Abstract
Plant root architecture is highly plastic during development and can adapt to many environmental stresses. The proper distribution of roots within the soil under various conditions such as salinity, water deficit, and nutrient deficiency greatly affects plant survival. Salinity profoundly affects the root system architecture of Arabidopsis (Arabidopsis thaliana). However, despite the inhibitory effects of salinity on root length and the number of roots, very little is known concerning influence of salinity on root growth direction and the underlying mechanisms. Here we show that salt modulates root growth direction by reducing the gravity response. Exposure to salt stress causes rapid degradation of amyloplasts in root columella cells of Arabidopsis. The altered root growth direction in response to salt was found to be correlated with PIN-FORMED2 (PIN2) messenger RNA abundance and expression and localization of the protein. Furthermore, responsiveness to gravity of salt overly sensitive (sos) mutants is substantially reduced, indicating that salt-induced altered gravitropism of root growth is mediated by ion disequilibrium. Mutation of SOS genes also leads to reduced amyloplast degradation in root tip columella cells and the defects in PIN2 gene expression in response to salt stress. These results indicate that the SOS pathway may mediate the decrease of PIN2 messenger RNA in salinity-induced modification of gravitropic response in Arabidopsis roots. Our findings provide new insights into the development of a root system necessary for plant adaptation to high salinity and implicate an important role of the SOS signaling pathway in this process.
Under normal conditions, after germination, the primary root exhibits gravitropism by growing downward. Studies on gravity perception and the tropic response function of the root cap at the primary root of Arabidopsis (Arabidopsis thaliana) strongly support the starch statolith hypothesis to explain gravity sensing (Kiss et al., 1989; Blancaflor et al., 1998; Morita and Tasaka, 2004). In this hypothesis, the columella cells in the root cap, which contain sedimentable amyloplasts, are the gravity-perceptive site in roots. Among the cells of the columella, the inner cells of the second tier have been proposed as making the greatest contribution to root gravitropism (Blancaflor et al., 1998). These cells have the fastest amyloplast sedimentation rates and generate the largest increase in gravisignaling-related cytoplasmic pH change in response to gravistimulation (Blancaflor et al., 1998; Scott and Allen, 1999; Fasano et al., 2001). Although the mechanosensing receptors and/or channels that sense amyloplast movement are still unknown, evidence suggests that cytosolic ions such as Ca2+ and rapid cytoplasmic alkalization may be involved in gravity signal transduction (Legue et al., 1997; Scott and Allen, 1999; Fasano et al., 2001; Johannes et al., 2001; Plieth and Trewavas, 2002; Hou et al., 2004). Asymmetric distribution of auxin in roots caused by basipetal transport mainly through the auxin efflux carrier PIN-FORMED2 (PIN2), which is distributed asymmetrically within the cells, results in gravitropic root response of the root elongation zone (Muller et al., 1998; Friml and Palme, 2002; Moore, 2002; Morita and Tasaka, 2004; Abas et al., 2006; Jaillais et al., 2006). This is the well-known Cholodny-Went hypothesis, which has been strongly supported by recent molecular genetic and biochemical evidence. However, there are still many unanswered questions about this process, such as how the signal generated by amyloplast sedimentation during gravistimulation is transmitted to cause auxin asymmetric distribution.
Owing to their sessile nature, plant roots constantly encounter various environmental stimuli in the soil, such as physical obstacles, and imbalanced distribution of water and/or nutrients. Therefore, plants have evolved highly adaptive regulatory mechanisms to sense and respond to both internal and external signals in an intricate and precise way. Plant root systems show high plasticity in development and can adapt their architecture in response to a variety of external stimuli to maintain optimal growth patterns (Lynch, 1995; Malamy, 2005). To do so, plant roots need to overcome the signal from gravity and reorient their growth direction to navigate across or around physical obstacles or toward water and nutrients. Plant roots are thus able to exhibit negative gravitropism and move away from the direction of the gravity vector. For example, roots show hydrotropism in response to moisture gradients, which represents an example of cross talk between the gravity signal and another environmental cue. Hydrotropism plays a very important role in plant growth and development to tolerate drought stress (Jaffe et al., 1985; Takahashi, 1997; Mizuno et al., 2002; Takahashi et al., 2003). Perception and subsequent response to interaction between gravitropism and hydrotropism may enable plants to direct root growth in the direction that maximizes the acquisition of water, thereby providing a fitness advantage during root development under drought conditions. Increased root length with strong root growth resulting from hydrotropism is one of the most important traits of drought-tolerant crops and has been used in breeding for crops with higher drought tolerance. A recent study showed that a moisture gradient generated by water stress caused immediate degradation of the amyloplasts in root columella cells of plants, resulting in reduced responsiveness to gravity and subsequent hydrotropism (Takahashi et al., 2003). However, how a gravity signal interacts with other environmental cues to modulate the direction of root growth is still largely unknown.
Another major constraint to root system development is soil salinity, which limits the productivity of agricultural crops and the distribution of plant species (Flowers and Yeo, 1995; Zhu, 2002, 2003; Chinnusamy et al., 2005). Sodium accumulation in the cells and the resulting disturbed balance of ions is the primary cause for inhibition of plant growth and subsequent yield reduction (Zhu, 2003). Thus, maintenance of low intracellular sodium is critical for plant adaptation to saline stress. Plants use different strategies to avoid salt injury at all levels of organization, from the cellular, biochemical, and molecular levels to the anatomical, morphological, and phenological levels. At cellular and molecular levels, plants maintain low cytosolic Na+ by regulating compartmentalization, ion influx, and efflux of ions (Zhu, 2002, 2003). Recent advances using molecular tools have led to the discovery of the SOS (salt overly sensitive) signaling pathway, which is the most understood ion-specific signaling regulatory mechanism pathway that controls Na+ efflux and K+ acquisition in plants (Zhu, 2002, 2003; Chinnusamy et al., 2005). In this pathway, perception of a highly saline environment by an unknown sensor induces cytosolic calcium signals, which are transduced by the SOS3-SOS2 kinase complex. Activated SOS2 kinase then controls sodium/proton antiporters, such as SOS1 and NHX1, to regulate sodium efflux and vacuole sequestration. The SOS pathway may also play a role in organ and plant responses to salt stress, including exclusion of sodium ions from meristems and from leaves of stressed plants.
We have observed that roots of Arabidopsis exhibit reduced gravitropism under salt stress, growing against the gravity vector. We speculated that the reduced gravitropism of root growth might be an important adaptive mechanism through which plants regulate root system architecture to avoid damage of elevated salt. Therefore, our objective was to better understand interaction of the ion and gravity-sensing pathways and the relative roles in the control of root growth direction. To do so, we analyzed the growth of Arabidopsis roots in response to simultaneous gravity and salt stimulation. We found that roots respond to salinity with a change in growth direction in a way that represents an apparent adaptive compromise between gravitropic and salt stimulation. These results suggest that gravitropic signaling and responses in the root cap are controlled, at least in part, by the SOS pathway. Therefore, the ion-sensing SOS signaling pathway may interact with the gravity-sensing system in the columella cells to direct root growth in a coordinated manner. Such an understanding could help breed salt-tolerant plant varieties for agricultural production.
RESULTS
Salt Stress Affects Gravitropic Curvature of Arabidopsis Roots
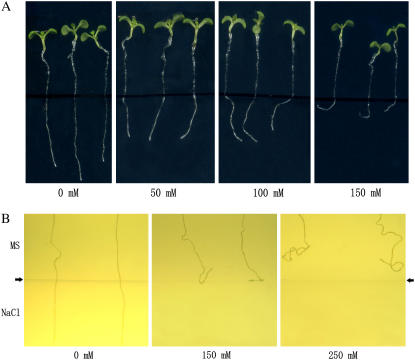
It is known that salt stress inhibits growth of primary roots in Arabidopsis seedlings. Interestingly, in our experiments, we observed that salt stress also modulates gravitropism of the primary roots of young seedlings. When planted vertically, the 5-d-old seedlings germinated normally on Murashige and Skoog (MS) medium containing various concentrations of NaCl, growth direction of the root changed along with increasing NaCl concentrations, and the roots of the stressed plants curved on 150 mm NaCl medium (Figs. 1A and 2B). To confirm the effect of salt on root growth direction, we repeated this experiment in darkness to exclude the possible influence of light. Essentially, the same results were obtained (data not shown). We also designed a two-layer medium experiment in which media supplemented with different concentrations of NaCl was on the bottom and normal MS medium on the top. As shown in Figure 1B, the roots of Columbia-0 (Col-0) seedlings penetrated the interface of the layers and grew straight downwards when both layers were MS media. In contrast, changes in the root growth direction of the seedlings were observed in response to salt stress from the bottom medium. When the bottom medium contained 150 mm NaCl, a small change in root growth direction of the Col-0 seedlings was observed. The primary roots did not pass the interface between normal MS and salt medium, and they grew either along the interface or became curved (Fig. 1B). When the salt concentration in the medium on the bottom was increased to 250 mm, loss of root gravitropism was observed at an early stage, and the root tips bent upwards before they met the MS-salt medium interface (Fig. 1B).
Figure 1.
Salt induces agravitropic response in Arabidopsis. Five-day-old wild-type Col-0 seedlings germinated on MS medium were transferred onto medium containing different concentrations of NaCl (0, 50, 100, 150 mm) in petri dishes and then were placed vertically (A). B, A two-layer medium. MS medium was on the top and salt on the bottom, and the arrows indicate the interface between the two media. The pictures were taken 6 d after transferring (A) or germination (B).
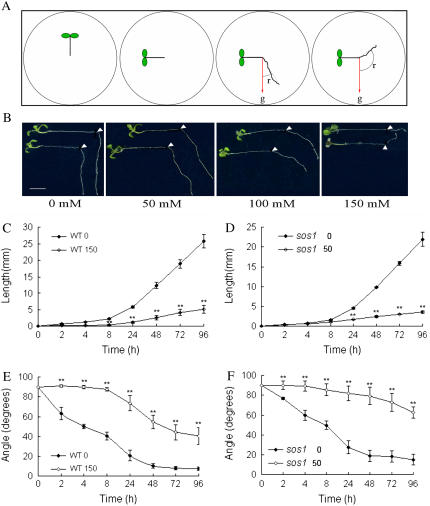
Figure 2.
Experimental system of salt-affected root growth and agravitropic response. A, Diagram shows an experimental design for the study of interaction between salt stress and gravitropism. Five-day-old wild-type Col-0 or sos1 mutant seedlings germinated on MS medium were transferred onto media containing indicated concentrations of NaCl in petri dishes vertically. The plates were then rotated 90° and photographed at intervals following gravistimulation and salt treatment. The angles (r) between gravity (g) and the roots were measured. B, Salt-induced alteration of gravitropic growth on different concentrations of NaCl; the picture was taken 4 d after treatments. The arrowhead indicates the position of the root tips when treatment was initiated. Bars = 0.5 cm. C to F, The time course of root growth (C and D) and root curvature (E and F) of the seedlings during treatments. WT0 and sos10, The wild-type Col-0 and sos1 seedlings grown under normal conditions; WT150 and sos150, responses of the wild-type and sos1 seedlings to 150 mm and 50 mm NaCl, respectively. Data are the means of measurements from three independent experiments (total n = 120), and bars represent the se. Statistical analysis indicates significant differences (**, P < 0.01) compared with the control using two-tailed Student's t test. [See online article for color version of this figure.]
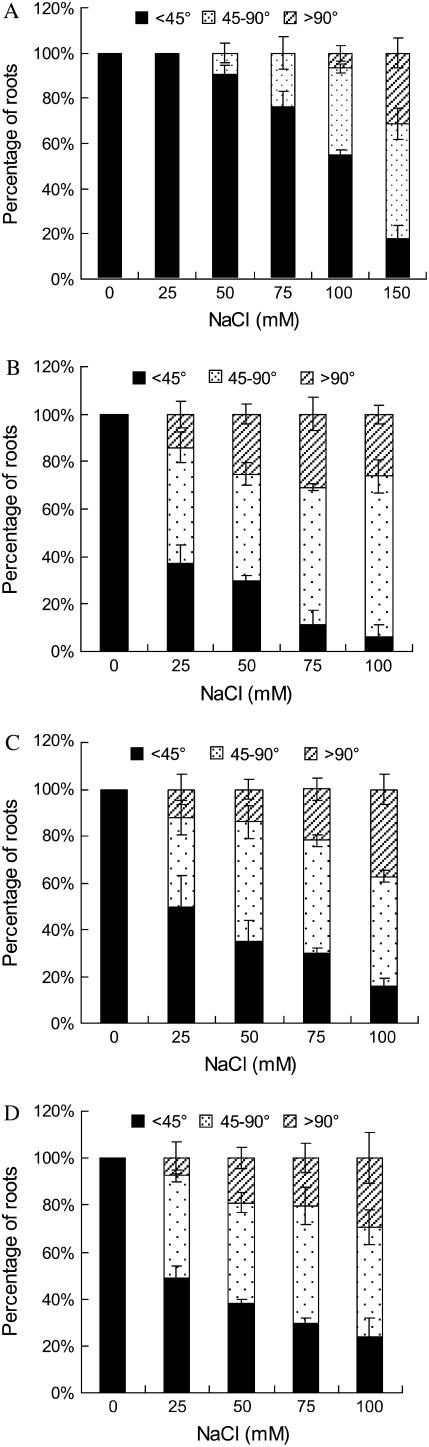
To better understand this tropistic response of Arabidopsis roots to salt, a dose response curve for elongation of gravitropic curvature of Arabidopsis Col-0 primary roots was investigated at various NaCl concentrations (25, 50, 75, 100, and 150 mm). Extreme high salt (NaCl concentrations > 200 mm) was not included in the experiments, because root growth was almost completely inhibited at these concentrations. The alteration of gravitropic response was presented as curvature of root (Fig. 2A). As shown in Figures 2B and 3A, the curvature was increased by salt treatment at the concentrations tested in a dose-dependent manner. At 25 mm NaCl, the primary roots of Arabidopsis seedlings showed normal gravitropism. Loss of gravitropism in roots started to be observed at 50 mm NaCl and was greatest at 150 mm NaCl with more than 85% of the seedlings showing a negative gravitropic response. The results suggest that salt stress and salt-induced signal transduction modulates root growth direction without regard to gravity.
Figure 3.
Tropistic responses of the wild-type Col-0 and sos mutant roots to salt stress. Five-day-old Col-0, sos1-1, sos2-1, and sos3-1 seedlings germinated on MS medium were transferred on media containing various concentrations of NaCl and then horizontal reorientated by 90°. The curvatures of the roots were measured 4 d after treatments for Col-0 (A), sos1-1 (B), sos2-1 (C), and sos3-1 (D). Data represent means of measurements from >60 individuals from three independent experiments. Bars represent se.
Salt Stress Caused Rapid Degradation of Amyloplasts in Columella Cells
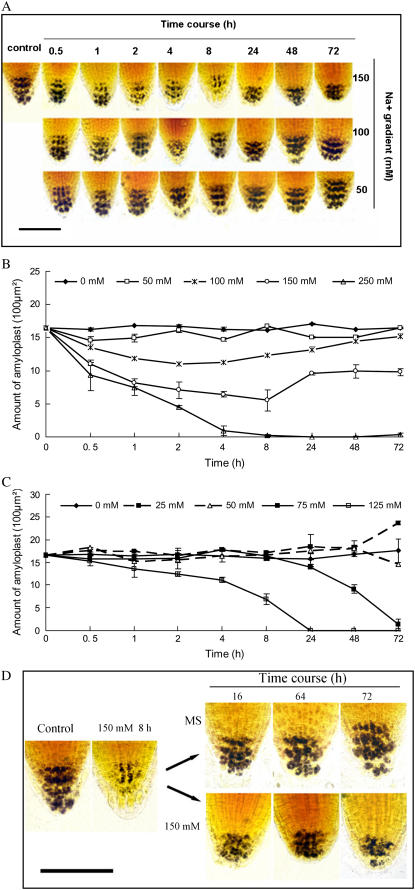
Amyloplasts in columella cells of primary roots have been proposed to play a critical role in gravity sensing in both roots and shoots. To examine whether salt alters amyloplasts in roots, the amyloplasts were visualized by staining with iodine-potassium iodide solution. We found that the amount of amyloplasts in columella cells of roots was substantially decreased with increasing concentrations of NaCl (Fig. 4A). A time course investigation showed that the reduction of amyloplasts was directly correlated with the level of salt stress (Fig. 4, A and B). Under salt stress (NaCl > 50 mm) digestion of amyloplasts was evident about 0.5 to 1 h after treatment, and the amount of amyloplasts continued to decline with prolonged time of treatment. The minimum amount of amyloplasts was observed at about 8 h after exposure to salt.
Figure 4.
Changes in amyloplasts of the columella cells of Col-0 and sos1-1 roots in response to salt and recovery treatments. A and B, Dose response and time course of amyloplast degradation under salt stress in Col-0 roots. Amyloplast starch was stained with iodine-potassium iodide solution, observed under a light microscope, and measured using a computer-assisted image analysis. Bar = 100 μm. C, Kinetics of amyloplasts in sos1-1 root tips under salt stress. D, The Col-0 seedlings treated with 150 mm NaCl for 8 h were transferred to MS medium or 150 mm NaCl for the indicated time. Bar = 100 μm. In B and C, each data point is the mean of measurements of at least 60 individuals from three independent experiments. Bars represent se. [See online article for color version of this figure.]
Interestingly, the amount of amyloplasts in the columella cells was gradually restored to nearly the original level within 48 to 72 h if the seedlings continued to be exposed to salt stress at 100 mm or lower. Prolonged exposure to high salt at 150 mm NaCl resulted in restoration of the amyloplast level to some degree but not to the level of untreated control plants (Fig. 4, A and B). However, the gravitropic growth response of the stressed roots was not recovered under these circumstances, suggesting that salt stress suppressed the gravisensing and the subsequent gravitropic growth of the roots. By contrast, gravitropism recovered when salt-stressed plants were transferred back to MS medium (normal conditions). Starch content was also completely restored to the levels prior to salt stress within 24 h (Fig. 4D). Together, these results suggest that salt-induced changes in starch metabolism in root tip cells may contribute to both altered gravisensing and adaptation of plants to saline environments.
Agravitropic Response in Arabidopsis Roots Is Caused by Ion Effects
Arabidopsis sos mutants (sos1-1, sos2-1, and sos3-1) were subjected to gravitropic analyses and statolith staining. Because these mutants are hypersensitive to salt stress, NaCl concentrations in the media were correspondingly reduced. The results showed that salt stress induced a pronounced agravitropic response in primary roots of the sos mutant seedlings based on the root curvature measurement (Fig. 3, B–D). Compared with the wild-type seedlings, loss of gravitropism in sos seedlings occurred at a lower concentration (25 mm) of NaCl. When grown on 25 mm NaCl, 50% to 65% of sos mutant roots showed altered gravitropic growth, whereas 100% of the wild type responded normally to gravity. Exposure to 100 mm NaCl resulted in about 50% of wild-type seedlings losing their gravitropism (Fig. 3A), whereas 80% to 90% sos mutant roots lost their gravitropism (Fig. 3, B–D). Also, there were more plant roots with a larger degree of curvature, at certain concentrations of NaCl from 50 to 100 mm, in sos mutants than wild-type seedlings. Furthermore, the percentage of seedlings showing an altered gravitropic response was highest in sos1, followed by sos2 and sos3 (Fig. 3, B–D), which correlates well with their salt sensitivity (Zhu et al., 1998).
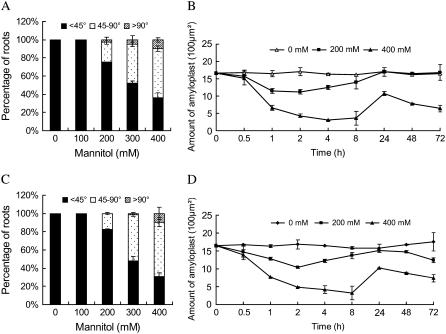
To further confirm that the increased loss of gravitropism displayed by sos mutants is related to ion sensitivity, we examined the root gravitropic growth response under osmotic stress using mannitol as the osmotic stress. We found that nonionic osmotic stress also suppressed the gravitropic response of roots. The percentage of roots losing gravitropism increased with increasing concentration of mannitol in a dose-dependent manner in wild-type Arabidopsis (Fig. 5A). The sos1-1 mutant roots exhibited a similar pattern of gravitropic response (Fig. 5C) and amyloplast metabolism compared to the wild type (Fig. 5, B and D). No difference in root gravitropic response among sos1-1, sos2-1, and sos3-1 mutant plants was observed (data not shown). These results confirm that SOS1, SOS2, and SOS3 are not involved in nonionic osmotic stress-induced agravitropic growth of Arabidopsis roots. Salt-induced modification of gravitropic growth appears to be mediated by ion disequilibrium and can be modulated by the SOS signaling pathway. Alteration in root growth direction against the gravity vector appears to be an adaptive mechanism that adjusts the root distribution to cope with saline conditions.
Figure 5.
The sos1-1 mutants act similarly as the wild-type plants in response to osmotic stress. Five-day-old wild-type (Col-0) and sos1-1 seedlings germinated on MS medium were transferred on medium containing various concentrations of mannitol and then reorientated by 90° with unilateral topside light stimulus. The curvatures of root growth and the amounts of amyloplasts in columella cells were measured as described previously for the wild-type plants (A and B) and sos1-1 mutants (C and D), respectively. The data in B and D are the means of measurements of at least 60 individuals from three independent experiments. Bars represent se.
Growth Arrest of Roots Is Regulated Independently from Root Curvature
To precisely examine the effects of salt on root tropism and how this is related to root growth, we performed a time course analysis of root growth and curvature. The Arabidopsis seedlings were transferred to a medium containing 150 mm NaCl and then gravitropism was stimulated by reorienting the plates by 90°. Figure 2, C to E, shows that, in salt-treated wild-type seedlings, root elongation and root curving commenced at about 8 h after salt treatment and gravitropic stimulation compared with immediate action in the untreated control seedlings. The majority of roots exhibited agravitropic response and amyloplasts in the columella cells showed rapid degradation. The difference in growth inhibition between treated and untreated roots was more pronounced during a prolonged period of treatment (Fig. 2, C–E), and the curvature of roots was also substantially increased under salt stress, and the maximum increase in curvature recorded was approximately 55° at 24 h after reorientation.
The results also showed greater curvature in salt-treated roots than that in untreated control roots at an equivalent root length. As shown in Figure 2, C to F, the wild-type seedling grown on 0 mm NaCl medium for 24 h and on 150 mm NaCl medium for 96 h had almost the same quantity of elongation (about 5 mm). However, the root curvature (40°) under salt stress was much larger that that on 0 mm NaCl medium (20°). This result suggests that salt stress affects root gravitropism more profoundly than simply root growth or elongation. This is further indicated by the observation that some seedlings even exhibited negative and not just reduced gravitropism in response to 150 mm NaCl. Furthermore, examination of the sos1-1 mutant seedlings on 50 mm NaCl showed that root curvature was markedly increased in the stressed plants compared with the wild-type control plants although their root elongation rates were similar (Fig. 2, C–F). These results indicate that increased curvature in salt-treated roots is not likely to be a secondary effect of arrested root growth.
Rapid Amyloplast Degradation Is Impaired in sos Mutants under Salt Stress
To investigate whether amyloplast degradation in columella cells of sos mutants is more susceptible to salt stress than the wild-type plants, we stained amyloplast in sos mutant seedlings. To our surprise, no rapid starch degradation in the columella cells was observed in any of the sos mutant roots at salt concentrations where they showed a strong alteration in gravitropic response (Fig. 4C). Because all of the sos mutants displayed similar responses, only the data for sos1-1 is shown. The salt-induced degradation-synthesis pattern of starch metabolism in Col-0 was not observed in sos mutants within a 72-h period. This observation indicates that sos mutants are likely to be impaired in rapid starch degradation during early stress acclimation conditions and that the SOS pathway also plays an important role in root starch degradation in Arabidopsis.
Interestingly, we found that at 75 mm NaCl, starch in sos1 root caps gradually degraded and disappeared and that there was no recovery in starch synthesis with prolonged treatment (Fig. 4C). To determine if this phenomenon is caused by ion toxicity, we investigated the starch degradation of wild type, sos1-1, sos2-1, and sos3-1. As shown in Figure 4, B and C, the sos mutants exhibited similar trends and dynamic changes in degradation of amyloplasts as observed in the wild type (Col-0) at extremely high and lethal doses of salt. There was a significant positive correlation between timing/salt dose of this particular response and salt sensitivity of the genotypes. For example, the starch reduction started to be observed at 75 and 300 mm NaCl for sos1 and the wild-type seedlings, respectively, and the NaCl levels for sos2 and sos3 fell between these two concentrations (data not shown). Starch amyloplasts could be completely restored if the plants were transferred from the extreme high salt concentrations back to normal conditions. Under very high salt levels, starch completely degraded and plants eventually died. The result again suggests that degradation of the majority of amyloplasts in columella cells of Arabidopsis roots is a general adaptive response to ion disequilibrium.
PIN2 Expression Shows the Similar Kinetics as Amyloplast Reduction
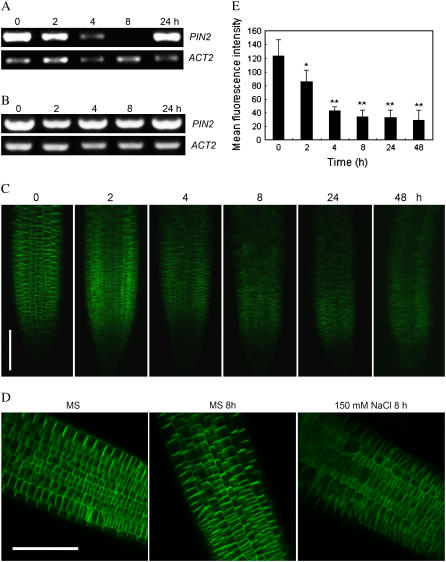
It has been demonstrated that the Arabidopsis auxin efflux carrier PIN2, which is distributed asymmetrically within the cells, plays an important role in regulating basipetal auxin transport and root gravitropic response of the root elongation zone (Ottenschlager et al., 2003). To test the effect of salt on PIN2 gene expression, we analyzed mRNA abundance of PIN2 in 6-d-old seedlings treated with 150 mm NaCl. Time course analysis showed that transcription of PIN2 was first down-regulated by a high concentration of NaCl (Fig. 6A). Reduction of PIN2 transcript was detectable at 2 h after treatment, and the minimum level of PIN2 was observed in the seedlings treated with NaCl for 8 h. However, the PIN2 transcript was then up-regulated and restored to nearly the level before treatment. The trend of PIN2 expression modulation by salt was similar to that of amyloplast degradation under salt stress, indicating that PIN2 abundance may play a role in mediation of root response to salt stress.
Figure 6.
PIN2 response to both salt and gravity stimulations. A and B, Reverse transcription-PCR analysis of mRNA abundance of PIN2 in response to salt stress. Five-day-old Col-0 (A) and sos1-1 (B) seedlings were treated with 150 mm and 100 mm NaCl, respectively. RNA was extracted from the treated roots on the indicated time after salt treatment. Abundance of ACT2 mRNA gene was used as loading control. The data are from a single experiment and are representative of two replicates. C, Expression and localization of PIN2 in the reoriented primary roots at specified time points. Five-day-old Arabidopsis seedlings expressing the PIN2∷PIN2:GFP germinated on MS medium were transferred to the media containing 0 or 150 mm NaCl with 90° horizontal reorientation. GFP signals were visualized by a confocal microscope. Bar = 100 μm. D, Close-up observation of epidermis and cortex cells revealed basipetal localization and expression and diffusion of PIN2 in control seedlings or with 150 mm NaCl at 8 h after treatments. Bar = 100 μm. E, Quantification of GFP fluorescence intensity in root tip cells. n = 10 for each experiment, repeated in triplicate. Statistical analysis indicates significant differences (*, P < 0.05; **, P < 0.01) compared with the control using one-tailed Student's t test. [See online article for color version of this figure.]
We then tested the PIN2 gene expression in sos mutant background. Interestingly, we found that the salt-induced PIN2 expression pattern was blocked in sos1-1 mutant roots (Fig. 6B). The results further demonstrated that the SOS pathway might modulate root response to salt by regulating PIN2 abundance and the subsequent auxin asymmetric distribution.
Salt Stress-Induced Gravitropic Responses Are Related to PIN2 Protein Levels
PIN2 protein level and localization play an important role in redistribution of auxin and the subsequent gravitropic response (Paciorek et al., 2005; Abas, et al., 2006). To test whether the expression/localization of the PIN2 protein is modified by salt stress, we monitored the alterations of PIN2-GFP signal in the wild-type seedlings expressing PIN2∷PIN2:GFP construct during the process. Under normal conditions, PIN2-GFP was found to be restricted to the upper transverse side of epidermal cells and to the lower sides of cortical cells (Fig. 6, C and D). The expression and polar localization of PIN2-GFP were detectable when the Col-0 seedlings were reoriented by 90° on MS medium at about 2 h upon to gravistimulation (data not shown), which is in good agreement with the previous results (Abas et al., 2006). In contrast, PIN2 abundance was dramatically reduced when the plants were subjected to 150 mm NaCl treatment and simultaneous gravistimulation, though PIN2 showed the same localization pattern. The lowest level of PIN2-GFP signal was detected at 8 h after treatment (Fig. 6, C–E). Unlike PIN2 transcripts, the PIN2-GFP abundance was not restored during a prolonged treatment, and PIN2 protein became diffuse in the stressed root tip cells. Distribution of PIN2 from the root tip to the elongation zone was substantially inhibited by salt treatment. Importantly, asymmetric distribution of PIN2 was not seen clearly after 2 h of gravistimulation and salt treatment. These results suggest that salt stress represses the abundance and distribution of PIN2 in response to gravity.
DISCUSSION
Gravitropic response is often overwhelming, particularly during root development. To investigate the interplay between gravitropism and salt stress in determining root growth direction, we developed a method for stimulating salt and gravity responses simultaneously (Fig. 2A). Curvature measurements indicated that gravitropic response of the stressed plant roots is greatly reduced upon exposure to salt stimuli in a dose-dependent manner (Figs. 1–4). We also established a two-layer assay (Fig. 1B), which may create an ion gradient (Eapen et al., 2003). In this method, a normal nutrient medium was in the top of a growth container and a salt stress medium was in the bottom of the container. At the beginning of the experiment, roots of seedlings on the normal medium grew downward, exhibiting gravitropism, but then curved and grew upward toward the lower level of salt. Therefore, we discovered that Arabidopsis roots are hypersensitive to an ion gradient and exhibit reduced gravitropic response to the elevated levels of salt in the bottom layer and curved away from the salt stimuli. The questions of whether or not this directed growth in response to salinity represents a tropism specific to salinity (referred to as halotropism), the role in regulating orientation of the root growth, and the molecular mechanism underlying the halotropism, remain to be investigated.
Our results further show that the negative gravitropism of Arabidopsis roots under salt stress is caused by ion disequilibrium. This conclusion is based on the findings that salt-induced agravitropic response is more pronounced in Arabidopsis sos mutants (Fig. 4). It has been shown that SOS1, SOS2, and SOS3 are essential for Na+ and K+ homeostasis and that sos mutations render plants more sensitive to high Na+ and low K+ in the growth environment (Shi et al., 2000; Zhu, 2002, 2003). We observed that in addition to root growth inhibition, sos mutants exhibited a more profound agravitropic response to salt stress compared to wild-type plants (Fig. 3). However, wild type and sos1-1, sos2-1, and sos3-1 mutants displayed similar responsiveness in gravitropic growth in response to osmotic stress (Fig. 5). The results indicate that ion disequilibrium, rather than osmotic stress, is likely to be an initiator of salt-induced negative gravitropic growth of roots in Arabidopsis. The SOS pathway may mediate this specific tropism in roots that is tailored to the particular saline stress condition.
Analysis of curvature of roots of the wild-type seedlings under salt stress showed that root bending started to be observed approximately 8 h after treatment (Fig. 2, C–F), indicating that gravisensitivity was delayed by salt. Changes in amyloplasts in culumella cells also are delayed to the same extent, indicating a strong correlation between the effects of NaCl on both amyloplast degradation and gravisensing (Fig. 4, A and B). This is consistent with the proposed role of columella cell amyloplasts in gravisensing (Takahashi et al., 2003; Ma and Hasenstein, 2005). However, our results indicate that salt-induced rapid degradation of amyloplasts, the proposed gravisensor, in the columella cells is not likely the main reason for a negative gravitropic response, because sos mutant roots exhibited profound negative gravitropic growth without apparent rapid digestion of amyloplasts (Fig. 4C). It seems that independent sensing and signal transduction pathways exist: one for immediate amyloplast metabolism (degradation and subsequent biosynthesis) and the other for agravitropic growth in response to salt stress. Our results favor the hypothesis that gravity sensing may exist outside of the root cap (Wolverton et al., 2002) but does not exclude the possibilities that salinity could directly affect the pathway for differential growth.
After perception of signals, PIN2-mediated asymmetric distribution of auxin is known to contribute to root bending during tropistic responses, such as gravitropism. These effects are potentially modulated through regulation of the expression of PIN2 at both transcriptional and posttranscriptional levels (Paciorek et al., 2005; Abas et al., 2006). Our findings support this hypothesis, demonstrating that combination of transcriptional and posttranscriptional regulatory mechanisms is crucial for the complex changes in PIN2 abundance and polar distribution during the gravitropic response under salt stress. Posttranscriptional control of PIN2 appears to play a more important role in the process. This is supported by the observation that roots display reduced gravitropic growth, even when PIN2 gene expression was restored but the level of PIN2 protein remains reduced during a prolonged treatment period (Fig. 6, A and C). The alterations in PIN2 gene expression and PIN2 abundance and polar localization under salt stress may interfere with the distribution of auxin within root meristem cells and auxin transport to root elongation zone (Petrásek et al., 2006; Wisniewska et al., 2006). Such a change may promote agravitropic growth to modify the direction of root growth away from the stress stimuli. Further, our findings by analysis of sos mutants (Fig. 6, A and B) indicate that the SOS pathway may be required for the regulation of PIN2 gene expression and modification of gravitropic response under saline conditions. However, the issue of how PIN2 is regulated at the posttranscriptional level in this process still needs to be explored. Further studies are also required to determine whether other PINs are involved in regulation of plant root growth and growth direction.
Our results show that salt not only inhibits root elongation but also greatly affects root growth direction (Fig. 2, C–F). The fact that in the wild-type seedlings, the stressed roots showed about an 8-h delay in both root elongation and curving indicates that root elongation and curving may be related. However, the curvatures were much greater in the stressed roots than that of the untreated controls when their root lengths were similar, suggesting that root curving and root growth might not be closely linked. Examination of curvature and root lengths of sos1-1 mutant supports this hypothesis. The significant difference in root curvatures between the wild-type and sos1-1 mutant roots under salt stress when they had similar root elongation rates indicates that the two processes are independent. Both processes are likely regulated by different branches downstream of the SOS signaling pathway under salt stress.
Our data indicates that degradation of amyloplasts in the columella cells may not play a key role in the alteration of gravisensing, but two types of response in amyloplast metabolism were observed in response to the severity of salt stress. Exposure to moderate salt stress (<200 mm NaCl) resulted in immediate degradation of amyloplasts in Arabidopsis (Fig. 4A). The amyloplast level was markedly reduced within 30 min, and minimum amyloplast content was seen after 8 h of salt treatment. The starch grains started to reform during prolonged treatment of moderate salt stress (>24 h), though the levels of restoration varied depending on the severity of stress. However, starch in columella cells completely degraded within 8 h, and no recovery was observed in response to high salinity (≥250 mm NaCl; Fig. 4B). These findings raised the important questions of what are the functions of amyloplasts in the columella cells and why do they show two types of metabolism in response to different levels of salt stress? Based on the previous evidence and our findings, we proposed that amyloplasts in root tips may have three roles. First, amyloplasts are not detectable within the first several hours of imbibition and appear at the onset of gravisensing. This result suggests a role of amyloplasts in gravisensing (Ma and Hasenstein, 2005). Second, amyloplasts are a local carbohydrate reserve that can release sugar to protect root tips from stresses that inhibit photosynthesis. The rate of metabolism of amyloplasts, when exposed to mild stress, shows an active adjustment during stress adaptation. However, disappearance of starch when salt stress becomes severe enough indicates the end of sugar availability, which is lethal. That might be the main reason for cell death of the primary root tips (Huh et al., 2002). Thus, the starch disappearance might be the central event controlling root tip growth under salt stress. Third, increased sugars and metabolites produced from starch degradation may be involved in osmoregulation, as osmolytes, in the cells. Under conditions of low osmotic potential or high ionic strength, osmolytes have been thought to function to restore osmotic potential of the cytoplasm to drive water uptake to maintain cell turgor, stabilize protein complexes and membranes, and scavenge reactive oxygen species, etc. (Cushman, 2001). It is likely that degradation of amyloplasts might be a possible mechanism of rapid osmoregulation, by which columella cells could maintain correct amounts of water and salt concentration in the cells. This is consistent with previous studies that demonstrate that salt stress-induced accumulation of soluble sugars enhances stress tolerance (Feng et al., 2002; Garg et al., 2002).
The regulatory mechanism of starch metabolism in root columella cells in the salt-stressed plants is currently not clear. Our results demonstrate that the SOS signaling pathway regulates rapid starch digestion induced by salt, because immediate degradation of amyloplasts in columella cells was not observed in sos mutant root tips under salt stress (Fig. 4D). Together, the observations suggest that the SOS pathway plays an important role in early adaptation to salt stress by regulating rapid starch degradation in columella cells of root tips. These findings are the first, to our knowledge, to show that columella cells are highly susceptible to high ionic strength that causes immediate degradation of amyloplasts.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 homozygous lines expressing PIN2∷PIN2:GFP (Xu and Scheres, 2005) and sos1-1, sos2-1, and sos3-1 mutants were used in this study. The seeds were sterilized and plated on MS medium (Murashige and Skoog, 1962) supplemented with 2% Suc at 4°C in darkness for 2 d of stratification, then transferred to a growth chamber under conditions of 20°C to 22°C with a 16-h-light/8-h-dark cycle. Five-d-old seedlings with relative straight roots, 1.0 to 1.5 cm in length, were used for most experiments. For the two-layer medium experiment, Arabidopsis seeds were directly planted on the top MS medium; both media were 1 cm in height.
Salt Treatment and Gravity Stimulation
For petri dish culture, 5-d-old Arabidopsis seedlings were placed on the agar plates containing media supplemented with various concentrations of NaCl or mannitol. The seedling roots were positioned vertically and the initial sites of root tips were marked. The gravitropic response of primary roots in salt stress media was stimulated by reorienting the petri dishes by 90°. Pictures of seedlings were digitized using a scanner (Epson Perfection 1670, SEIKO Epson) at the specified time intervals after gravistimulation. Growth length and curvature of roots were measured using ImageJ software version 1.38 (http://rsbweb.nih.gov/ij/download.html). Root curvature was measured as the angle of deviation from the initial straight line of the seedling root as shown in Figure 2A. Roots of sos1-1, sos2-1, and sos3-1 mutants were also subjected to salt stress or osmotic stress using mannitol as the osmo agent. At least 20 seedlings of three independent lines were used in the experiments. Statistical differences were determined using Student's two-tailed t test.
Statolith Staining and Light Microscopy Observation
Five-day-old Arabidopsis seedling with similar root lengths were selected and transferred to MS medium supplemented with NaCl gradients for 0.5- to 72-h time gradient treatments. Observation and measurement of the amyloplasts in the columella cells of the root cap were made according to the method described by Takahashi et al. (2003).
Reverse Transcription-PCR Analysis
Reverse transcription-PCR analyses were performed to study the transcription of PIN2 gene in Arabidopsis wild-type Col-0 and sos1-1 mutant seedlings without or with NaCl treatment for 2, 4, 8, and 24 h. Total RNA was extracted from plant root samples using the Trizol reagent (Invitrogen). Two micrograms of DNAse-treated (RQ1 DNAse; Promega) total RNA was used as a template for first-strand cDNA synthesis with Superscript II (Invitrogen) and an oligo(dT) primer. The ACTIN2 (locus no. At3g18780) gene was used as a positive internal control with primers 5′-CCTTCGTCTTGATCTTGCGG-3′ and 5′-AGCGATGGCTGGAACAGAAC-3′. The following gene-specific primers were used to detect PIN2 transcript: 5′-AAGTCACGTACATGCATGTG-3′ and 5′-AGATGCCAACGATAATGAGTG-3′. Ten-microliter reactions were set up for each sample and amplified through 25 cycles. Eight microliters of the product was run on an agarose gel and the results were documented.
Determination of PIN2 Expression and Distribution
For visualization of GFP, 5-d-old transgenic seedlings expressing PIN2∷PIN2:GFP were transferred to MS or 150 mm NaCl medium. After 2, 4, 8, 24, and 48 h treatment with gravistimulation and salt as described earlier, the excised roots were mounted immediately and examined with a Zeiss LSM 510 Confocal laser scanning microscope (Carl Zeiss MicroImaging) with a 488-nm excitation line and a 530-nm emission filter. All images were taken under same conditions. Integrated optical density and area of root tip (450 μm in length as shown in Fig. 6C) were measured using ImageJ software. The mean fluorescence intensity was calculated as integrated optical density/area.
Acknowledgments
We thank Hongtao Ji, Yiliang Xu, and Yunqiao Shu for their technique assistance for measurement of amyloplasts. We also thank the Arabidopsis Resource Center at Ohio State University for providing the Col-0 seeds used in this study, Dr. Jiankang Zhu, Department of Botany and Plant Pathology, University of California, Riverside, for the homozygous sos mutant lines, and Dr. Ben Scheres, Department of Molecular Cell Biology, Utrecht University, The Netherlands, for kindly providing us seeds expressing PIN2∷PIN2:GFP. Finally, we thank Dr. Ray Bressan, Purdue University, for his critical reading of the manuscript.
This work was supported by the One Hundred Talent Program of Chinese Academy of Sciences, and by the National Natural Science Foundation of China (grant no. 30570143).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Xia Li (xli@genetics.ac.cn).
Some figures in this article are displayed in color online but in black and white in the print edition.
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8 249–256 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45 437–448 [Google Scholar]
- Cushman JC (2001) Osmoregulation in plants: implications for agriculture. Am Zool 41 758–769 [Google Scholar]
- Eapen D, Barroso ML, Campos ME, Ponce G, Corkidi G, Dubrovsky JG, Cassab GI (2003) A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis. Plant Physiol 131 536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12 185–190 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Yeo AR (1995) Breeding for salinity resistance in crop plants: where next? Aust J Plant Physiol 22 875–884 [Google Scholar]
- Friml J, Palme K (2002) Polar auxin transport: old questions and new concepts? Plant Mol Biol 49 273–284 [PubMed] [Google Scholar]
- Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 9 15898–15903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Kramer VL, Wang YS, Chen R, Perbal G, Gilroy S, Blancaflor EB (2004) The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J 39 113–125 [DOI] [PubMed] [Google Scholar]
- Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J 29 649–659 [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Takahashi H, Biro RL (1985) A pea mutant for the study of hydrotropism in roots. Science 230 445–447 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miege C, Rollin C, Gaude T (2006) AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443 106–109 [DOI] [PubMed] [Google Scholar]
- Johannes E, Collings DA, Rink JC, Allen NS (2001) Cytoplasmic pH dynamics in maize pulvinal cells induced by gravity vector changes. Plant Physiol 127 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD (1989) Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177 198–206 [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S (1997) Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 114 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J (1995) Root architecture and plant productivity. Plant Physiol 109 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Hasenstein KH (2005) The onset of gravisensitivity in the embryonic root of flax. Plant Physiol 140 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE (2005) Intrinsic and environmental response pathways that regulate system architecture. Plant Cell Environ 28 67–77 [DOI] [PubMed] [Google Scholar]
- Mizuno H, Kobayashi A, Fujii N, Yamashita M, Takahashi H (2002) Hydrotropic response and expression pattern of auxin-inducible gene, CS-IAA1, in the primary roots of clinorotated cucumber seedlings. Plant Cell Physiol 43 793–801 [DOI] [PubMed] [Google Scholar]
- Moore I (2002) Gravitropism lateral thinking in auxin transport. Curr Biol 12 R452–R454 [DOI] [PubMed] [Google Scholar]
- Morita MT, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7 712–718 [DOI] [PubMed] [Google Scholar]
- Muller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, et al (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435 1251–1256 [DOI] [PubMed] [Google Scholar]
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312 914–918 [DOI] [PubMed] [Google Scholar]
- Plieth C, Trewavas AJ (2002) Reorientation of seedlings in the earth's gravitational field induces cytosolic calcium transients. Plant Physiol 129 786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AC, Allen NS (1999) Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol 121 1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H (1997) Hydrotropism: the current state of our knowledge. J Plant Res 110 163–169 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Yamazaki Y, Kobayashi A, Higashitani A, Takahashi H (2003) Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol 132 805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertová D, Brewer PB, Ruzicka K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312 883. [DOI] [PubMed] [Google Scholar]
- Wolverton C, Ishikawa H, Evans ML (2002) The kinetics of root gravitropism: dual motors and sensors. J Plant Growth Regul 21 102–112 [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6 441–445 [DOI] [PubMed] [Google Scholar]
- Zhu JK, Liu J, Xiong L (1998) Genetic analysis of salt tolerance in Arabidopsis: evidence for a role of potassium nutrition. Plant Cell 10 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]