Abstract
We identify here the Arabidopsis (Arabidopsis thaliana) gene encoding the third enzyme in the biotin biosynthetic pathway, dethiobiotin synthetase (BIO3; At5g57600). This gene is positioned immediately upstream of BIO1, which is known to be associated with the second reaction in the pathway. Reverse genetic analysis demonstrates that bio3 insertion mutants have a similar phenotype to the bio1 and bio2 auxotrophs identified using forward genetic screens for arrested embryos rescued on enriched nutrient medium. Unexpectedly, bio3 and bio1 mutants define a single genetic complementation group. Reverse transcription-polymerase chain reaction analysis demonstrates that separate BIO3 and BIO1 transcripts and two different types of chimeric BIO3-BIO1 transcripts are produced. Consistent with genetic data, one of the fused transcripts is monocistronic and encodes a bifunctional fusion protein. A splice variant is bicistronic, with distinct but overlapping reading frames. The dual functionality of the monocistronic transcript was confirmed by complementing the orthologous auxotrophs of Escherichia coli (bioD and bioA). BIO3-BIO1 transcripts from other plants provide further evidence for differential splicing, existence of a fusion protein, and localization of both enzymatic reactions to mitochondria. In contrast to most biosynthetic enzymes in eukaryotes, which are encoded by genes dispersed throughout the genome, biotin biosynthesis in Arabidopsis provides an intriguing example of a bifunctional locus that catalyzes two sequential reactions in the same metabolic pathway. This complex locus exhibits several unusual features that distinguish it from biotin operons in bacteria and from other genes known to encode bifunctional enzymes in plants.
Biotin is a vitamin that functions as an enzyme cofactor in cellular metabolism to facilitate CO2 transfer during carboxylation and decarboxylation reactions. Biosynthesis of biotin from pimeloyl-CoA and Ala, first elucidated in bacteria more than 40 years ago, occurs through four reactions that result in the sequential production of 7-keto-8-aminopelargonic acid (KAPA), 7,8-diaminopelargonic acid (DAPA), dethiobiotin (DTB), and ultimately biotin. In Escherichia coli, four genes that encode these enzymes (bioF, bioA, bioD, bioB) are clustered into an operon whose structure and function has been examined in detail (DeMoll, 1996). Biosynthesis of biotin in plants occurs through a similar pathway but is divided between two compartments. The initial production of KAPA occurs in the cytosol (Pinon et al., 2005), whereas the final conversion of DTB to biotin occurs in mitochondria (Weaver et al., 1996; Baldet et al., 1997; Picciocchi et al., 2003; Arnal et al., 2006). Intracellular localization of the intermediate reactions remains unresolved (Rébeillé et al., 2007). Metabolic enzymes that require biotin as a cofactor are located in four different compartments: chloroplasts, mitochondria, protein bodies, and the cytosol (Nikolau et al., 2003). Plants must therefore possess transport mechanisms for delivering biotin and related intermediates to their proper locations in the cell.
Two auxotrophic mutants of Arabidopsis (Arabidopsis thaliana) have played an important role in the analysis of biotin biosynthesis in plants. The bio1-1 mutant was isolated following a forward genetic screen designed to identify embryo-defective (emb) mutants in which arrested embryos were rescued on an enriched nutrient medium (Schneider et al., 1989). Aborted seeds from heterozygous siliques contain reduced levels of biotin, consistent with a defect in biotin synthesis (Shellhammer and Meinke, 1990). Embryo rescue experiments and subsequent complementation with the bioA ortholog from E. coli demonstrated that mutant embryos are defective in the conversion of KAPA to DAPA (Schneider et al., 1989; Patton et al., 1996). The bio2-1 mutant was isolated through a similar genetic screen for embryo defectives and was shown to be disrupted in the final reaction of the pathway (Patton et al., 1998). A second allele (bio2-2) with an insertion identified through reverse genetics has recently been described (Arnal et al., 2006). Because the original bio2-1 mutant contains a deletion that includes an adjacent gene (FPA) required for flowering (Schomburg et al., 2001), rescued bio2-1 homozygotes produce giant rosettes under long days. Rescued bio1-1 and bio2-2 homozygotes, in contrast, appear normal when supplemented with biotin. A complete list of Arabidopsis genes and mutants involved in biotin synthesis is presented in Table I.
Table I.
Biotin biosynthetic genes and auxotrophic mutants of Arabidopsisa
| Arabidopsis Gene | Bacterial Ortholog | Arabidopsis Locus | Enzymatic Product | Allele | Mutagen | Line No. | Reference on Mutant |
|---|---|---|---|---|---|---|---|
| BIO4 | bioF | At5g04620 | KAPA | NAb | NAb | NAb | None identified |
| BIO1 | bioA | At5g57590 | DAPA | bio1-1 | EMS | 122G-E | Schneider et al. (1989) |
| bio1-2 | T-DNA | 36172 | www.SeedGenes.orgc | ||||
| bio1-3 | T-DNA | 46455 | www.SeedGenes.orgc | ||||
| BIO3 | bioD | At5g57600 | DTB | bio3-1 | T-DNA | RATM53-2665-1G | This article |
| bio3-2 | T-DNA | RATM53-3000-1G | This article | ||||
| bio3-3 | T-DNA | SALK_023399 | This article | ||||
| BIO2 | bioB | At2g43360 | Biotin | bio2-1 | EMS | emb49 | Patton et al. (1998) |
| bio2-2 | T-DNA | GABI_100C11 | Arnal et al. (2006) |
Genes are listed in order of their function in the pathway.
Not applicable (NA) because no mutants have been identified.
Refer also to McElver et al. (2001) and Tzafrir et al. (2004).
Several years ago, we initiated a large-scale T-DNA insertional mutagenesis project with colleagues at Syngenta that was designed to identify genes required for embryo development in Arabidopsis (McElver et al., 2001; Tzafrir et al., 2004). Two additional alleles of bio1 uncovered through that forward genetic screen are described in this article. We also began to pursue reverse genetic approaches to identify EMB genes missed through forward genetics. One approach was to focus on nonredundant genes associated with metabolic pathways that were known to be required for embryo development. The effectiveness of this strategy is illustrated by the recent identification of multiple His auxotrophs defective in embryo development (Muralla et al., 2007). In addition, we focused again on the biotin pathway and the bioD ortholog (BIO3) required for the conversion of DAPA to DTB. This eventually led to the unexpected discovery that BIO3 and BIO1 are positioned adjacent to each other on the chromosome, in the same orientation as found in a variety of microorganisms, and that differential splicing results in production of two types of full-length transcripts, one with the potential to encode separate proteins and the other capable of producing a bifunctional fusion protein. We document here the structure and function of this unusual locus, provide indirect evidence that both of the corresponding enzymatic reactions take place within mitochondria, examine related genes and transcripts from other plants and fungi, and present the results of a genome-wide scan for similar types of complex loci associated with metabolic pathways in Arabidopsis.
RESULTS
Molecular Identification of the BIO1 Locus
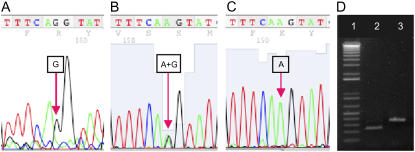
Three candidate BIO1 genes were identified based on the genetic map location of the bio1-1 mutant allele (Patton et al., 1991) and BLASTP searches of the Arabidopsis genome queried with the BioA ortholog from E. coli. Two of these candidates, At5g46180 and At5g63570, were eliminated from consideration because they encode known enzymes, Orn-δ-aminotransferase (Roosens et al., 1998) and chloroplastic Glu-1-semialdehyde 2,1-aminomutase (Ilag et al., 1994). The remaining candidate, At5g57590, is predicted to encode an aminotransferase class III protein that shares 26% sequence identity with BioA. Experimental confirmation that this gene corresponds to BIO1 was obtained by sequencing PCR-amplified genomic fragments from rescued bio1-1 homozygotes grown on biotin. A single nucleotide substitution (G to A) that modifies the 3′ acceptor site of the final intron was found in mutant plants. To ensure that this polymorphism was not due to a sequencing error, we amplified and sequenced this genomic region from progeny plants derived from a single heterozygote. Three different genotypes of plants were found at the expected frequencies in this population. Heterozygotes and homozygotes exhibited the predicted polymorphism (A and G) at the mutation site (Fig. 1, A–C). These results confirmed that At5g57590 corresponds to the BIO1 gene.
Figure 1.
BIO1 gene identification and nature of the point mutation in bio1-1. The 3′ end of the last intron of At5g57590 in wild-type plants (TTTCAG) is modified in bio1-1 homozygotes (TTTCAA). The 5′ end of the last exon (GTAT) remains unchanged. Refer to Supplemental Figure S1 for additional details on the location of this sequence polymorphism. A, Sequencing of genomic DNA from wild-type plants reveals a G nucleotide at the mutation site. B, Genomic DNA from heterozygotes yields a doublet peak that results from the expected mixture of A and G nucleotides at the mutation site. C, Rescued homozygotes exhibit a single peak, consistent with the G to A substitution. D, RT-PCR products obtained from this region demonstrate that transcripts from rescued homozygotes (lane 3) are longer than normal because they include the final intron (confirmed by sequencing) not found in transcripts from wild-type plants (lane 2). The five smallest bands in the DNA ladder (lane 1) range from 100 to 500 nucleotides.
Characterization of Additional bio1 Mutant Alleles
Two embryo-defective mutants identified through a forward genetic screen of T-DNA insertion lines generated at Syngenta (McElver et al., 2001) were found to contain insertions in the BIO1 region. Genetic complementation tests revealed that both mutants (bio1-2 and bio1-3) were allelic to the original ethyl methanesulfonate (EMS) allele (bio1-1). Mutant embryos from immature siliques of bio1-2 heterozygotes were rescued in culture on DAPA, DTB, or biotin, consistent with the results of previous experiments with bio1-1 (Schneider et al., 1989; Shellhammer, 1991). Mutant embryos from both insertion lines exhibited a range of phenotypes similar to bio1-1 and less severe than either bio2-1 or bio2-2.
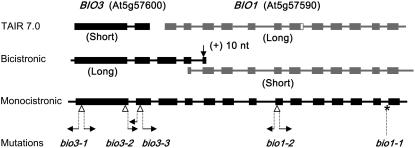
The locations of mutation sites in bio1 mutant alleles in relation to different annotated versions of the BIO3-BIO1 locus are presented in Figure 2. The bio1-2 mutant represents a putative null allele because the insertion is located within an exon in the middle of the BIO1 coding region. Flanking sequences obtained from both sides of the insert revealed a small deletion associated with the insertion (Supplemental Fig. S1). The point mutation in bio1-1 results in a longer transcript but a shorter open reading frame (ORF), which leads to a defective protein lacking the normal C terminus (Fig. 1D). Based on a comparison of mutant phenotypes, this altered protein appears to retain little BIO1 function. The bio1-3 insertion is located downstream of the BIO1 coding region, but the precise location remains unresolved because flanking sequences obtained from both sides of the insert gave contradictory information (Supplemental Fig. S1). The failure of bio1-3 to complement either bio1-1 or bio1-2 in genetic crosses nevertheless confirms that BIO1 function in this mutant is disrupted.
Figure 2.
Genome annotation and mutation sites for the BIO3-BIO1 region. Two separate genes (BIO3 short and BIO1 long) are predicted at TAIR (www.arabidopsis.org). Bicistronic cDNA has the potential to encode two distinct proteins (BIO3 long and BIO1 short). Monocistronic cDNA contains a single ORF that encodes a bifunctional fusion protein. Monocistronic (−10) and bicistronic (+10) transcripts differ with respect to the presence or absence of 10 nucleotides (arrow) at the end of intron 4. White triangles represent insertion sites for T-DNA mutant alleles. Horizontal arrows designate the locations of flanking sequences obtained. The asterisk marks a single nucleotide substitution that disrupts splicing of the last intron in the bio1-1 allele. The final 33 nucleotides of BIO1 exon 7 in the TAIR model (white rectangle) are differentially spliced and are not present in the full-length cDNAs.
Isolation and Characterization of bio3 Mutant Alleles
A candidate BIO3 gene (At5g57600) was identified in the Arabidopsis genome based on sequence homology to the BioD protein of E. coli. Three insertion lines that disrupted the coding region were obtained from the Arabidopsis Biological Resource Center (bio3-3) and the RIKEN Bioresource Center in Japan (bio3-1 and bio3-2). All three lines generated heterozygous plants that produced siliques with approximately 25% aborted seeds. Linkage between the T-DNA insert and mutant phenotype was confirmed by PCR genotyping of individual plants. Genetic complementation tests demonstrated that all three mutants are allelic (Table II). Phenotypes of bio3-arrested embryos are similar to bio1 alleles and less severe than bio2 alleles (Table III). Mutant embryos are pale and typically block at the transition to cotyledon stages of development. All three bio3 mutants are likely to be nulls based on insert locations. It therefore appears that interfering with the initial reactions in biotin synthesis, catalyzed by BIO1 and BIO3, is less detrimental to embryo development than elimination of the final (BIO2) step. One possible explanation is that maternal supplies of DAPA and DTB may contribute somewhat to continued development of bio1/bio1 and bio3/bio3 embryos.
Table II.
Results of genetic complementation testsa
| Female Parent | Male Parent | Siliques Screened | Results Obtained |
|---|---|---|---|
| bio1-2 | bio1-3 | 5 | Allelic |
| bio1-1 | bio3-1 | 4 | Allelic |
| bio1-1 | bio3-2 | 4 | Allelic |
| bio1-1 | bio3-3 | 2 | Allelic |
| bio3-3 | bio1-1 | 3 | Allelic |
| bio1-2 | bio3-1 | 3 | Allelic |
| bio1-2 | bio3-2 | 3 | Allelic |
| bio1-2 | bio3-3 | 5 | Allelic |
| bio3-3 | bio1-2 | 7 | Allelic |
| bio3-1 | bio3-2 | 4 | Allelic |
| bio3-2 | bio3-1 | 4 | Allelic |
| bio3-3 | bio3-1 | 2 | Allelic |
| bio3-3 | bio3-2 | 2 | Allelic |
Crosses were performed between heterozygotes and the resulting siliques were screened for aborted seeds prior to maturity.
Table III.
Phenotypes of arrested embryos recovered from mutant seedsa
| Mutant Allele | Mutant Seeds | Average Embryo Size | Phenotypic Classes of Mutant Embryos
|
|||
|---|---|---|---|---|---|---|
| Preglobular | Globular | Transition | Cotyledon | |||
| % | μm ± (sd) | |||||
| bio1-1 | 23.1 | 270 (80) | 0 | 1 | 2 | 97 |
| bio1-2 | 27.0 | 300 (100) | 2 | 9 | 0 | 89 |
| bio1-3 | 34.8 | 320 (90) | 0 | 9 | 4 | 87 |
| bio3-1 | 23.5 | 210 (80) | 0 | 6 | 29 | 65 |
| bio3-2 | 23.8 | 190 (60) | 0 | 3 | 36 | 61 |
| bio3-3 | 24.3 | 230 (110) | 9 | 12 | 13 | 66 |
| bio2-1 | 27.6 | 50 (40) | 22 | 64 | 5 | 9 |
Embryos were removed from 100 aborted seeds and classified as described at www.seedgenes.org.
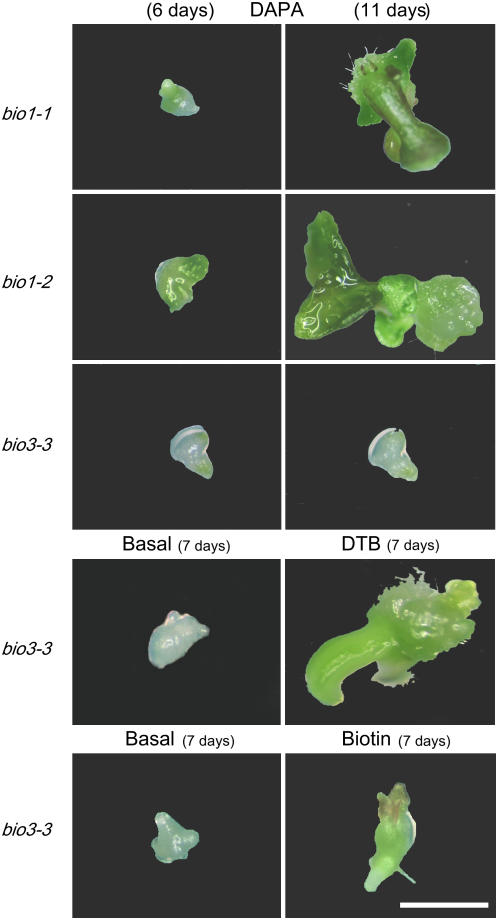
Mutant embryos from parental bio1 and bio3 heterozygotes were rescued by watering plants with biotin (Table IV). Progeny seedlings derived from rescued siliques exhibited the expected 1:2:1 ratio of genotypes (wild type, heterozygote, and homozygote). Responses of mutant embryos in culture are illustrated in Figure 3 and Table V. Immature embryos from bio3 heterozygotes were fully rescued on DTB and biotin but not on DAPA, consistent with the predicted role of BIO3 in biotin synthesis. Biological activity of the DAPA used in culture experiments was confirmed by successful rescue of bio1 mutant embryos. Failure to rescue bio2 embryos indicated that DAPA stocks were not contaminated with biotin. We conclude from these experiments that bio3 mutants of Arabidopsis are defective in the conversion of DAPA to DTB.
Table IV.
Rescue of mutant embryos in siliques of heterozygous plants supplemented with 1 mm biotina
| Mutant | Plants Rescued | Progeny Seedlings Transplanted | Total Seedlings Genotyped | WT | HET | HMZ |
|---|---|---|---|---|---|---|
| bio3-1 | 7 | 111 | 71 | 14 | 40 | 17 |
| bio3-2 | 6 | 121 | 90 | 23 | 38 | 29 |
| bio1-2 | 6 | 131 | 89 | 17 | 46 | 26 |
| Total | 19 | 363 | 250 | 54 | 124 | 72 |
Rescued heterozygotes were identified by the absence of aborted seeds in developing siliques. Mature seeds from three rescued siliques (per mutant) were germinated on agar plates containing 0.1 μm biotin. The resulting seedlings, which all appeared normal, were transplanted to pots watered with 100 μm biotin and PCR genotyped. WT, Wild type; HET, heterozygote; HMZ, homozygote.
Figure 3.
Responses of mutant embryos in culture. Immature embryos were removed from heterozygous siliques, plated on agar medium containing the supplements noted, and observed after the specified number of days in culture. Top, Dual images of three embryos at two different time points. DAPA, a biotin intermediate, was expected to rescue bio1 embryos but not bio3 embryos. DTB, a later intermediate, was expected to rescue bio3 embryos. See Table V for additional details. Scale bar = 1 mm.
Table V.
Response of mutant embryos cultured on DAPAa
| Genotype | Embryos Cultured | Extent of Embryo Response in Culture
|
||||
|---|---|---|---|---|---|---|
| A | B | C | D | F | ||
| bio1-1 | 60 | 44 | 6 | 10 | 0 | 0 |
| bio1-2 | 60 | 36 | 8 | 12 | 0 | 4 |
| bio3-3 | 60 | 0 | 0 | 1 | 52 | 7 |
| bio2-1 | 20 | 0 | 0 | 0 | 0 | 20 |
| Wild type | 40 | 25 | 5 | 2 | 8 | 0 |
Embryos were removed from immature siliques of heterozygotes and cultured on 1 to 2 μm DAPA. Embryo stages were noted at the time of culture. Responses were ranked after 21 d: A, extensive green callus with shoots; B, green callus with small shoots; C, green callus without shoots; D, trace amount of callus (unpigmented); F, no change in culture.
Allelism between bio1 and bio3 Heterozygotes
An unexpected result was obtained when genetic complementation tests were performed between bio1 and bio3 heterozygotes (Table II). In every combination examined, mutants failed to complement, suggesting that a single gene was disrupted. Because these results were initially analyzed without knowledge of the types of transcripts produced, we reasoned that T-DNA insertions in BIO3 might be reducing expression of the downstream BIO1 gene. We then attempted to locate EMS mutations in the BIO3 coding region by searching the Arabidopsis TILLING database (Henikoff et al., 2004) with the hope that point mutations would have more limited effects. Unfortunately, no candidate mutations in the appropriate region were identified. We therefore concluded, based on genetic evidence alone, that BIO1 and BIO3 define a single genetic locus.
BIO1 and BIO3 Define a Single Locus That Exhibits Differential Splicing
Molecular evidence in support of a single chimeric locus was obtained by characterizing cDNAs derived from this region of the genome. The Arabidopsis EST clone RZ128g09R (GenBank accession no. AV551591) was first sequenced and found to match the exon structure of At5g57590. Sequencing of a 3′-RACE product derived from this cDNA identified a 121-nucleotide 3′-untranslated region (UTR). A 5′-RACE product was then sequenced and found to contain a single ORF that included both BIO3 (At5g57600) and BIO1 (At5g57590). The 78-nucleotide 5′-UTR was later confirmed in a cap-dependent RACE experiment (Maruyama and Sugano, 1994). These combined results demonstrate the existence of monocistronic, full-length cDNA (GenBank accession no. EU089963) capable of encoding a single fusion protein (833 amino acids) with potentially two different catalytic activities.
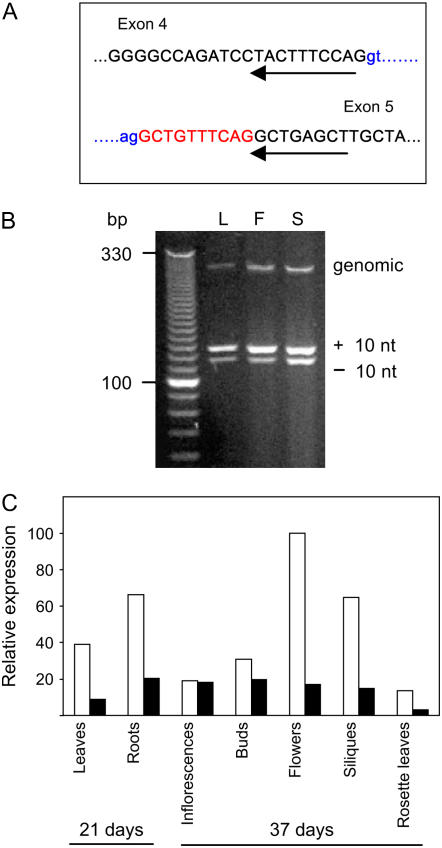
Two additional full-length cDNAs spanning the BIO3-BIO1 locus were found in public databases: RAFL22-07-J07 (Seki et al., 2002) and BX842298 (Castelli et al., 2004). The RAFL22 clone is bicistronic and contains separate BIO3 and BIO1 ORFs. The BX842298 clone includes small insertions and deletions that disrupt the BIO1 ORF. Whether these single nucleotide polymorphisms reflect true differences in transcripts or represent artifacts of sequencing remains unresolved. Another bicistronic cDNA was identified in the Nikolau laboratory (GenBank accession no. EU090805). Sequence alignments revealed a 10-nucleotide region in the bicistronic clones that is missing in the monocistronic clone (Supplemental Fig. S2). This short sequence (5′-GCTGTTTCAG-3′) provides an alternative 3′-splice acceptor site that corresponds to the end of intron 4 in monocistronic (−10) transcripts and the start of exon 5 in bicistronic (+10) transcripts.
Four different ORFs can be identified within this region based on gene models and cDNA sequences (Figs. 2 and 4). The BIO3 (long) ORF found in the bicistronic clones terminates right after the (+10) sequence. The Arabidopsis Information Resource (TAIR) 7.0 annotation of the BIO3 (short) ORF utilizes an upstream stop codon that is predicted in other models to be part of intron 2. The resulting protein is not likely to be functional because it lacks a region conserved in orthologs from a variety of microorganisms. The TAIR 7.0 annotation of BIO1 (long) requires that a (−10) transcript be produced. In contrast, the BIO1 (short) protein encoded by the bicistronic transcript requires a (+10) transcript. This shortened protein also lacks conserved regions shared among microorganisms. The long BIO1 and BIO3 proteins are therefore most likely to be functional, if they are indeed produced.
Figure 4.
Region of the Arabidopsis genome spanning the BIO3-BIO1 junction. Exons are shown in capital letters (orange) and introns in lower case (blue). Boxed and underlined sequences identify potential start and stop codons for bicistronic and single gene transcripts. The long BIO1 start site is located upstream of the long BIO3 stop site. Alternative BIO3 stop and BIO1 start sites for translation are underlined. The large boxed (+10) region is differentially spliced from the full-length monocistronic transcript (GenBank accession no. EU089963). RT-PCR primers are noted beneath the sequence with black (BIO3, forward), green (BIO1, long), violet (BIO1, short), and red (BIO3, reverse) arrows. Flanking sequences from bio3-3 begin at green (Oklahoma State sequence) and red (Salk sequence) arrowheads located within the second exon.
Because evidence of a monocistronic, full-length transcript was at first limited to a single 5′-RACE experiment, we designed additional reverse transcription (RT)-PCR primers capable of distinguishing between (+10) and (−10) mRNAs. The results obtained (Fig. 5) confirmed that significant amounts of both types of transcripts are present. The (+10) version is somewhat more abundant than the (−10) version in most parts of the plant. Although the (+10) sequence can be found in both the bicistronic full-length transcript and BIO1 single gene transcripts, the (−10) sequence appears to be limited to monocistronic full-length transcripts. Arabidopsis therefore has the potential to produce a full-length BIO3-BIO1 transcript that encodes a bifunctional fusion protein capable of catalyzing two sequential reactions in biotin biosynthesis.
Figure 5.
RT-PCR confirmation of (+10) and (−10) transcripts. A, One strategy used a reverse primer (underlined) that spanned the fourth and fifth exons and skipped the 10 nucleotides (red) that are alternatively spliced. Sequencing confirmed that the single product obtained was derived from the (−10) transcript. B, A second strategy used a reverse primer located in a downstream exon. As expected, two products that differed in length by 10 nucleotides were obtained from leaves (L), flowers (F), and siliques (S). Sequencing confirmed that these products differed with respect to the 10 nucleotides in question. A small amount of genomic DNA was also amplified. C, Semiquantitative RT-PCR analysis of the (+10; white rectangles) and (−10; black rectangles) products obtained using a reverse primer that spanned two downstream exons. Ubiquitin served as an internal standard. Band intensities were quantified using ImageJ (http://rsb.info.nih.gov/ij) and normalized relative to the maximal intensity in the flower sample.
Organization of BIO1 and BIO3 Orthologs in Flowering Plants
Further evidence of differential splicing and the presence of a monocistronic transcript encoding a bifunctional protein was obtained by searching GenBank for homologous sequences from other plant species that spanned the junction region. Two different types of rice (Oryza sativa) transcripts were identified. A full-length cDNA (accession no. AK100945) and EST (accession no. AU0033128) confirm the presence of a monocistronic transcript. Another full-length cDNA (accession no. AK241284) and EST (accession no. CT857795) provide evidence for an alternative splice variant that does not encode either a fusion protein or a functional BIO1 protein. The only source of BIO1 activity in rice therefore appears to be the bifunctional protein. The main difference between the two types of transcripts is a region 37 nucleotides in length that provides an alternative 3′-acceptor site for splicing. This rice sequence (5′-gcaatttttgtagcctaaatttctctttgctcattag-3′) aligns in part with the (+10) region from Arabidopsis. Monocistronic transcripts were also identified from EST databases with a TBLASTN search using a query (WWTQGPDPTFQAELAREMGY) based on the junction region of the predicted Arabidopsis bifunctional protein. This search identified ESTs from snapdragon (Antirrhinum majus; accession no. AJ788704), Jerusalem artichoke (Helianthus tuberosus; accession no. EL452781), and barley (Hordeum vulgare; accession no. CA029744) that appeared to encode a bifunctional protein. We have therefore found evidence to support the widespread occurrence of transcripts capable of producing a bifunctional DAPA synthase/DTB synthetase protein in a variety of plants.
Organization of Biotin Biosynthetic Genes in Microorganisms
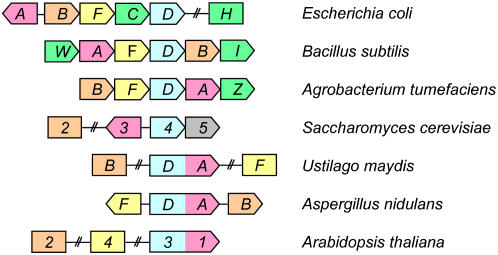
Several recent studies have surveyed the organization of biotin biosynthetic genes in microorganisms (Rodionov et al., 2002; Streit and Entcheva, 2003; Hall and Dietrich, 2007). We focused on the identification of BIO3 and BIO1 orthologs in bacteria and fungi to search for additional evidence of a bifunctional fusion protein and determine whether this region of the Arabidopsis genome might represent a novel remnant of a bacterial operon. Figure 6 illustrates some of the different types of gene organization encountered. The BIO3 ortholog (bioD) is located just upstream of bioA (BIO1) and in the same orientation in a wide range of bacteria, including Agrobacterium tumefaciens, Staphylococcus aureus, and Bacillus sphaericus. Twenty examples of this pattern of organization are included among the 90 eubacterial and archaeal genomes characterized by Rodionov et al. (2002). However, no evidence of a BioD-BioA fusion protein can be found among these sequenced genomes or in public databases (www.igs.cnrs-mrs.fr/FusionDB) of prokaryotic gene fusion events (Suhre and Claverie, 2004). This novel feature of biotin gene organization and enzyme function therefore appears to be limited to eukaryotes.
Figure 6.
Genomic organization of biotin biosynthetic genes in microorganisms. Orthologs are depicted using the same color. Gene names (bio; BIO) and directions of transcription (pointed edge) are noted. Adjacent genes are abutted, genes that are close but not adjacent are joined by a thin line, and unlinked genes are separated by a hatched line. Green rectangles represent dissimilar genes involved in the biosynthesis of the initial biotin precursors. The BIO5 gene of yeast is involved in transport. A BIO3-BIO1 fusion protein is found in some fungi but not in yeasts.
BIO3 and BIO1 orthologs are adjacent but oriented in opposite directions in yeast (Saccharomyces cerevisiae). Separate orthologs are also found in a variety of hemiascomycete fungi, including Candida albicans but not Yarrowia lipolytica. A different situation is encountered in the basidiomycetes and filamentous fungi. Evidence of a single ORF encoding a bifunctional protein can be found in at least 18 different species, including Aspergillus nidulans, Ustilago maydis, and Cryptococcus neoformans. This list includes data from Hall and Dietrich (2007) and additional examples obtained from BLASTP searches at GenBank using Arabidopsis and Aspergillus fusion proteins as queries. A significant match was also found with a protein from the sequenced genome of the green alga, Ostreococcus tauri. The existence of a bifunctional BIO3-BIO1 fusion protein in flowering plants is therefore supported by extensive sequence data from lower eukaryotes.
Functional Complementation of E. coli Biotin Auxotrophs
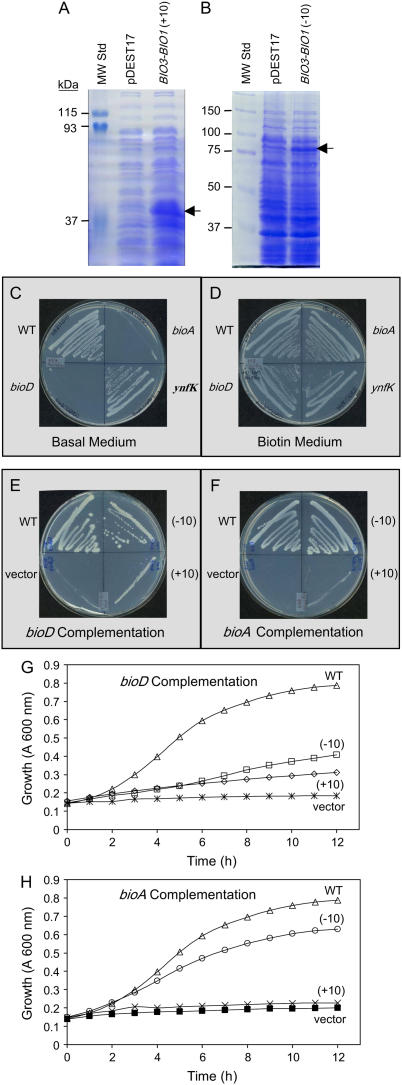
To assess the functions of different BIO3-BIO1 gene products, Arabidopsis proteins encoded by the monocistronic (−10) and bicistronic (+10) full-length cDNAs were produced in E. coli using a Gateway expression vector (pDEST17) that fused an N-terminal 6×-His tag to each recombinant protein. This added about 3 kD to the molecular mass of each product. The (−10) construct was therefore expected to produce a 95-kD BIO3-BIO1 fusion protein and the (+10) version a 48-kD BIO3 protein. Plasmid DNA from the expression clones was transformed into E. coli and evaluated for expression of targeted proteins (Fig. 7, A and B). Expression of the (+10) construct in E. coli strain BL21(λDE3) resulted in accumulation of the BIO3 protein (48 ± 4 kD). Expression of the (−10) construct in strain C41(λDE3) (Miroux and Walker, 1996) generated a fusion protein (95 ± 8 kD). Both polypeptides uniquely reacted with anti-His tag antibodies (data not shown). The failure of E. coli cells carrying the (+10) construct to accumulate BIO1 (short) protein suggests that bacterial ribosomes are unable to initiate translation effectively at the required internal AUG site.
Figure 7.
Heterologous expression (A and B) and functional characterization (C–H) of BIO3-BIO1 gene products in E. coli. A and B, Protein extracts from isopropylthio-β-galactoside-induced E. coli cultures harboring either the empty vector (pDEST17) or the BIO3-BIO1 bicistronic (+10) or monocistronic (−10) full-length cDNA were subjected to SDS-PAGE and stained with Coomassie Blue. Putative BIO3 (A) and fusion (B) proteins are marked with arrows. C and D, Expected responses of wild-type (WT) and mutant strains of E. coli in the presence and absence of biotin. E and F, Responses of a wild-type control strain and bioD (E) and bioA (F) mutants transformed with either the pDEST17 (empty) vector or recombinant vectors containing the (−10) or (+10) cDNA. G and H, Responses of wild-type and transformed bioD (G) and bioA (H) strains in liquid cultures. E to H, Strains were lysogenized with λDE3 and cultured on a kanamycin medium without biotin.
Functional properties of recombinant proteins were evaluated by introducing each construct into E. coli strains bioD (JW0761) and bioA (JW0757) obtained from the Keio collection of single gene knockouts (Baba et al., 2006). We also examined the ynfK knockout (JW5264) because this gene encodes a DTB synthase-like protein that shares 50% sequence identity with BioD. The bioD and bioA mutants exhibited the expected biotin requirement for growth, whereas the ynfK mutant grew on basal medium (Fig. 7, C and D). We therefore used only the bioD and bioA mutants for subsequent complementation studies.
Because the expression vector used for complementation utilizes the T7 RNA polymerase promoter to control expression of the targeted sequence, auxotrophic E. coli strains were first lysogenized with λ(DE3) to introduce the required T7 RNA polymerase. The resulting strains were then transformed with (+10) and (−10) constructs and with a negative control (pDEST17) and evaluated for growth in the absence of biotin. Both the (−10) and (+10) constructs complemented the bioD mutant and supported growth on basal medium, although the (+10) construct was less effective than the (−10) construct (Fig. 7, E and G). In contrast, only the (−10) construct complemented the bioA mutant (Fig. 7, F and H). As expected, transformation with the control pDEST17 vector resulted in no growth on basal medium. We therefore conclude that the monocistronic (−10) transcript encodes a 92-kD fusion protein that is bifunctional, catalyzing both the DTB synthetase (BioD/BIO3) and DAPA aminotransferase (BioA/BIO1) reactions. The (+10) transcript, in contrast, encodes a smaller protein that exhibits only DTB synthase (BioD/BIO3) activity.
Evidence for Distinct BIO1 and BIO3 Transcripts in Arabidopsis
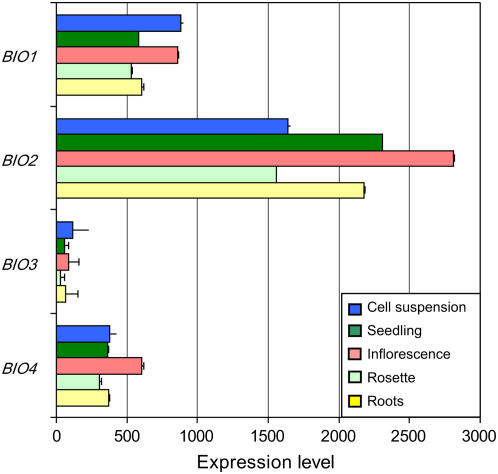
Having established that an Arabidopsis fusion protein produced from the monocistronic transcript is bifunctional in E. coli and that similar proteins should be present in a variety of plants and fungi, we next sought to determine whether single gene transcripts capable of producing distinct BIO1 and BIO3 proteins are produced in Arabidopsis. We were initially surprised by the striking differences in expression levels for BIO1 and BIO3 in public microarray databases. If full-length transcripts alone are produced, then the relative levels of transcripts identified using primers localized to different regions of this locus should be the same. However, as shown in Figure 8, multiple microarray experiments indicate that BIO1 expression is consistently above BIO3 levels.
Figure 8.
Summary of expression data for BIO genes of Arabidopsis. Results of microarray experiments were obtained from https://www.genevestigator.ethz.ch. BIO3 expression levels are low in all tissues examined.
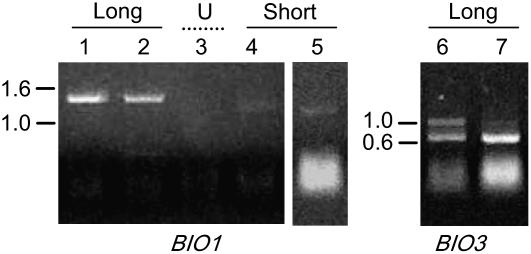
RT-PCR primers were therefore designed to distinguish between single gene and full-length transcripts based on the assumption that portions of the 3′-UTR for BIO3 transcripts and the 5′-UTR for BIO1 transcripts were positioned within introns of full-length transcripts. The locations of primers used in these experiments are illustrated in Figure 4. A PCR product of expected size (approximately 0.7 kb) was obtained when a BIO3 forward primer (9385) joining the first two exons was used in combination with a reverse primer (9387) located in the fifth intron of the BIO3-BIO1 locus, downstream of the BIO3 (long) ORF and within the putative 3′-UTR of the BIO3 (long) transcript (Fig. 9, lanes 6 and 7). Sequencing of this product (lower band) confirmed that a BIO3 (long) single gene transcript is produced. We did not attempt to identify BIO3 (short) single gene transcripts because the resulting protein would not likely be functional.
Figure 9.
Semiquantitative RT-PCR evidence of BIO1 and BIO3 single gene transcripts in extracts from wild-type plants. BIO1 forward primers were located either in the predicted 5′-UTR for the long transcript (lanes 1 and 2) or short transcript (lanes 4 and 5) or upstream (U) of the 5′-UTR for the short transcript (lane 3), but in all cases within an intron for the BIO3-BIO1 transcript. The BIO3 reverse primer was located in the predicted 3′-UTR for the long transcript but in an intron for the BIO3-BIO1 transcript. The lower band in lanes 6 and 7 was confirmed by sequencing to be the expected product. This band is less abundant than the BIO1 (long) product (lanes 1 and 2) when gels are run under equivalent conditions. The top band in lanes 6 and 7 represents contaminating genomic DNA. Plant extracts were prepared from leaves (lanes 1–6) and flowers (lane 7).
No product was obtained when BIO1 forward primers (9381 and 9414) located in BIO3-BIO1 intron 3 and far upstream of the predicted ATG for BIO1 (short) were used in combination with a reverse primer that spanned two downstream exons (e.g. Fig. 9, lane 3). We concluded that these forward primers were located beyond the start of the BIO1 (short) 5′-UTR. Additional primers (9415 and 9382) located further downstream gave a small amount of product of expected size (approximately 1.2 kb) that was confirmed by sequencing to represent BIO1 (short) single gene (+10) transcripts (Fig. 9, lanes 4 and 5). A more dramatic result was obtained when forward primers (9412 and 9413) located in the predicted 5′-UTR for BIO1 (long) were combined with the same reverse primer spanning downstream exons (Fig. 9, lanes 1 and 2). Sequencing of this product (approximately 1.4 kb) of expected size confirmed the presence of a (+10) BIO1 (long) single gene transcript. The abundance of this transcript may explain in part the high BIO1 signal observed in microarray experiments. What remains surprising is the consistently low BIO3 signal observed in multiple microarrays. This appears to indicate that much of the BIO1 signal observed in microarrays corresponds to single gene transcripts and that only trace levels of the BIO3-BIO1 monocistronic transcript and bifunctional protein are present in Arabidopsis plants.
Predicted Intracellular Localization of Biotin Biosynthetic Enzymes
The BIO3 protein contains an N-terminal sequence that is predicted to target the protein to mitochondria. All of the major prediction programs for intracellular localization of plant proteins (Emanuelsson et al., 2007) support this conclusion. It therefore appears that the bifunctional protein produced from the monocistronic, full-length transcript and whatever BIO3 (long) protein is produced from the single gene transcript all function in mitochondria. In contrast, the BIO1 (long) protein encoded by the single gene transcript does not appear to contain a mitochondrial localization signal. The short version of this protein encoded by the predominant (+10) single gene transcript is also missing conserved N-terminal sequences found in microorganisms. BIO1 activity required for biotin biosynthesis in Arabidopsis therefore appears to be associated primarily with the bifunctional protein. Whether alternative pathways exist for production of small amounts of cytosolic DAPA utilizing related S-adenosyl Met transaminases remains to be explored.
These results help to explain the failure of bio3 and bio1 mutants to complement. BIO1 activity is disrupted by insertion mutations in either the BIO3 or BIO1 coding regions. Following genetic crosses between BIO3/bio3 and BIO1/bio1 heterozygotes, embryos with a bio3-BIO1/BIO3-bio1 genotype become arrested because the bio3 insertion mutation disrupts the function of the adjacent copy of BIO1, whereas the second copy (bio1) located on the homologous chromosome is altered by mutation. The BIO3 (long) protein produced from single gene transcripts should be functional based on bacterial complementation experiments. But this protein cannot rescue mutant embryos devoid of BIO1 activity in complementation tests. Whether a majority of BIO3 activity in Arabidopsis is associated with the bifunctional protein or with the BIO3 (long) protein remains an open question. Comparative studies with fungi nevertheless suggest that the bifunctional protein is ancestral and may therefore be predominant.
A Genome-Wide Survey for Gene Clusters with Related Metabolic Functions
Three approaches were taken to search for additional examples of gene clusters encoding proteins with related metabolic functions in Arabidopsis. We first searched for full-length cDNAs that covered two distinct genes based on TAIR 7.0 annotation of the genome, which updated the list of 58 complex loci published by Thimmapuram et al. (2005). We then compared this dataset with genes listed in the AraCyc (Zhang et al., 2005) database of metabolic pathways at TAIR. This approach uncovered only the BIO3-BIO1 locus described here. Next, we scanned the Arabidopsis genome for examples of adjacent loci that encoded proteins with related metabolic functions but did not produce a full-length transcript that covered both genes. This identified 99 adjacent sets comprising 262 total genes. The vast majority of these cases are tandem duplications involving genes annotated as having the same enzymatic function. Several of these clusters contain more than two adjacent genes. Two clusters of interest were identified in addition to the BIO3-BIO1 locus. One is required for chorismate biosynthesis in plastids: At1g48850 (chorismate synthase) and At1g48860 (5-enolpyruvylshikimate-3-phosphate synthase). The other involves branched-chain amino acid catabolism in mitochondria: At3g06850 (branched-chain keto-acid dehydrogenase) and At3g06860 (enoyl-CoA hydratase).
Because the success of these initial strategies for identifying clusters of interest is dependent on correct annotation of gene function, we pursued a third approach by looking for Arabidopsis orthologs of clustered yeast genes with related metabolic functions. A recent study by Hall and Dietrich (2007) identified 14 examples of such clusters in the sequenced genome of yeast. BLASTP (Altschul et al., 1997) analyses and Kyoto Encyclopedia of Genes and Genomes database (Ogata et al., 1999) searches failed to identify any definitive clusters of putative orthologs in Arabidopsis. We therefore conclude that with respect to the regulation of metabolic pathways, the BIO3-BIO1 locus described here provides an interesting and atypical example of gene organization and function in plants.
DISCUSSION
Gene Clusters with Related Metabolic Functions
Clusters of genes with related metabolic functions are a defining feature of prokaryotic genomes. Eukaryotic orthologs of these genes, in contrast, tend to be dispersed throughout the genome and do not typically produce a polycistronic transcript. A significant number of eukaryotic operons have been described over the years (Blumenthal, 2004), particularly in Caenorhabditis elegans, but most of these are not involved in basic metabolism. Even in yeast, there are few known examples of gene clusters that produce enzymes with related metabolic functions (Hall and Dietrich, 2007). The locus described here provides an interesting example in Arabidopsis of two adjacent genes involved in sequential reactions of the same pathway that produce a combination of separate and chimeric transcripts. This locus does not appear to be an evolutionary remnant of a prokaryotic operon. Instead, it defines a genomic region in Arabidopsis that represents both a single gene for a bifunctional enzyme and two adjacent genes that produce multiple, distinct types of transcripts.
There are numerous examples of adjacent genes in Arabidopsis that produce a chimeric transcript (Thimmapuram et al., 2005). These transcripts can be either monocistronic, encoding a single bifunctional protein, or bicistronic, encoding two distinct polypeptides. Transcripts limited to individual genes can also be produced from these loci. The BIO3-BIO1 region is not part of the original list of 60 loci characterized by Thimmapuram et al. (2005) because the corresponding full-length cDNAs were not yet deposited in GenBank. The distinctive feature of the BIO3-BIO1 locus is the involvement of both gene products in the same metabolic pathway. The updated survey described here failed to identify additional examples of adjacent genes, based on current genome annotation, that produce a chimeric transcript for related but distinct enzymes. Even the broader search for adjacent genes involved in the same metabolic pathway, but not necessarily transcribed as a unit, identified only two new candidates. One of these clusters (At1g48850 and At1g48860), involved in aromatic amino acid biosynthesis, is of special interest because At1g48850 is required for embryo development (EMB1144; www.seedgenes.org) and At1g48860 (5-enolpyruvylshikimate-3-phosphate synthase) represents the well-characterized target for glyphosate herbicides. Orthologs of these two genes (aroA and aroC) are dispersed in the E. coli chromosome, whereas in Saccharomyces and Neurospora they constitute part of a complex (arom) locus that encodes a large, pentafunctional polypeptide (Duncan and Coggins, 1986). The second cluster of Arabidopsis genes (At3g06850 and At3g06860), involved in the metabolism of branched-chain amino acids, is more difficult to analyze from a comparative perspective because a definitive ortholog of At3g06860 (enoyl-CoA hydratase) remains to be identified in fungi and bacteria. The general conclusion, however, is that the Arabidopsis genome contains few examples of adjacent genes with sequential roles in metabolism.
Bifunctional Enzymes in Arabidopsis
At least 19 examples of bifunctional enzymes associated with cellular metabolism have been identified in Arabidopsis (Moore, 2004). In each case, a single gene product catalyzes more than one reaction in a common pathway. Six of these enzymes are involved with amino acid metabolism, six with lipid and carbohydrate metabolism, and the remainder with miscellaneous biochemical pathways. Some of these enzymes have well-characterized, bifunctional counterparts in lower eukaryotes. Two factors argue against the simple interpretation that BIO3-BIO1 should be viewed as another bifunctional plant protein that was incorrectly annotated in the Arabidopsis genome and previously escaped detection in biochemical studies. First, the predominant full-length transcript from this locus is bicistronic and encodes separate BIO3 and BIO1 proteins, not the bifunctional protein. Furthermore, single gene transcripts can also be produced, although the BIO1 transcript does not appear to encode a functional protein. Some bifunctional Arabidopsis proteins, however, are also encoded by complex loci that produce more than one type of transcript. One intriguing example with notable similarities to the case described here is the bifunctional Lys ketoglutarate reductase-saccharopine dehydrogenase enzyme that catalyzes the initial reactions in Lys degradation. In some plants, including Arabidopsis, this enzyme is encoded by a complex locus with an internal promoter that allows expression of the monofunctional (downstream) saccharopine dehydrogenase, as well as internal polyadenylation sites that result is the production of monofunctional (upstream) Lys ketoglutarate reductase (Tang et al., 2002).
Origin of the BIO3-BIO1 Bifunctional Protein
Although the presence of adjacent genes oriented in the same direction and associated with a single metabolic pathway is reminiscent of gene organization in bacterial operons, the probable origin of the BIO3-BIO1 locus of Arabidopsis is a gene fusion event that occurred early in the evolution of eukaryotes. This conclusion is supported by evidence of a bifunctional protein from whole-genome sequencing of O. tauri, a basal member of the green alga lineage that gave rise to land plants (Derelle et al., 2006) and Cyanidioschyzon merolae, a primitive red alga (Matsuzaki et al., 2004) used for comparative studies of plant evolution (Misumi et al., 2005), and from extensive sequence data derived from a wide range of basidiomycetes and filamentous fungi. Hall and Dietrich (2007) propose that much of the biotin pathway was lost in fungal ancestors of Saccharomyces and Candida, and that BIO3 and BIO1 orthologs were reacquired through separate, horizontal gene transfer from an unspecified prokaryotic donor. The ability to produce a bicistronic transcript and separate gene products through differential splicing appears to have been a more recent event because it is limited, based on available sequence data, to selected angiosperms, including Arabidopsis, Brassica, and rice. Two examples of adjacent Arabidopsis genes with related functions in amino acid metabolism identified here are different in that rice orthologs of these genes are not physically adjacent. We are therefore unable to point to a single example of adjacent genes with related but distinct metabolic functions in Arabidopsis that remain adjacent in unrelated angiosperms but do not produce a chimeric transcript or encode a fusion protein.
Implications for Biotin Biosynthesis in Plants
The intracellular localization of intermediate reactions in biotin synthesis in plants has remained unresolved despite the demonstration that the first reaction catalyzed by KAPA synthase occurs in the cytosol and the final reaction involving biotin synthase occurs in mitochondria (Rébeillé et al., 2007). Results presented here provide strong evidence that both of the intermediate steps catalyzed by the bifunctional BIO3-BIO1 protein and the monofunctional BIO3 protein also take place in mitochondria. This underscores the central role that mitochondria serve in the biosynthesis of vitamin coenzymes (Rébeillé et al., 2007). The membrane transport system that delivers KAPA into the mitochondrion of plant cells remains to be identified. The proteins involved may be difficult to identify through sequence homology because of differences in the compartmentalization of the biotin biosynthetic pathway in plants and microorganisms. The ability of a single plant protein to convert KAPA into DAPA and then DTB raises interesting questions about enzyme mechanics that remain to be addressed. Improved catalytic efficiency of the bifunctional enzyme may be advantageous in light of the trace amounts of intermediates available. The presence of a bifunctional enzyme also has implications for ongoing efforts to design herbicides that interfere with biotin production (Ashkenazi et al., 2007) and with biotechnological strategies to increase biotin levels in crop plants.
Another issue that needs to be reconciled is the ability of an E. coli bioA transgene to rescue the phenotype of the Arabidopsis bio1-1 point mutant (Patton et al., 1996). Because the bacterial protein introduced into mutant plants did not include a mitochondrial localization signal, the enzymatic conversion of KAPA to DAPA probably took place in the cytosol, with the product transported into mitochondria. BIO3 activity in this case must have been provided either by a defective fusion protein altered only at a site associated with BIO1 activity, or by small amounts of monofunctional BIO3 protein. The incomplete rescue observed in these experiments, despite high levels of transgene expression, is consistent with inefficient reaction and transport mechanisms related to aberrant localization of biotin intermediates.
The presence of a bifunctional BIO3-BIO1 protein in cell extracts from Arabidopsis plants remains to be definitively established. Based on the low transcript levels detected in multiple microarray and RT-PCR experiments, and the small amounts of biotin produced in plant cells, this may be a challenging task. Indeed, our initial efforts to generate and utilize antibodies against purified Arabidopsis BIO3 and BIO1 proteins produced in E. coli have not been successful. These Arabidopsis proteins have also not been identified in a broad survey of the plant mitochondrial proteome (Heazlewood and Millar, 2005). Detailed biochemical studies on the enzymatic production of DAPA and DTB in plants may therefore need to focus initially on protein generated in E. coli rather than isolated from plant extracts. The work presented here provides an important framework for a variety of future studies on the BIO3-BIO1 protein of Arabidopsis, the regulation of biotin synthesis in plants, and the role of mitochondria in plant growth and development.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All three bio1 alleles (Columbia ecotype) and the bio3-3 insertion line (Columbia ecotype) from the Salk Institute (Alonso et al., 2003) can be obtained through the Arabidopsis Biological Resource Center at Ohio State University. We used internal seed stocks for the bio1-1 EMS allele identified in the Meinke laboratory (Schneider et al., 1989) and the bio1-2 and bio1-3 insertion lines generated at Syngenta (McElver et al., 2001). The Syngenta lines are distinct from the SAIL population (Sessions et al., 2002) designed for reverse genetics. The bio3-1 and bio3-2 mutants (No-0 ecotype) were obtained from the RIKEN Bioresource Center in Japan. Plants at Oklahoma State University were grown in a soil mixture and placed in a growth room (24°C ± 2°C) under fluorescent lights (16-h light/8-h dark cycles) as described by Berg et al. (2005). At Iowa State University, seeds were first germinated on Murashige and Skoog agar medium (Invitrogen) containing 0.1% Suc. Seedlings were then transferred to LC1 Sunshine Mix soil (Sun Gro Horticulture) and grown to maturity under continuous illumination (170 μmol m−2 s−1) at 22°C. Heterozygous plants were identified by screening siliques for the presence of aborted seeds. Allelism tests were performed by crossing two heterozygotes and screening immature F1 siliques for the presence of aborted seeds. Detailed information on the methods used to characterize mutant seeds is presented in the tutorial section at www.seedgenes.org.
PCR Genotyping of Plants
Gene-specific primers for each mutant were designed using the SIGnAL iSect Primer Design program at http://signal.salk.edu and were purchased from IDT. Primers for the left T-DNA border in Salk and Syngenta lines and the Ds border in the RIKEN lines were used in combination with the appropriate gene-specific primers to detect and confirm insertions. A complete list of primers used is presented in Supplemental Table S1. Genomic DNA was isolated in the Meinke lab using a modified cetyl-trimethyl-ammonium bromide protocol (Lukowitz et al., 2000) and in the Nikolau lab using an SDS-phenol-chloroform extraction protocol. Two different PCR parameters were used: 94°C for 2 min, followed by 30 cycles of 94°C for 30 s, 56°C for 40 s, 72°C for 80 s, and a final elongation step of 72°C for 10 min (Meinke); and 96°C for 10 min followed by 35 cycles at 94°C for 15 s, 65°C for 30 s, and then at 72°C for 4 min for final extension (Nikolau). Reactions were performed with a Biometra Uno II (Meinke) or an MJ Research (Nikolau) thermocycler. Amplified products were separated in 1.0% agarose gels, stained with ethidium bromide, gel purified (Qiagen), and sequenced at the Oklahoma State University Recombinant DNA/Protein Resource Facility or the DNA Facility at Iowa State University to confirm insert locations.
Biotin Rescue Experiments
Biotin rescue of mutant seeds in heterozygous plants grown in soil was accomplished by daily watering of plants (20–40 mL/pot) with a solution of fertilizer (Berg et al., 2005) supplemented with 1 mm biotin. The solution was refrigerated between applications to limit microbial contamination. Heterozygous plants chosen for treatment were first identified by screening immature siliques for aborted seeds and then trimmed to remove excess branches and stems before supplementation began. Embryo rescue experiments were performed under aseptic conditions (Schneider et al., 1989) on plates containing Murashige and Skoog salts, 3% (w/v) Glc, 0.8% (w/v) agar, 0.1 mg/L 1-naphthylacetic acid, and 1.0 mg/L 6-benzylaminopurine. Two different sources of DAPA were used. The first (adjusted to 2 μm final concentration in the culture medium) was derived from an ethanol stock prepared 15 years ago (Shellhammer, 1991) using a powdered sample of DAPA provided by Nicholas Shaw and stored since that time at −20°C. The second sample (1 μm final concentration) also originated from the Shaw laboratory, but was provided in 2005 by Peter Roach (University of Southampton) in powdered form and then dissolved (8.0 mg in 10 mL 50% ethanol) to form a concentrated stock. Both sources of DAPA gave similar responses in culture. D,l-desthiobiotin (2 μm final concentration) and d-biotin (1 μm final concentration) were both obtained from Sigma Chemical Company.
Sequencing of the bio1-1 Mutant Allele
Genomic DNA was isolated from leaf tissue of plants homozygous for the bio1-1 allele and grown in the presence of 1 mm biotin. Using a set of primers that spanned the At5g57590 gene, PCR was used to generate a series of overlapping amplicons that were directly sequenced and compared to the wild-type Arabidopsis (Arabidopsis thaliana) genome (TAIR 7.0). Any polymorphisms identified between the sequences derived from bio1-1 plants and the published genomic sequences were confirmed by PCR amplifying the homologous DNA fragment from wild-type Columbia plants.
RT-PCR Analysis of Transcript Diversity
Cauline leaves, young flowers, and siliques with embryos up to the transition stage were harvested from plants grown in soil, flash frozen in liquid nitrogen, and stored at −80°C without thawing until RNA extraction. Frozen tissue (0.1 g) was homogenized in liquid nitrogen. Total RNA was prepared from powdered tissues using the RNeasy plant mini kit (Qiagen), treated with RNase-free DNase I (TaKaRa Bio), quantified with a Shimadzu UV-160 spectrophotometer, and visualized on a 1.0% formaldehyde agarose gel. For the two-step RT-PCR reaction, 5 μg total RNA was reverse transcribed using the SuperScript first-strand synthesis system (Invitrogen). A 1-μL aliquot of reverse transcribed reaction was then used as a template for the second-step PCR with REDTaq DNA polymerase (Sigma-Aldrich). Flower cDNA was used as a template to amplify separate BIO3 and BIO1 transcripts. Leaf, flower, and silique cDNAs were used to amplify (−10) and (+10) chimeric transcripts. Reactions were performed with a Biometra Uno II thermocycler. Primers are listed in Supplemental Table S1. PCR parameters were: 94°C for 1 min, followed by 30 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 2 min, and a final elongation step at 72°C for 10 min. Amplified products from (−10) transcripts (112 bp) and (+10) transcripts (122 bp) were separated using a high-resolution 4% MetaPhor (Cambrex Bio Science) agarose gel.
5′- and 3′-RACE Experiments
Initial 5′-RACE experiments designed to identify the full-length mRNA sequence that corresponded to EST clone RZ128g09R were conducted with the Invitrogen 5′-RACE system. The 5′ and 3′ ends of the BIO3-BIO1 mRNA were authenticated with an RNA ligase-mediated rapid amplification method (Maruyama and Sugano, 1994) using the GeneRacer kit (Invitrogen).
Complementation of Bacterial Biotin Auxotrophs
Escherichia coli strains carrying mutations in biotin biosynthetic genes were obtained from the Keio collection of single gene knockouts (http://ecoli.aist-nara.ac.jp/gb6/Resources/deletion/deletion.html), which replaced each coding region with a kanamycin resistance gene (Baba et al., 2006). Four different strains were used in these studies: BW25113 (wild-type parental strain), JW0761 (bioD knockout), JW0757 (bioA knockout), and JW5264 (ynfK knockout). Mutant strains were confirmed by their ability to grow on kanamycin and by sequencing of PCR products that amplified the mutant allele. Strains were first lysogenized with λ(DE3) to introduce the required T7 RNA polymerase and then transformed with pDEST17-derivative plasmids that carried either the BIO3-BIO1 (+10) or (−10) cDNA versions. These plasmids were constructed using PCR products corresponding to full-length Arabidopsis transcripts that were amplified using forward (5′-CACCATGATACCCGTAACCGC-3′) and reverse (5′-AGCTGGAGAGAGAGTTTTGGGT-3′) primers spanning the entire BIO1-BIO3 locus and then cloned in pENTR vector (Invitrogen). The (+10) and (−10) splice variants were identified by sequencing. Constructs were then moved from pENTR to pDEST17 using Gateway Technology as recommended by the manufacturer. E. coli strains were grown in isopropylthio-β-galactoside (0.1 mm) and kanamycin containing solid or liquid (M9 Glc) medium that were either depleted of biotin by the addition of 50 μg mL−1 of avidin (basal medium) or supplemented with 1 mm biotin.
Sequence Alignments and Genome Analyses
BLASTP and TBLASTN searches of GenBank datasets were performed using default settings at the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov). Sequences were aligned using the MultAlin program (http://bioinfo.genopole-toulouse.prd.fr/multalin/multalin.html) as described by Corpet (1988). Potential coding regions were identified with ORF finder at the National Center for Biotechnology Information. Putative orthologs of selected Arabidopsis genes were identified using the Kyoto Encyclopedia of Genes and Genomes (www.genome.ad.jp/en/gn_kegg.html) database. The AraCyc dataset (Zhang et al., 2005) of metabolic pathways in Arabidopsis was used to search for adjacent genes with related functions. Two different AraCyc datasets were downloaded in February and July, 2007, from TAIR 7.0 (www.arabidopsis.org): aracyc_dump_20070213 and aracyc_dump_20070703. These datasets were then analyzed using a computer program that we developed to first read into memory the AraCyc pathway designation for each gene and then scan down the ordered list of Arabidopsis Genome Initiative locus identifiers to look for instances where sequential genes shared the same pathway designation. The output was compared with the original list of complex loci obtained from Thimmapuram et al. (2005), with an updated list of such loci provided by David Swarbeck at TAIR, and with detailed AraCyc assignments of Arabidopsis genes to specific metabolic reactions.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EU089963 and EU090805.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Region of the Arabidopsis genome that includes the entire BIO3-BIO1 locus (At5g57600/At5g57590) and part of the downstream gene (At5g57580).
Supplemental Figure S2. Sequence alignments between four different full-length cDNAs from the BIO3-BIO1 locus: Nikolau (−10; GenBank EU089963, monocistronic); Nikolau (+10; GenBank EU090805, bicistronic); RIKEN (+10; RAFL22-07-J07, bicistronic); and French (+10; BX842298, bicistronic).
Supplemental Table S1. Primers for RT-PCR and genotype analyses.
Supplementary Material
Acknowledgments
We thank Nicholas Shaw (Biotechnology, Lonza AG, Switzerland) and Peter Roach (University of Southampton, UK) for providing samples of DAPA, and Johnny Lloyd (Oklahoma State University) for assistance with plant maintenance.
This work was supported by the National Science Foundation Metabolic Biochemistry Program (grant no. 0416730 to B.J.N.) and Arabidopsis 2010 Program (grants no. 0114866 and no. 0618166 to D.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David Meinke (meinke@okstate.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal N, Alban C, Quadrado M, Grandjean O, Mireau H (2006) The Arabidopsis Bio2 protein requires mitochondrial targeting for activity. Plant Mol Biol 62 471–479 [DOI] [PubMed] [Google Scholar]
- Ashkenazi T, Pinkert D, Nudelman A, Widberg A, Wexler B, Wittenbach V, Flint D, Nudelman A (2007) Aryl chain analogues of the biotin vitamers as potential herbicides. Part 3. Pest Manag Sci 63 974–1001 [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldet P, Alban C, Douce R (1997) Biotin synthesis in higher plants: purification and characterization of bioB gene product equivalent from Arabidopsis thaliana overexpressed in Escherichia coli and its subcellular localization in pea leaf cells. FEBS Lett 419 206–210 [DOI] [PubMed] [Google Scholar]
- Berg M, Rogers R, Muralla R, Meinke D (2005) Requirement of aminoacyl-tRNA synthetases for gametogenesis and embryo development in Arabidopsis. Plant J 44 866–878 [DOI] [PubMed] [Google Scholar]
- Blumenthal T (2004) Operons in eukaryotes. Brief Funct Genomics Proteomics 3 199–211 [DOI] [PubMed] [Google Scholar]
- Castelli V, Aury JM, Jaillon O, Wincker P, Clepet C, Menard M, Cruaud C, Quétier F, Scarpelli C, Schächter V, et al (2004) Whole genome sequence comparisons and “full-length” cDNA sequences: a combined approach to evaluate and improve Arabidopsis genome annotation. Genome Res 14 406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16 10881–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoll E (1996) Biosynthesis of biotin and lipoic acid. In FC Neidhardt, ed, Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press, Washington, DC, pp 704–709
- Derelle E, Ferraz C, Rombauts S, Rouzé P, Worden AZ, Robbens S, Partensky F, Degroeve S, Echeynié S, Cooke R, et al (2006) Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci USA 103 11647–11652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Coggins JR (1986) The serC-aroA operon of Escherichia coli. Biochem J 234 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protocols 2 953–971 [DOI] [PubMed] [Google Scholar]
- Hall C, Dietrich FS (2007) The reacquisition of biotin prototrophy in Saccharomyces cerevisiae involved horizontal gene transfer, gene duplication, and gene clustering. Genetics 177 2293–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood JL, Millar AH (2005) AMPDB: the Arabidopsis mitochondrial protein database. Nucleic Acids Res 33 D605–D610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Till BJ, Comai L (2004) TILLING: traditional mutagenesis meets functional genomics. Plant Physiol 135 630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilag LL, Kumar AM, Soll D (1994) Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell 6 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR (2000) Positional cloning in Arabidopsis: why it feels good to have a genome initiative working for you. Plant Physiol 123 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Sugano S (1994) Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides. Gene 138 171–174 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Misumi O, Shin-I T, Maruyama S, Takahara M, Miyagishima SY, Mori T, Nishida K, Yagisawa F, Nishida K, et al (2004) Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428 653–657 [DOI] [PubMed] [Google Scholar]
- McElver J, Tzafrir I, Aux G, Rogers R, Ashby C, Smith K, Thomas C, Schetter A, Zhou Q, Cushman MA, et al (2001) Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics 159 1751–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroux B, Walker JE (1996) Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol 260 289–298 [DOI] [PubMed] [Google Scholar]
- Misumi O, Matsuzaki M, Nozaki H, Miyagishima SY, Mori T, Nishida K, Yagisawa F, Yoshida Y, Kuroiwa H, Kuroiwa T (2005) Cyanidioschyzon merolae genome: a tool for facilitating comparable studies on organelle biogenesis in photosynthetic eukaryotes. Plant Physiol 137 567–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B (2004) Bifunctional and moonlighting enzymes: lighting the way to regulatory control. Trends Plant Sci 9 221–228 [DOI] [PubMed] [Google Scholar]
- Muralla R, Sweeney C, Stepansky A, Leustek T, Meinke D (2007) Genetic dissection of histidine biosynthesis in Arabidopsis. Plant Physiol 144 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau BJ, Ohlrogge JB, Wurtele ES (2003) Plant biotin-containing carboxylases. Arch Biochem Biophys 414 211–222 [DOI] [PubMed] [Google Scholar]
- Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M (1999) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 27 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DA, Franzmann LH, Meinke DW (1991) Mapping genes essential for embryo development in Arabidopsis thaliana. Mol Gen Genet 227 337–347 [DOI] [PubMed] [Google Scholar]
- Patton DA, Schetter AL, Franzmann LH, Nelson K, Ward ER, Meinke DW (1998) An embryo-defective mutant of Arabidopsis disrupted in the final step of biotin synthesis. Plant Physiol 116 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DA, Volrath S, Ward ER (1996) Complementation of an Arabidopsis thaliana biotin auxotroph with an Escherichia coli biotin biosynthetic gene. Mol Gen Genet 251 261–266 [DOI] [PubMed] [Google Scholar]
- Picciocchi A, Douce R, Alban C (2003) The plant biotin synthase reaction: identification and characterization of essential mitochondrial accessory protein components. J Biol Chem 278 24966–24975 [DOI] [PubMed] [Google Scholar]
- Pinon V, Ravanel S, Douce R, Alban C (2005) Biotin synthesis in plants: the first committed step of the pathway is catalyzed by a cytosolic 7-keto-8-aminopelargonic acid synthase. Plant Physiol 139 1666–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébeillé F, Alban C, Bourguignon J, Ravannel S, Douce R (2007) The role of plant mitochondria in the biosynthesis of coenzymes. Photosynth Res 92 149–162 [DOI] [PubMed] [Google Scholar]
- Rodionov DA, Mironov AA, Gelfand MS (2002) Conservation of the biotin regulon and the BirA regulatory signal in eubacteria and archaea. Genome Res 12 1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosens NHCJ, Thu TT, Iskander HM, Jacobs M (1998) Isolation of the ornithine-δ-aminotransferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol 117 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Dinkins R, Robinson K, Shellhammer J, Meinke DW (1989) An embryo-lethal mutant of Arabidopsis thaliana is a biotin auxotroph. Dev Biol 131 161–167 [DOI] [PubMed] [Google Scholar]
- Schomburg FM, Patton DA, Meinke DW, Amasino RM (2001) FPA, a gene involved in floral induction in Arabidopsis, encodes a protein containing RNA-recognition motifs. Plant Cell 13 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Kamiya A, Ishida J, Satou M, Sakurai T, Nakajima M, Enju A, Akiyama K, Oono Y, et al (2002) Functional annotation of a full-length Arabidopsis cDNA collection. Science 296 141–145 [DOI] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellhammer AJ (1991) Analysis of a biotin auxotroph of Arabidopsis thaliana. PhD thesis. Oklahoma State University, Stillwater, OK
- Shellhammer J, Meinke DW (1990) Arrested embryos from the bio1 auxotroph of Arabidopsis contain reduced levels of biotin. Plant Physiol 93 1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WR, Entcheva P (2003) Biotin in microbes, the genes involved in its biosynthesis, its biochemical role and perspectives for biotechnological production. Appl Microbiol Biotechnol 61 21–31 [DOI] [PubMed] [Google Scholar]
- Suhre K, Claverie JM (2004) FusionDB: a database for in-depth analysis of prokaryotic gene fusion events. Nucleic Acids Res 32 D273–D276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Zhu X, Gakiere B, Levanony H, Kahana A, Galili G (2002) The bifunctional LKR/SDH locus of plants also encodes a highly active monofunctional lysine-ketoglutarate reductase using a polyadenylation signal located within an intron. Plant Physiol 130 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmapuram J, Duan H, Liu L, Schuler MA (2005) Bicistronic and fused monocistronic transcripts are derived from adjacent loci in the Arabidopsis genome. RNA 11 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D, et al (2004) Identification of genes required for embryo development in Arabidopsis. Plant Physiol 135 1206–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Yu F, Wurtele ES, Nikolau BJ (1996) Characterization of the cDNA and gene coding for the biotin synthase of Arabidopsis thaliana. Plant Physiol 110 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Foerster H, Tissier CP, Mueller L, Paley S, Karp PD, Rhee SY (2005) MetaCyc and AraCyc: metabolic pathway databases for plant research. Plant Physiol 138 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.