 dai) and old (21 dai) syncytia. As a control, pieces of noninfected Arabidopsis roots without lateral and apical meristems were used. According to MapMan 2.0.0 software, 56 genes are known to be involved in starch metabolism in Arabidopsis. The most highly up-regulated genes are listed in Table I, ranked by their fold-change expression of 15-dai syncytia compared with control roots. The complete dataset is presented as Supplemental Table S1. The most highly up-regulated gene at all studied time points was GRANULE-BOUND STARCH SYNTHASE1 (GBSS1), followed by several genes involved in both starch synthesis and degradation. With a minimum threshold of 1.5-fold regulation (log2), almost one-half of the listed genes in Supplemental Table S1 were up-regulated and only two genes (both UDP-glycosyltransferases) were down-regulated at 15 dai.
Table I.
Genes of the starch metabolic pathway most highly up-regulated in cyst nematode-induced syncytia
Normalized values of expression levels on a log2 scale. Changes were obtained by comparing 5-, 15-, and 21-d-old micro-aspirated syncytial protoplasts in relation to control roots (con) and displayed as fold change (log2 ratios; n = 4 for 5 dai, n = 3 for 15 dai, n = 2 for 21 dai, n = 4 for con). Asterisks indicate significance differences determined by a Benjamini-Hochberg multiple testing correction (* = q < 10%; ** = q < 5%; see “Materials and Methods” for details). For qPCR, syncytia containing root segments were compared to control roots. (Values are means, n = 3.)
| Synonyms | Chip Data
|
qPCR
|
Name | Function | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normalized Values (log2)
|
Fold Change (log2)
|
Fold Change (log2)
|
||||||||||||||
| con | 5 dai | 15 dai | 21 dai | 5 dai | q | 15 dai | q | 21 dai | q | 5 dai | 10 dai | 15 dai | 20 dai | |||
| At1g32900 | 0.15 | 1.10 | 2.39 | 0.94 | 2.9 | ** | 4.0 | ** | 2.6 | * | 3.6 | 3.9 | 3.5 | 1.1 | GBSS1 | Starch synthase |
| At5g24300 | 0.21 | 1.29 | 1.69 | 0.80 | 2.6 | ** | 3.0 | ** | 2.0 | * | 2.6 | 2.4 | 2.6 | 1.1 | SS1 | Starch synthase |
| At3g29320 | 0.25 | 1.70 | 1.94 | 0.87 | 2.8 | ** | 3.0 | 1.8 | * | 2.3 | 2.4 | 2.1 | −1.0 | PHS1 | Starch phosphorylase | |
| At5g03650 | 0.52 | 1.53 | 3.32 | 1.31 | 1.6 | ** | 2.7 | ** | 1.3 | 1.2 | 1.4 | 2.0 | 0.5 | SBE2 | Starch-branching enzyme | |
| At4g39210 | 0.28 | 1.41 | 1.51 | 0.86 | 2.3 | ** | 2.4 | ** | 1.6 | 3.3 | 2.8 | 2.8 | 0.8 | APL3 | ADP-Glc pyrophosphorylase | |
| At3g46970 | 0.28 | 1.16 | 1.35 | 0.66 | 2.1 | ** | 2.3 | ** | 1.3 | 1.7 | 1.8 | 1.9 | −0.2 | PHS2 | Starch phosphorylase | |
Comparing 5- and 15-d-old syncytia, there were hardly any differentially expressed genes; some genes were expressed at lower levels at 21 dai compared with 15 dai. Most of the 56 recorded genes were markedly up-regulated at 15 dai. Only six genes showed the highest expression level at 5 dai and none at 21 dai. However, at all three time points, we found genes coding for enzymes involved in different steps of starch synthesis and degradation. Therefore, we could not assign particular developmental stages to particular parts of starch metabolism at the gene expression level.
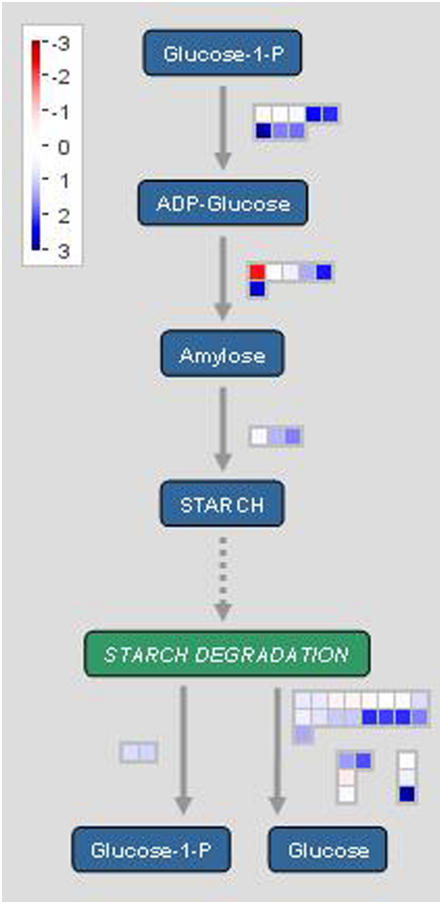
To visualize the involvement of the different genes in starch metabolism, we used MapMan 2.0.0 software that offers a concise tool to illustrate gene expression in different metabolic pathways and is publicly available at the GABI home page (http://gabi.rzpd/projects/MapMan). We applied the software to our gene dataset of 15-dai syncytia (Fig. 3; datasets of 5 and 21 dai are presented as Supplemental Fig. S1) to monitor the stages of starch metabolism and their intermediate products. Transformation of Glc-1-P into ADP-Glc is the main regulatory step during starch synthesis because ADP-Glc is the exclusive substrate of starch synthesis (Smith et al., 2004). ADP-Glc is bound subsequently to the amylase chain by the activity of starch synthases, whereas starch-branching enzymes (SBEs) are responsible for amylopectin formation. Starch is degraded either by starch phosphorylase cleaving single Glc-1-P molecules from the amylopectin chain or debranching enzymes and amylases (Lloyd et al., 2005). Small color-coded squares in the chart represent the expression levels of particular genes compared with noninfected root pieces. Blue squares indicate up-regulation, whereas red squares report down-regulation. The figure illustrates that only one of the genes involved in starch metabolism is considerably down-regulated, but many of the genes are up-regulated in syncytia. These results indicate that in all enzymatic steps at least one gene is up-regulated in both starch synthesis and starch degradation.
Figure 3.
MapMan 2.0.0 software was used to illustrate expression of genes involved in the starch metabolic pathway in nematode-induced syncytia (15 dai) compared to noninfected control roots (log2). Single squares represent one gene each. The blue-colored boxes indicate up-regulated genes; red boxes indicate down-regulated genes.
Quantitative Reverse Transcription-PCR Analysis
To confirm the gene chip data, expression of several highly regulated genes involved in starch synthesis and degradation was tested by quantitative reverse transcription (qRT)-PCR and compared with the results obtained from the chip (Table I). In most cases, both values matched very well, even though samples for the gene chip data were derived from micro-aspirated material, whereas dissected syncytia containing surrounding root cells were used for qRT-PCR. Only the data of 20-dai syncytia obtained by qPCR were consistently lower than the ones from the chip.
Results from both techniques indicate that the expression of genes involved in starch metabolism is minimal in cells surrounding the syncytia; this confirms our microscopic observations. The selected genes coding for enzymes involved in starch synthesis and degradation were up-regulated in 5-dai syncytia already. Five of the six genes analyzed were consistently expressed at 5, 10, and 15 dai; the sixth showed a slight increase in expression from 5 to 15 dai. However, at 20 dai, the fold changes of all genes decreased.
Infection Test
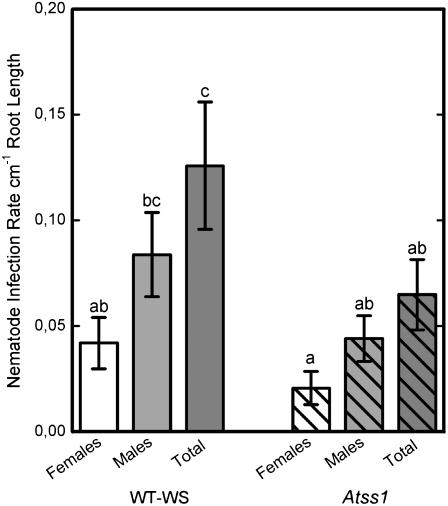
The functional role of one of the genes differentially regulated in syncytia, STARCH SYNTHASE1 (SS1), was evaluated in a nematode infection assay using a T-DNA insertion line (Atss1). This mutant line was shown previously to contain 23% less starch in leaves at the end of the illumination period (Delvallé et al., 2005). The loss-of-function insert in this line was located in the intron of the genomic sequence, but a protein could not be detected. We tested the seeds for homozygosity and grew plants on Knop medium without sugar supplement (see “Materials and Methods”). Two weeks after inoculation, when females and males can be discriminated clearly, nematodes were counted and the infection rate was calculated per centimeter of root length. Results of the infection assay showed a reduction in the number of males and females and a significant reduction (P < 0.05) in the total number of developing juveniles on the Atss1 plants when compared with the wild type (Fig. 4).
Figure 4.
Nematode infection and development on an Atss1 T-DNA insertion mutant line was compared to Arabidopsis wild-type Wassilewskija plants. Plants were grown on sugar-free medium to ensure that development of the nematode depended on the plant's sugar supply. Infection rate is indicated as number of nematodes per centimeter root length. Values are means ± se, n = 15. Different letters indicate significant variations (P < 0.05).
DISCUSSION
During coevolution, cyst nematodes achieved the ability to induce feeding sites that serve as an interface to orchestrate plant transport mechanisms and supply them with nutrients in the appropriate quantity and quality. Assuming soluble carbohydrates are an essential source of energy, syncytial feeding sites have to contain high levels of sugars that can be taken up by the nematodes. In fact, this has been shown recently (Hofmann et al., 2007). On the other hand, it is improbable that sugar transport into syncytia is entirely adapted to the temporal and quantitative demand of the parasites. Thus, we hypothesized that starch acts as intermediate storage to compensate for fluctuating sugar levels and demands that occur during the different phases of nematode feeding and development. To examine this, we monitored the presence of starch and examined starch metabolism in syncytia.
Plants generally compensate for excess sugar due to photosynthetic activity during the daytime by starch synthesis. During the dark period, plants switch to heterotrophy and degrade the accumulated starch. In this process, plants are thought to respond to altering sugar levels rather than to light-to-dark transition (Fondy and Geiger, 1982).
Starch acts as a buffer against excessive sugar levels in plant cells. At first sight, it seems astonishing that it is synthesized in syncytia, which serve as the exclusive nutrient source of the feeding nematodes. In fact, a massive transfer of sugars from the host plant's phloem into syncytia occurs, which leads to the necessary high levels of sugars (Hofmann et al., 2007). Sugar import has been shown to be performed via transporters at the beginning of syncytium formation and via plasmodesmata at later stages (starting around 10 dai; Juergensen et al., 2003; Hoth et al., 2005; Hofmann and Grundler, 2006, 2007a; Hofmann et al., 2007). It could be anticipated that continuous sugar demand induces an equivalent transport, thus establishing physiological equilibrium. However, sugar demand by the nematodes fluctuates due to their feeding behavior and their developmental phases. Nematode feeding consists of repeated cycles, during which the stylet is inserted into the syncytium, secretions are released through the stylet, nutrients are withdrawn from the syncytium, and the stylet is retracted again. Depending on the developmental stage, a single feeding cycle lasts for 1.5 to 2 h with short feeding interruptions (Wyss, 1992). However, these intermissions might be too short to induce the deposition of starch granules in the syncytia. To grow, the nematodes have to molt their cuticle, including the stylet. Therefore, during molting, no food is withdrawn. Molting, however, lasts for about 20 h and is even longer than a usual light period. During this phase, the import of plant solutes into syncytia may remain constant, thus leading to an excess of sugar in syncytia (Hofmann et al., 2007). In this way, a situation occurs in which starch synthesis is induced as a common response of the plant cells. Further studies have to be performed to prove this concept.
The amount of nutrients withdrawn by the nematodes may also play a major role in the dynamics of starch metabolism. Nutrient uptake by nematodes increases considerably during their development. Whereas young J2s take up comparatively small volumes (0.4–0.6 times the syncytium volume/day), adult females withdraw as much as 4-fold the syncytium content per day (Sijmons et al., 1991). Moreover, compared to male nematodes, females feed for a much longer period and thus take up more nutrients. In Brassica napus, their total calculated food consumption was about 29 times higher than that of males (Müller et al., 1981). Further, adult females stay alive for about 2 months until egg production is completed and they finally die. Therefore, we conclude that high sugar levels in young syncytia could induce the accumulation of starch; starch will be degraded during later stages of nematode development. As shown in Figure 1, starch content was highest in syncytia at 10 dai when plasmodesmata are formed between syncytia and the phloem, and thereafter decreased constantly.
Knowledge of starch metabolism in nematode feeding sites is very limited. Among the few published transcriptome analyses of feeding sites, only Bar-Or et al. (2005) mentioned the expression of the tomato GBSSII, SBE, and two putative starch synthases upon infection of root-knot nematodes. Here, we present detailed analysis of gene expression using micro-aspirated syncytia and Affymetrix gene chip technology that enabled us to study the involvement of all genes related to starch formation and degradation in syncytia according to their present annotation and their availability on the chip.
Smith et al. (2004) suggested that genes regulated in the same metabolic pathways should show clustered expression. They found, however, no such expression pattern during the circadian cycle. In this work, expression of the majority of up-regulated genes peaked at 15 dai. Interestingly, these genes include those coding for enzymes responsible for both synthesizing and degrading starch, indicating either a high turnover of starch and/or a distinct role of fine tuning on the post-transcriptional level.
The initial and thus decisive regulatory enzymatic reaction for starch synthesis in leaves is catalyzed by ADP-Glc pyrophosphorylase (AGPase; Smith et al., 2004). This enzyme consists of a large subunit (APL) that is encoded by four genes (APL1–4) in Arabidopsis and a small subunit (APS) encoded by two genes (but only APS1 encodes a functional AGPase). Mutational analyses have shown that most of the AGPase activity in leaves derived from APL1 and APS1 (for review, see Smith et al., 2004). In this study, it was shown that APL2 to APL4 were weakly expressed in noninfected Arabidopsis roots, whereas APL1 was moderately expressed. In syncytia, APL3 was the highest up-regulated gene among the APL family. The transcript levels of APL2 and APL3 have been shown to increase several fold after Glc or Suc application into Arabidopsis leaves in the dark (Sokolov et al., 1998). This indicates that the high Suc level in nematode-induced syncytia affects the expression of these genes. Two other genes (At1g74910 and At2g04650) belong to the AGPase gene family, but there is no further information so their function is a matter of speculation.
The next step in the starch metabolic pathway is the formation of amylose, during which ADP-Glc is linked to the nonreducing end of an α-glucan chain. This reaction relies on the activity of the starch synthase that is encoded by five genes in Arabidopsis. The transcript level of one of these starch synthases, GBSS1, is highly elevated at the beginning of the illumination phase and changes dramatically during the photoperiod (Smith et al., 2004). In contrast to other starch synthases, GBSS1 is bound to starch granules. Smith et al. (2004) suggested that GBSS1 is destroyed during starch degradation because it is released from the granules and has to be produced again during starch synthesis. In this work, high up-regulation of GBSS1 was found in nematode-induced syncytia, which may indicate high turnover of starch in syncytia.
In the step for amylose formation, in addition to GBSS1, another gene involved in amylopectin synthesis, SS1, encoding a plastidial enzyme (Delvallé et al., 2005), was also up-regulated. Whereas Delvallé et al. (2005) reported that SS1 mRNA levels increased at the onset of the light period, SS1 transcript levels were found by Smith et al. (2004) to be almost constant during circadian rhythm. However, the level of SS1 mRNA did not correlate with fluctuations of the protein content and with enzyme activity levels, suggesting post-transcriptional regulation of this enzyme (Delvallé et al., 2005). Due to the up-regulation of SS1, we selected Atss1 T-DNA insertion lines for a loss-of-function analysis in a nematode infection assay. Our results on the Atss1 T-DNA insertion line revealed a decreased number of nematodes developing on these plants, thus indicating that this gene plays a role in syncytium metabolism. Both males and females are affected by the loss of function. This indicates that starch is important to both types of syncytia, but does not influence sex determination. However, it is difficult to assess the full importance of starch synthase because the corresponding gene family may allow compensation of loss of function of one gene by functional redundancy.
The last step in starch synthesis is the formation of amylopectin, which is performed by SBEs. There are three SBE genes in Arabidopsis. SBE1 has no apparent function in starch metabolism in Arabidopsis leaves, but SBE2 and SBE3 are required for starch synthesis (Dumez et al., 2006). SBE3 gene expression changes within the diurnal cycle, whereas SBE1 and SBE2 expression undergoes minor fluctuations (Smith et al., 2004). SBE1 and SBE3 transcript levels were unchanged in syncytia, whereas SBE2 was up-regulated significantly.
Because nematodes are unable to take up large and insoluble starch granules, starch degradation is essential to make sugars again available to the parasites. The initial steps of starch degradation are regulated by GWD and phosphoglucan-water dikinase (PWD) activity (Kötting et al., 2005). Whereas expression of GWD is considerably up-regulated in syncytia, PWD is weakly expressed or even down-regulated at 5 dai. This supports the concept that starch synthesis, rather than starch degradation, is more pronounced during the early stages of syncytia development. Although intensive research on starch metabolism in recent years has revealed new insights into the complex process of starch degradation, various questions still remain unanswered (for review, see Lloyd et al., 2005). Obviously amylases, isoamylases, and pullulanases play a role in starch degradation (Dennis and Blakeley, 2000). Starch phosphorylase exists in the two isoforms PHS1 and PSH2. PSH1 appears to be dispensable for starch degradation. Zeeman et al. (2002) suggested that PHS1 is important for tolerance against abiotic stress because Atphs1 mutants were susceptible for transient water deficiency. Both PHS isoforms, however, were highly regulated during the diurnal cycle and PHS2 is of special interest because it is cytosolic and might play a role in the conversion from starch via maltose into Glc (Smith et al., 2004). Both PHS genes were among the most highly up-regulated genes in syncytia. Because the function of both genes is still unclear, it is difficult to determine their role in syncytia. It might be related to intermittent water deficiency during feeding cycles.
CONCLUSION
Regulation of the starch metabolic pathway is highly complex because no obvious correlations between expression of involved genes, levels of encoded proteins, and fluxes in starch metabolism have been found (Smith et al., 2004). In this study, however, 20 of 56 genes known to be related to starch synthesis and degradation were up-regulated in nematode-induced syncytia, indicating the importance of this pathway for the function of the feeding sites. The altered starch metabolism represents a completely new feature of these highly specialized plant structures. It is yet another example of the intriguing coevolution of plants and sedentary nematodes in which the nematodes have developed a strategy to exploit plant assimilate sources by inducing feeding cells.
MATERIALS AND METHODS
Plant Growth Conditions and Nematode Culture
Sterile Arabidopsis (Columbia-0) seeds were germinated under sterile conditions on 0.2% Knop medium and grown at 16/8-h photoperiod, 150 μmol m−2 s−1 at 25°C. Twelve-day-old plants were inoculated with 50 freshly hatched J2s (Sijmons et al., 1991) of Heterodera schachtii obtained from sterile stock culture. Inoculated plants were kept in the dark overnight and put back into the growth chamber the next day. For measuring starch content in syncytia and noninfected roots, plants were grown in sand/soil (1:2 [v/v]) culture in 24-well plates. In each well, five to 10 plants were grown and each well was inoculated after 12 d with approximately 500 J2s.
RNA Isolation and RT
At 5, 10, 15, and 20 dai, about 200 syncytia and pieces of control roots without root tips of noninfected plants were dissected in the middle of the light phase and immediately frozen in liquid nitrogen. For each time point, three independent samples were collected in three independent experiments. Total RNA was isolated using an RNeasy plant mini kit (Qiagen) according to the manufacturer's instructions, including DNaseI (Qiagen) digestion. Quality of the RNA was checked with an Agilent 2100 bioanalyzer (Agilent Technologies). RT was performed with a SuperScript III reverse transcriptase (Invitrogen) and random primers [oligo(dN)6] according to the manufacturer's instructions.
qPCR
Relative expression differences of genes involved in starch synthesis and degradation between nematode-induced syncytia and noninfected control roots were analyzed using an ABI PRISM 7300 sequence detector (Applied BioSystems). Primers were selected using Primer Express Version 2.0 software (Applied BioSystems). Primer sequences and PCR efficiencies are given in Supplemental Table S2. Each qPCR sample contained 12.5 μL of platinum SYBR Green qPCR SuperMix with UDG and ROX (Invitrogen), 30 mm MgCl2, 0.75 μL of forward and reverse primers (10 mm), 2 μL of cDNA, and water to reach 25 μL total reaction volume. All samples were tested in triplicate; water was used as a control to rule out false-positive signals. In addition, dissociation runs were performed to control the possible formation of primer dimers. As internal references, 18S rRNA and UBP22 were used, which were known to be stably expressed in syncytia (Hofmann and Grundler, 2007b). Samples were diluted 1:3 and 1:100 when performed for 18S rRNA. Results were obtained using Sequence Detection Software (SDS Version 2.0; Applied BioSystems). Relative expression was calculated by the (1 + E)−ΔΔCt method.
Gene Chip Analysis and Statistical Evaluation
For gene chip analysis, micro-aspirated syncytial protoplasts collected from 5-, 15-, and 21-d-old syncytia were used. The cytoplasm of syncytia was extracted with a microcapillary and a micromanipulator without contamination from uninfected root cells or nematodes (Juergensen et al., 2003). Noninfected root pieces cut from the elongation zone without root tips or lateral root primordia were used as control samples. Sample preparation was performed as published earlier (Wieczorek et al., 2006, 2008) Biotin-labeled probes were synthesized according to the Affymetrix protocol. ATH1 Arabidopsis gene chips (Affymetrix) were hybridized by Deutsches Ressourcenzentrum für Genomforschung GmbH according to the manufacturer's protocols. To test biological variability, four samples of control roots and 5-dai syncytia, three samples of 15-dai syncytia, and two samples of 21-dai syncytia were hybridized to the chips. Chip raw data are presented as Supplemental Table S3; the complete dataset will be published elsewhere. Affymetrix.CEL files (from GCOS 1.2) were imported into the software program GeneSpring Version 7.2 (Silicon Genetics) using the Robust Multi-Array Average normalization preprocessor. Additionally, per-gene normalization (normalize to median) was performed. To simulate Gaussian distribution, data were log transformed by using the log-of-ratio mode of analysis.
To identify changes in gene expression profiles during syncytium development, pair-wise comparisons for the set of 56 genes present on the chip were conducted. We calculated the fold change between two conditions by using the geometric mean of the normalized ratios within each condition. We then excluded genes with a fold-change factor <1.5 as a further quality control for unreliable genes. Finally, we used the filtered genes for a parametric test comparing two conditions each, coupled with the Benjamini and Hochberg multiple testing correction to control the false discovery rate (Benjamini and Hochberg, 1995).
The significance P-value cutoff was set at 0.1. Significant differences are shown as fold-change log2 ratio values. Finally, the fold-change data were imported into MapMan 2.0.0 software that is publicly available at the GABI home page (http://gabi.rzpd/projects/MapMan).
Starch Extraction and Quantification by HPLC
Starch content in nematode-induced syncytia and noninfected control roots was analyzed in plants grown in sand/soil culture as described above. Eighteen to 127 mg of fresh syncytia or root material were collected in the middle of the light phase and immediately frozen in liquid nitrogen. Plant material was ground into fine powder and washed with 60% (v/v) ethanol at 60°C for 30 min to remove soluble carbohydrates. The remaining pellet was washed again with 50% (v/v) ethanol at room temperature and with 80% (v/v) and twice with absolute ethanol at 60°C and dried under vacuum. The dried pellet was then suspended in 500 μL of a heat-stable α-amylase solution from Bacillus licheniformis (500 units mL−1; Sigma-Aldrich) and incubated for 45 min at 85°C. After centrifugation (13,000g for 10 min), 100 μL of the supernatant were transferred to a new tube and mixed with 250 μL of amyloglucosidase solution (20 units mL−1 in sodium-acetate buffer; Sigma-Aldrich). After incubation at 55°C for 30 min, 250 μL of chloroform were added to the mixture and aliquots of the aqueous phase were analyzed by HPLC-pulsed-amperometric detection on a 3- × 150-mm Carbopac PA20 column (Dionex) using a Dionex ICS3000 chromatography system. The column and the detector were thermostatically maintained at 30°C and Glc equivalents were eluted with 100 mm NaOH at a flow rate of 0.5 mL min−1.
Microscopy
Seeds of Arabidopsis (Landsberg erecta) were grown aseptically as described above and inoculated after 2 weeks with 100 J2s. After inoculation, the aerial parts of plants were cut off and plates with root systems were kept in darkness. Because invasion of plant roots by J2 and their development is not temporarily synchronized, samples for electron microscopic examination, consisting of nematode-induced syncytia and surrounding root tissues as well as attached juvenile, were collected according to the nematode developmental stage and sex at different time points after inoculation. Dissected samples were immediately immersed in a fixative composed of 2% (w/v) paraformaldehyde and 2% (v/v) glutaraldehyde in 0.05 m cacodylic buffer (pH 7.2) and processed further for embedding and sectioning as described by Golinowski et al. (1996) and Sobczak et al. (1997).
Nematode Infection Test
Seedlings of a mutant Arabidopsis line lacking the soluble starch synthase (At5g24300) Atss1 were tested as described by Delvallé et al. (2005). To test the importance of AtSS1 for nematode development, we compared numbers of juveniles developing on wild-type (Wassilewskija) plants with the number developing on the loss-of-function mutant. Plants were grown on 0.2% Knop medium without Suc and reduced nitrogen levels as described above. Twelve-day-old plants with roots of a proper length as estimated according to Jürgensen (2001) were each inoculated with 50 J2s. Two weeks after inoculation, plants were screened for developing females and males. Significant differences were calculated using the LSD test.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. MapMan figures illustrating expression of genes involved in the starch metabolic pathway.
Supplemental Table S1. Complete gene chip dataset of the starch metabolic pathway of micro-aspirated syncytial protoplasts in comparison to noninfected roots.
Supplemental Table S2. PCR conditions and primer sequences for genes tested by qRT-PCR.
Supplemental Table S3. Chip raw data of all replicates, including mean values and sd.
Supplementary Material
Acknowledgments
We gratefully acknowledge Christophe D'Hulst, Université des Sciences et Technologies de Lille (France), for providing us the Atss1 T-DNA insertion lines and wild-type (Wassilewskija) seeds. We would also like to thank Professor David Kreil, University of Natural Resources and Applied Life Sciences, Vienna, Austria, for assisting in statistical analyses, and Professor Roland Perry, Rothamsted Research, Harpenden, UK, and one of the anonymous reviewers for helpful comments.
This work was supported by the Austrian Science Fond (project nos. P16897–B06 and P16296–B06).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Florian M.W. Grundler (florian.grundler@boku.ac.at).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bar-Or C, Kapulnik Y, Koltai H (2005) A broad characterization of the transcriptional profile of the compatible tomato response to the plant parasitic root knot nematode Meloidogyne javanica. Eur J Plant Pathol 111 181–192 [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57 289–300 [Google Scholar]
- Betka M, Grundler FMW, Wyss U (1991) Influence of changes in the nurse cell system (syncytium) on sex determination and development of the cyst nematode Heterodera schachtii: single amino acids. Phytopathology 81 75–79 [Google Scholar]
- Böckenhoff A (1995) Untersuchungen zur Physiologie der Nährstoffversorgung des Rübenzystennematoden Heterodera schachtii und der von ihm induzierten Nährzellen in Wurzeln von Arabidopsis thaliana unter Verwendung einer speziell adaptierten in situ Mikroinjektionstechnik. PhD thesis. Christian-Albrechts Universität, Kiel, Germany
- Delvallé D, Dumez S, Wattebled F, Roldán I, Planchot V, Berbezy P, Colonna P, Vyas D, Chatterjee M, Ball S, et al (2005) Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J 43 398–412 [DOI] [PubMed] [Google Scholar]
- Dennis DT, Blakeley SD (2000) Carbohydrate metabolism. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants, Ed 1. American Society of Plant Biologists, Rockville, MD, pp 630–675
- Dumez S, Wattebled F, Dauvillee D, Delvalle D, Planchot V, Ball SG, D'Hulst C (2006) Mutants of Arabidopsis lacking starch branching enzyme II substitute plastidial starch synthesis by cytoplasmic maltose accumulation. Plant Cell 18 2694–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy BR, Geiger DR (1982) Diurnal pattern of translocation and carbohydrate metabolism in source leaves of Beta vulgaris L. Plant Physiol 70 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golinowski W, Grundler FMW, Sobczak M (1996) Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 194 103–116 [Google Scholar]
- Grundler FMW, Betka M, Wyss U (1991) Influence of changes in the nurse cell system (syncytium) on sex determination and development of the cyst nematode Heterodera schachtii: total amounts of proteins and amino acids. Phytopathology 81 70–74 [Google Scholar]
- Grundler FMW, Sobczak M, Golinowski W (1998) Formation of wall openings in root cells of Arabidopsis thaliana following infection by the plant-parasitic nematode Heterodera schachtii. Eur J Plant Pathol 104 545–551 [Google Scholar]
- Hofmann J, Grundler FMW (2006) Females and males of root-parasitic cyst nematodes induce different symplasmic connections between their syncytial feeding cells and the phloem in Arabidopsis thaliana. Plant Physiol Biochem 44 430–433 [DOI] [PubMed] [Google Scholar]
- Hofmann J, Grundler FMW (2007. a) How do nematodes get their sweets: solute supply to sedentary plant parasitic nematodes. Nematology 9 451–458 [Google Scholar]
- Hofmann J, Grundler FMW (2007. b) Identification of reference genes for qRT-PCR studies of gene expression in giant cells and syncytia induced in Arabidopsis thaliana by Meloidogyne incognita and Heterodera schachtii. Nematology 9 317–323 [Google Scholar]
- Hofmann J, Wieczorek K, Blöchl A, Grundler FMW (2007) Sucrose supply to nematode-induced syncytia depends on the apoplasmic and the symplasmic pathway. J Exp Bot 58 1591–1601 [DOI] [PubMed] [Google Scholar]
- Hoth S, Schneidereit A, Lauterbach C, Scholz-Starke J, Sauer N (2005) Nematode infection triggers the de novo formation of unloading phloem that allows macromolecular trafficking of green fluorescent protein into syncytia. Plant Physiol 138 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MGK, Payne HL (1977) The structure of syncytia induced by the phytoparasitic nematode Nacobbus aberrans in tomato roots, and the possible role of plasmodesmata in their nutrition. J Cell Sci 23 299–313 [DOI] [PubMed] [Google Scholar]
- Jürgensen K (2001) Untersuchungen zum Assimilat- und Wassertransfer in der Interaktion zwischen Arabidopsis thaliana und Heterodera schachtii. PhD thesis. Christian-Albrechts Universität, Kiel, Germany
- Juergensen K, Scholz-Starke J, Sauer N, Hess P, van Bel AJE, Grundler FMW (2003) The companion cell-specific Arabidopsis disaccharide carrier AtSUC2 is expressed in nematode-induced syncytia. Plant Physiol 131 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G (2005) Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol 137 242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Kossmann J, Ritte G (2005) Leaf starch degradation comes out of the shadows. Trends Plant Sci 10 130–137 [DOI] [PubMed] [Google Scholar]
- Müller J, Rehbock K, Wyss U (1981) Growth of Heterodera schachtii with remarks on amounts of food consumed. Rev Nematol 4 227–234 [Google Scholar]
- Schuster M, Sandstedt R, Estes LW (1964) Starch formation induced by a plant parasitic nematode. Science 143 1342–1343 [DOI] [PubMed] [Google Scholar]
- Siedlecka A, Ciereszko I, Mellerowicz E, Martz F, Chen J, Kleczkowski LA (2003) The small subunit ADP-glucose pyrophosphorylase (ApS) promoter mediates okadaic acid-sensitive uidA expression in starch-synthesizing tissues and cells in Arabidopsis. Planta 217 184–192 [DOI] [PubMed] [Google Scholar]
- Sijmons PC, Grundler FMW, von Mende S, Burrows PR, Wyss U (1991) Arabidopsis thaliana as a new model host for plant parasitic nematodes. Plant J 1 245–254 [Google Scholar]
- Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, Dunstan H, Hylton C, Zeeman SC, Smith AM (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136 2687–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak M, Golinowski W, Grundler FMW (1997) Changes in the structure of Arabidopsis thaliana roots induced during development of males of the plant parasitic nematode Heterodera schachtii. Eur J Plant Pathol 103 113–124 [Google Scholar]
- Sokolov LN, Déjardin A, Kleczkowski LA (1998) Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress). Biochem J 336 681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek K, Golecki B, Gerdes L, Heinen P, Szakasits D, Durachko DM, Cosgrove DJ, Kreil DP, Puzio PS, Bohlmann H, et al (2006) Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. Plant J 48 98–112 [DOI] [PubMed] [Google Scholar]
- Wieczorek K, Hofmann J, Blöchl A, Szakasits D, Bohlmann H, Grundler FMW (2008) Arabidopsis endo-1,4-β-glucanases are involved in the formation of root syncytia induced by Heterodera schachtii. Plant J (in press) [DOI] [PubMed]
- Wyss U (1992) Observations of the feeding behaviour of Heterodera schachtii throughout development, including events during moulting. Fundam Appl Nematol 15 75–89 [Google Scholar]
- Zeeman SC, Thorneycroft D, Schupp N, Chapple A, Weck M, Dunstan H, Haldimann P, Bechtold N, Smith AM, Smith SM (2004) Plastidial α-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol 135 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Tiessen A, Pilling E, Kato KL, Donald AM, Smith AM (2002) Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiol 129 516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.