Abstract
Light promotes the expression of PHYTOCHROME KINASE SUBSTRATE1 (PKS1) in the root of Arabidopsis thaliana, but the function of PKS1 in this organ is unknown. Unilateral blue light induced a negative root phototropic response mediated by phototropin 1 in wild-type seedlings. This response was absent in pks1 mutants. In the wild type, unilateral blue light enhanced PKS1 expression in the subapical region of the root several hours before bending was detectable. The negative phototropism and the enhanced PKS1 expression in response to blue light required phytochrome A (phyA). In addition, the pks1 mutation enhanced the root gravitropic response when vertically oriented seedlings were placed horizontally. The negative regulation of gravitropism by PKS1 occurred even in dark-grown seedlings and did not require phyA. Blue light also failed to induce negative phototropism in pks1 under reduced gravitational stimulation, indicating that the effect of pks1 on phototropism is not simply the consequence of the counteracting effect of enhanced gravitropism. We propose a model where the background level of PKS1 reduces gravitropism. After a phyA-dependent increase in its expression, PKS1 positively affects root phototropism and both effects contribute to negative curvature in response to unilateral blue light.
Root tissues may be exposed to light due to light penetration into the upper layers of the soil (Mandoli et al., 1990) and tissue piping effects (Mandoli and Briggs, 1984). Whereas shoots bend toward the direction of incoming blue light, improving the chances of light-harvesting organs to collect light for photosynthesis, roots bend away from the direction of incoming blue light stimuli, avoiding the stressful conditions of the upper layers of the soil (Esmon et al., 2005). Phytochromes (Somers and Quail, 1995) and phototropins (Sakamoto and Briggs, 2002) are expressed in root as well as shoot tissues. Phototropin 1 (phot1) and phot2 are blue-light photoreceptors that play a major role in perception of the light gradient that initiates both shoot and root phototropism (Liscum and Briggs, 1995; Briggs and Christie, 2002). Roots of the phot1 mutant are less efficient in exploring the soil because they show more frequent random turns than the wild type and therefore require more growth in length to achieve the same depth (Galen et al., 2006). An apparent consequence of the deficient growth pattern of phot1 roots is the impaired plant biomass gain in dry soils, suggesting that genetic engineering of root negative phototropism could enhance productivity in arid environments (Galen et al., 2006).
Phytochromes A to E (phyA–phyE) are red-light and far-red-light photoreceptors that secondarily also absorb blue light. In higher plants, unilateral red or far-red light does not initiate phototropic responses in the shoot; however, red light induces weak positive phototropism in the root of Arabidopsis (Arabidopsis thaliana; Ruppel et al., 2001; Kiss et al., 2003b). In addition, phytochromes can modulate the ability of the hypocotyl to respond to the phototropic stimulus perceived by phototropins (Liscum and Briggs, 1996; Parks et al., 1996; Janoudi et al., 1997; Stowe-Evans et al., 2001). The root of phyA and phyA phyB mutants of Arabidopsis shows reduced response to unilateral blue light, a response that is unaffected by the phyB mutation (Kiss et al., 2003a).
PHYTOCHROME KINASE SUBSTRATE1 (PKS1) is a plasma membrane-associated protein (Lariguet et al., 2006) that physically interacts with and is phosphorylated by phytochromes in vitro (Fankhauser et al., 1999). PKS1 and its closest homolog, PKS2, are components of a complex network that modulates the very low-fluence-response branch of phyA signaling (Lariguet et al., 2003). PKS1 also binds phot1 and NONPHOTOTROPIC HYPOCOTYL3 (NPH3; Motchoulski and Liscum, 1999; Lariguet et al., 2006) and mutants deficient in PKS1, PKS2, and/or PKS4 (another member of the PKS1–PKS4 family in Arabidopsis) show severely reduced hypocotyl phototropism under low fluences of unilateral blue light (Lariguet et al., 2006). Blue light perceived by phyA induces expression of PKS1 in hypocotyls of Arabidopsis seedlings (Lariguet et al., 2006). Therefore, enhancement of the hypocotyl phototropic response by phyA (Parks et al., 1996; Janoudi et al., 1997) could result, at least in part, from the enhanced level of expression of PKS1 triggered by this photoreceptor. In the root, red light also promotes expression of PKS1, NPH3, and RPT2 (Molas et al., 2006), which are key players in tropic responses (Motchoulski and Liscum, 1999; Sakai et al., 2000).
The root shows a strong positive gravitropic response that orients growth toward deeper soil strata (Chen et al., 1999), where blue light no longer provides a signal (Mandoli et al., 1990). Light and gravitropic responses share some players downstream of the events related to signal perception and exhibit complex mutual interactions in the control of organ orientation (Correll and Kiss, 2002). Phototropic responses often involve deviation of the growth direction from the gravity vector and generate a gravitational stimulus that partially counteracts phototropism. Consequently, mutants with deficient gravitropic response show apparently enhanced root phototropism (Okada and Shimura, 1994; Vitha et al., 2000). In turn, light modulates the gravitropic response, but the effect can be positive or negative, depending on the species and organ (for review, see Correll and Kiss, 2002). In maize (Zea mays) roots, for instance, red light stimulates gravitropism (Feldman and Briggs, 1987). However, light perceived by phyA and phyB reduces the gravitropic response of the hypocotyls in Arabidopsis (Liscum and Hangarter, 1993; Poppe et al., 1996). Roots from phyA phyB or phyB mutants have reduced gravitropic response compared with the wild type (Correll and Kiss, 2005).
Although red and far-red light induce expression of PKS1 in the root, no function of PKS1 in the root has been identified (Lariguet et al., 2003). PKS1 physically interacts with phot1 and NPH3 and regulates shoot phototropism (Lariguet et al., 2006), but root and shoot show different patterns of tropic responses. This scenario prompted us to investigate whether PKS1 is important for phot1-mediated root phototropism and for gravitropism.
RESULTS
Negative Root Phototropism Requires PKS1
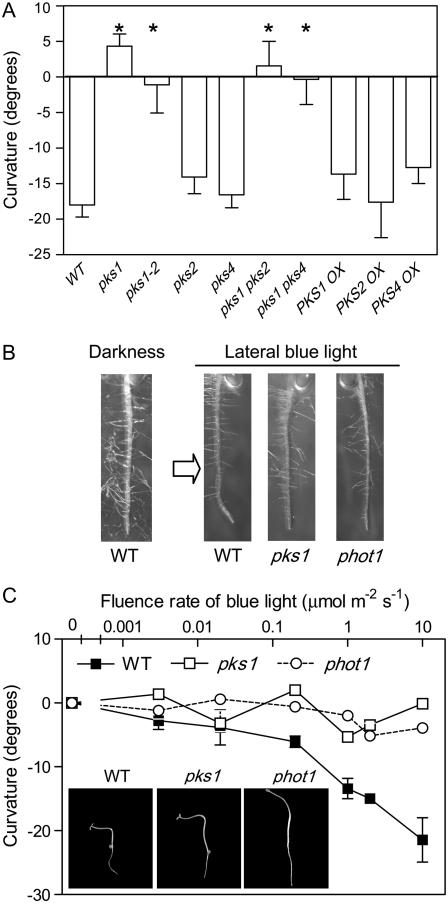
To investigate whether PKS family members play a role in negative root phototropism, we provided unilateral blue light to etiolated vertically oriented seedlings of different single and double pks mutants. The two pks1 mutant alleles used here presented no root curvature in response to 24 h of 1 μmol m−2 s−1 unilateral blue light (Fig. 1, A and B). pks2 and pks4 mutants showed wild-type root phototropism and the pks1 pks2 and pks1 pks4 double mutants behaved as the pks1 single mutant (Fig. 1A). Transgenic lines overexpressing PKS1, PKS2, or PKS4 also showed wild-type curvature in response to unilateral blue light (Fig. 1A). In wild-type seedlings, the degree of root curvature increased significantly with the fluence rate of blue light in the whole range tested here (0.003–10 μmol m−2 s−1). However, neither the phot1 mutant nor the pks1 mutant showed detectable root phototropic response (Fig. 1C). Unilateral blue-light treatments (10 μmol m−2 s−1) that failed to induce root phototropism in pks1 and phot1 mutants still caused significant positive phototropism of the hypocotyl (Fig. 1C, inset). As expected (Ruppel et al., 2001; Kiss et al., 2003b), unilateral red light (5 μmol m−2 s−1) caused a weak positive phototropic response of the root. The pks1 mutant failed to show this response (curvature, degrees, mean ± se; pks1 = 1 ± 1 against red light; wild type = 2 ± 1 toward red light).
Figure 1.
Root phototropic response to unilateral blue light requires PKS1. A, Reduced root phototropism in the pks1, pks1 pks2, and pks1 pks4 mutants and normal responses in the pks2 and pks4 mutants and PKS1, PKS2, and PKS4 overexpressors. B, Roots of representative seedlings. C, Response to fluence rate. Seedlings were grown in full darkness for 2 d and exposed to unilateral blue light for 24 h (1 μmol m−2 s−1 [A and B] or the indicated fluence rate in C) before measurements. Dark controls were measured simultaneously. Data are means and se of 20 replicate boxes.
Negative Root Phototropism and Blue-Light Induction of PKS1 Expression Require phyA
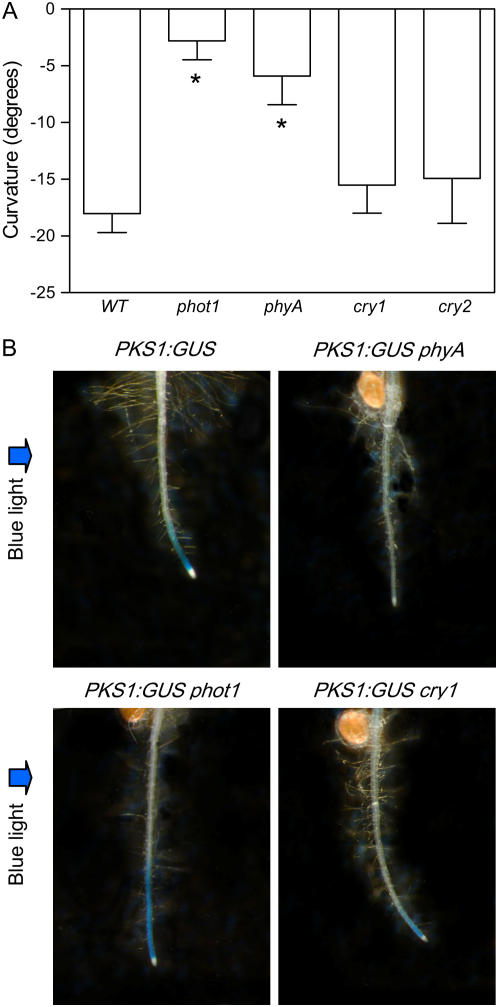
The phyA mutant showed significantly reduced root curvature in response to unilateral blue-light irradiation (Kiss et al., 2003a), whereas cryptochrome1 (cry1) and cry2 mutants presented normal root phototropism (Fig. 2A). PKS1 expression in the elongation zone of the root is promoted by white, red, or far-red light (Lariguet et al., 2003). To investigate whether the light stimulus that induces the negative phototropic response of the root also promotes activity of the PKS1 promoter, seedlings bearing a GUS reporter transgene fused to the PKS1 promoter were exposed to unilateral blue light. This light treatment strongly enhanced PKS1 promoter activity in the root (Fig. 2B). The PKS1-GUS construction was introduced in the phyA, cry1, and phot1 mutants by crosses. The absence of GUS staining in the phyA mutant and the normal staining observed in the cry1 and phot1 mutants indicates that the blue-light treatment inducing expression of the PKS1-GUS transgene was perceived largely by phyA (Fig. 2B).
Figure 2.
Negative phototropism and promotion of PKS1 promoter activity require phyA. A, Reduced phototropism in phyA and phot1 mutants. Data are means and se of 10 replicate boxes. B, Reduced induction of GUS driven by the PKS1 promoter in the root of phyA mutant seedlings. Seedlings were grown in full darkness for 2 d, transferred to unilateral blue light (1 μmol m−2 s−1), and measured or stained 24 h later.
Blue-Light Induction of PKS1 Expression Anticipates Negative Root Phototropism
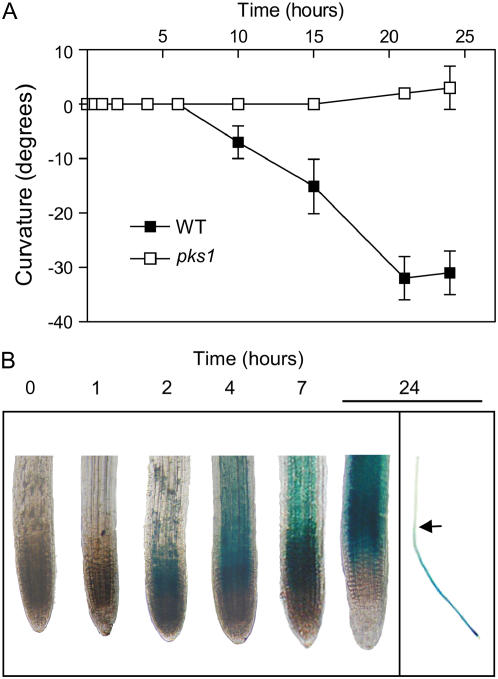
To investigate the kinetics of root curvature, 2-d-old, vertically oriented etiolated seedlings of the wild type and the pks1 mutant were exposed to unilateral blue light (1 μmol m−2 s−1). Root curvature was detected in wild-type seedlings 10 h later and reached a maximum after 20 h of treatment (Fig. 3A). The pks1 mutant failed to respond. The effect of unilateral blue light on GUS driven by the PKS1 promoter was already noted after 2 h of treatment (i.e. well before any phototropic curvature was detectable [Fig. 3B]). The PKS1 response anticipated by at least 5 h the phototropic response.
Figure 3.
Changes in PKS1 promoter activity anticipate PKS1-mediated effects on negative phototropism in roots. A, Time course of root bending after the beginning of unilateral blue light. Data are means and se of nine replicate boxes. B, Time course of GUS staining driven by the PKS1 promoter under unilateral blue light. In B, magnification is 100× (left box) and 7× (right box). The arrow indicates the position of the root tip at the beginning of the light treatment (this position was labeled in some boxes under dim green light). Seedlings were grow in full darkness for 2 d, transferred to unilateral blue light (1 μmol m−2 s−1), and measured or stained at the indicated time point.
The strongest GUS staining driven by the PKS1 promoter was observed in the subapical zone of the root (Fig. 3B). Labeling the place that the root tip had reached at the time when the light treatment started revealed that this point coincided with the place where the curvature occurred; therefore, the initial expression of PKS1 could act as a marker for the place of subsequent bending. GUS staining gradually extended to the rest of the elongation zone (4–7 h; Fig. 3B). After 24 h of blue-light treatment, staining extended to the curvature zone (Fig. 3B), but GUS stability might contribute to staining of these distant cells as they move away from the subapical region.
PKS1 Negatively Regulates Root Gravitropism
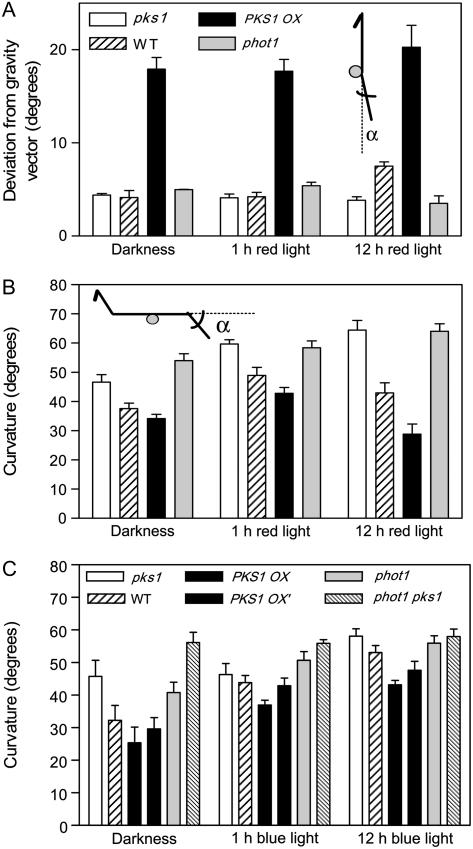
Phototropic and gravitropic responses share signaling components involved in the generation of the extension growth of the shoot and root axes (Correll and Kiss, 2002). To investigate whether, in addition to its effect on the negative phototropic response, PKS1 also affects root gravitropism, dark-grown seedlings of the wild type, the pks1 mutant, and a PKS1 overexpressor were exposed to either 1 h of red light followed by 11 h of darkness or to 12 h of red light, whereas dark controls remained without red-light treatment. Red light was provided from both sides to enhance PKS1 expression (Supplemental Fig. S1A), while avoiding induction of the phototropic response. In the wild type, the angle between the root and the gravity vector (randomization of root growth direction) was not affected by 1 h of red light compared to dark controls, but 12 h of red light significantly randomized root position and therefore increased the average deviation compared to the gravity vector (Fig. 4A). The pks1 mutant showed normal root angle in darkness or after 1 h of red light, but it failed to reduce the gravitropic orientation in response to 12 h of bilateral red light. The PKS1 overexpressor showed constitutive enhanced deviation in darkness without a significant response to red light. In additional experiments, a similar pattern was observed for a second, independent PKS1 overexpressor line (deviation from gravity vector in darkness, degrees, mean ± se; pks1 = 7 ± 1; wild type = 9 ± 1; PKS OX = 21 ± 3; PKS OX′ = 21 ± 3). Noteworthy is the fact that the phot1 mutant behaved as the pks1 mutant (i.e. it retained strong vertical orientation even after 12 h of red light).
Figure 4.
PKS1 negatively regulates root gravitropism. A, Root deviation from gravity in vertically grown seedlings exposed to 0, 1, or 12 h of bilateral red light (5 μmol m−2 s−1). Seedlings were grown vertically for 2 d and exposed to 1 h of bilateral red light followed by 23 h of darkness, 12 h of bilateral red light followed by 12 h of darkness, or left as dark controls. B, Deviation from the horizontal plane (gravitropic response) in seedlings transferred from the vertical to the horizontal position. Seedlings grown vertically for 2 d were transferred to the horizontal position and the change in root growth angle was measured 24 h later. Some seedlings were exposed to 1 h or 12 h of bilateral red light (5 μmol m−2 s−1) immediately prior to gravitropic stimulation. C, Experimental setting, as in B, but using bilateral blue light (1 μmol m−2 s−1). Data are means and se of at least five replicate boxes.
In a second experimental setting, dark-grown seedlings were shifted from the vertical to the horizontal position after 0, 1, or 12 h of bilateral red-light (Fig. 4B) or blue-light (Fig. 4C) treatment. A negative correlation between PKS1 levels and gravitropic response occurred in all the latter conditions, including darkness. In dark-grown seedlings, there is detectable PKS1 expression in the root, but resolution of the system does not allow us to conclude whether PKS1 expression is enhanced by the gravitropic stimulus. The phyA mutation did not enhance the gravitropic response in our conditions (Fig. 4C). Exposure to 1 h of red light increased PKS1 expression in the root (Supplemental Fig. S1B) and also enhanced the gravitropic response in all genotypes (Fig. 4B). Compared to 1 h of red light, exposure to 12 h of red light reduced the gravitropic response in the wild type, particularly in the PKS1 OX line, but not in the pks1 and phot1 mutants (Fig. 4B). In accordance with the experiments described in the above paragraph, the phot1 mutant behaved as the pks1 mutant. In darkness, the effects of phot1 and pks1 were additive (Fig. 4C).
Negative Root Phototropism Requires PKS1 Even under Reduced Gravitational Stimulus
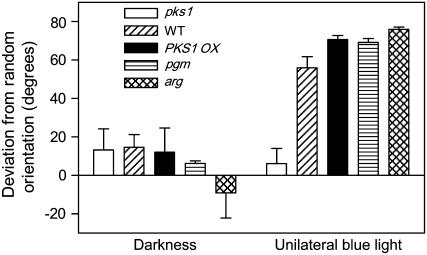
Seedlings of the phosphoglucomutase (pgm; Caspar and Pickard, 1989) and altered response to gravity (arg; Sedbrook et al., 1999) mutants, which have deficient gravitropic responses, were exposed to unilateral blue light. The phototropic bending induced by unilateral blue light was significantly higher in pgm (27.9 ± 1.0 degrees) and arg (25.7 ± 1.5 degrees) than in the wild type (16.6 ± 0.6 degrees). This and previous observations (Okada and Shimura, 1994; Vitha et al., 2000) suggest that the reduced phototropic response of pks1 might be caused by its enhanced gravitropism. To investigate this possibility, seedlings were incubated in a clinostat to abolish gravitational stimulation. Seedlings were rotated on an axis perpendicular to the gravity vector. In dark controls, the direction of root growth did not deviate significantly from random (Fig. 5). In wild-type seedlings exposed to 12 h of unilateral blue light, the root deviated significantly from random, adopting a position close to parallel to the irradiation axis (90 degrees in Fig. 5). When exposed to unilateral blue light, the root of the pks1 mutant retained a close-to-random position (Fig. 5), indicating that the phototropic response was severely reduced in pks1 even under reduced gravitational stimulus.
Figure 5.
Negative phototropism of the roots requires PKS1 even under reduced gravity. Clear plastic boxes containing chilled seeds were transferred to the clinostat (two revolutions/min) at 22°C and exposed for 12 h to white light to induce germination. Then, boxes were wrapped in black plastic (full darkness) or in black plastic with a window to allow unilateral irradiation with blue light (1 μmol m−2 s−1) normal to the axis of rotation of the clinostat. We measured the angle of the root to the gravitational axis. In dark-grown seedlings, the average angle tends to zero because divergent angles of deviation cancel each other. In the presence of unilateral blue light, the root grows parallel to the light axis. Data are means and se of three (darkness) or six (blue light) replicate boxes.
DISCUSSION
PKS1, originally discovered by its ability to interact with and become phosphorylated by phytochromes in vitro (Fankhauser et al., 1999), was later shown to interact also with phot1 and NPH3 (Lariguet et al., 2006). PKS1 and PKS2 regulate phytochrome-mediated photomorphogenesis (Lariguet et al., 2003) and hypocotyl phototropism (Lariguet et al., 2006). However, despite the observation that PKS1 is expressed in the roots in response to pulses of red or far-red light, no root phenotype had become obvious (Lariguet et al., 2003). The results presented here indicate that PKS1 positively regulates negative root phototropism induced by unilateral blue light and negatively regulates root gravitropism. These findings place the PKS family as central players in the control of organ orientation.
Seedlings of pks1 mutants exposed to 12 h of continuous blue light of up to 10 μmol m−2 s−1 from one side failed to show root curvature (Fig. 1). pks2 and pks4 mutants showed normal negative phototropism. The latter is different from the scenario observed for shoot phototropism, where PKS2 and PKS4 play a similar and partially redundant role, with PKS1 promoting hypocotyl phototropism (Lariguet et al., 2006), but it is consistent with the observation that at least PKS2 and PKS4 are expressed exclusively in aerial tissues (Lariguet et al., 2003; I. Schepens and C. Fankhauser, unpublished data). Unilateral blue light induces PKS1 expression in the root several hours before any root curvature response becomes detectable (Fig. 3). The place of early PKS1 expression coincides with the position where root curvature is observed later, indicating that PKS1 could be an early location marker. No radial gradient of PKS1 expression in response to unilateral blue light was apparent. This suggests that PKS1 is not acting directly in the main signaling stream downstream of phot1, which shows an apparent gradient of activation in response to unilateral blue light (Salomon et al., 1997), but rather as a regulator of these signaling events downstream of phot1. After introducing the PKS1-GUS transgene in the phyA, phot1, and cry1 mutant backgrounds, we conclude that phyA and not phot1 or cry1 mediates the response of PKS1 expression to unilateral blue light (Fig. 2B) similarly to what has been observed in shoots (Lariguet et al., 2006). In agreement with previous reports (Kiss et al., 2003a), the negative phototropic response of the root was reduced in the phyA mutant (Fig. 2A). Taken together, these observations indicate that blue light perceived by phyA increases PKS1 expression in the subapical region of the root, where bending is observed hours later, and this increase in PKS1 expression is necessary for the normal phototropic response, providing a nice example of coordination between phyA and phot1 activity. Coordination between phytochrome and phototropin appears particularly important for photomovement responses as the fern Adiantum capillus-veneris and the filamentous green alga Mougeotia scalaris bear chimeras of these photoreceptors, which have evolved independently (Suetsugu et al., 2005).
In addition to its effects on root phototropism, PKS1 also regulated root gravitropism (Fig. 4). Analysis of randomization of root growth in vertically grown seedlings and quantification of the curvature of the root in seedlings shifted from the vertical to the horizontal position revealed negative correlation between PKS1 levels (pks1 mutant, wild type, PKS1 OX) and gravitropic response in dark-grown, bilateral red-light-treated and blue-light-treated seedlings. In gravity-stimulated seedlings, promotion of PKS1 expression by red or blue light (Supplemental Fig. S1B; Fig. 2) is apparently not necessary for negative regulation of the gravitropic response by PKS1. First, red and blue light enhanced PKS1 expression, but not the difference between the wild type and pks1, with the exception of 12 h of red light (Fig. 4, B and C). Second, promotion of PKS1 expression by blue light requires phyA (Fig. 2B), but the phyA mutant showed no enhanced gravitropic response under blue light (Fig. 4C).The literature contains reports of positive as well as negative effects of light on gravitropism (for review, see Correll and Kiss, 2002). Here, we show that 12 h compared to 1 h of red light randomized root position in vertically grown seedlings (Fig. 4A) and reduced the curvature in gravity-stimulated seedlings (Fig. 4B) in the wild type but not in the pks1 mutant. Therefore, positive and negative effects of light on gravitropism can be separated temporarily and genetically in the same system.
Noteworthy is the fact that the phot1 mutant showed enhanced gravitropism not only in seedlings exposed to bilateral blue light, but also in seedlings grown in darkness or exposed to bilateral red light (i.e. in the absence of blue-light activation). This phenotype is manifested as reduced root deviation from the gravity vector in vertically grown seedlings (Fig. 4A) and increased curvature of the root when seedlings were placed horizontally (Fig. 4B). Clock (Devlin and Kay, 2000) and de-etiolation (Botto et al., 2003) phenotypes in the absence of blue light have also been reported for blue-light photoreceptor cryptochromes. PKS1 and phot1 interact physically (Lariguet et al., 2006) and both are required to induce root phototropism (Fig. 1) and reduce root gravitropism (Fig. 4); however, not all of these activities may require light activation of phot1.
Phototropic responses involve bending either toward or against the light gradient with consequent deviation from the gravity vector. As a result, the actual degree of bending depends on the phototropic response and a counteracting gravitropic response (Okada and Shimura, 1994; Vitha et al., 2000). The positive effect of PKS1 on the phototropic response could therefore be due, at least in part, to its negative effect on gravitropism (Fig. 6). However, the pks1 mutant failed to respond to blue light even under reduced gravitational stimulation (Fig. 5). This indicates that, in addition to the predicted indirect contribution of PKS1 to the phototropic response via its negative regulation of gravitropism, there is a more direct effect on phototropism itself (Fig. 6). The responses to gravity and unilateral blue light share late steps connected to the generation of a gradient of auxin and the modification of the direction of the growth response (for review, see Correll and Kiss, 2002). The contrasting effects of PKS1 on root phototropism and gravitropism suggest that PKS1 regulates these processes upstream of their convergence.
Figure 6.
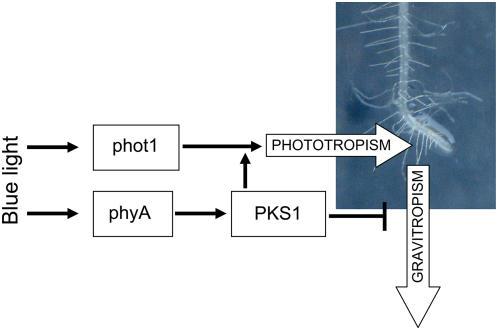
Working model of PKS1 function in root phototropism and gravitropism. Black arrows indicate positive regulatory processes and blunt-ended lines indicate negative regulatory processes. White arrows indicate phototropic and gravitropic forces acting in the direction of root growth. phyA-mediated induction of PKS1 is important for PKS1-mediated regulation of phototropism (as shown in Fig. 2), but not for the PKS1-mediated effect on gravitropism (as shown in Fig. 4). [See online article for color version of this figure.]
MATERIALS AND METHODS
Plant Material
The pks1-1 (Lariguet et al., 2003), pks1-2, pks2-1 (Lariguet et al., 2003), pks4-1 (Lariguet et al., 2006), phot1-5 (Huala et al., 1997), cry1-304, cry2-1 (Guo et al., 1998), and phyA-211 (Nagatani et al., 1993) mutants and the PKS1 OX, PKS2 OX, and PKS1∷GUS transgenic lines (Lariguet et al., 2003) used here are in the Arabidopsis (Arabidopsis thaliana) Columbia background. PKS4-overexpressing plants were obtained by transforming Columbia-0 seedlings with construct pIS007, which codes for the PKS4 cDNA driven by the cauliflower mosaic virus 35S promoter. PKS4 coding sequence flanked with BamHI sites was amplified by PCR (IS01 5′-gga tcc atg gcg caa act act gtc ac-3′ and IS02 5′-gga tcc tgg tat cca tca ttg cct tg-3′), and the BamHI-digested product was ligated into BamHI-digested pCGN18 to yield pIS007. All PCR-generated constructs were verified by sequencing. Two single insertion lines expressing elevated levels of PKS4 mRNA (data not shown) were selected for further analysis (IS07-5 and IS07-11). We obtained the pks1-2 insertion mutant from the GABI collection (line GABI 481C08; Rosso et al., 2003). T-DNA is inserted after the 53rd codon. No PKS1 was detected in pks1-2 (Supplemental Fig. S2). The PKS1:GUS transgene was introduced in different mutant backgrounds by crossing. PKS1:GUS phyA-211 was selected as tall seedlings under continuous far-red light (10 μmol m−2 s−1) in the F2 and F3 generations. PKS1:GUS cry1 was selected as tall under blue light (20 μmol m−2 s−1) in the F2 and F3 generations. PKS1:GUS phot1-5 was selected as nonbending seedlings under lateral blue-light irradiation (1 μmol m−2 s−1) in the F2 and F3 generations. Screening for the presence of a homozygous PKS1:GUS transgene was performed by sowing seeds on petri dishes containing one-half-strength Murashige and Skoog medium and selecting 100% BASTA resistance and 100% GUS-stained seedlings under white light in the F3 generation.
Light Treatments and Growth Conditions
Seeds were surface sterilized, sown on 0.8% agar-water in clear plastic boxes (42 × 35 mm2 × 20 mm), incubated in the dark at 4°C for 3 d, and exposed to 1 h of red light to induce homogeneous germination. Then, boxes were placed with the agar oriented vertically and kept in the dark for 2 d at 22°C before light treatments. A combination of fluorescent tubes and a blue filter (Rosco; filter no. 83) provided blue light (λmax = 440 nm). Different blue-light fluence rates were obtained by interposing different numbers of neutral filters and measured with a LiCor radiometer. The spectral photon distribution of the blue-light field was measured with a spectroradiometer (Analytical Spectral Devices Field Spec Pro FR). Fluorescent tubes in combination with red, yellow, orange (Lee filters; nos. 106, 101, and 105, respectively) and neutral filters provided bilateral red light (λmax = 640 nm; 5 μmol m−2 s−1).
Measurements of Curvature
Seedlings grew along the agar surface of the vertically oriented boxes. In phototropism experiments, the root of seedlings that grew toward the light source were given positive angles and the roots that grew away from the light were assigned negative angles. In gravitropism experiments, the angle between the gravitational vector and the root was assigned a positive value. Seedlings were photographed with a digital camera connected to a binocular loop. Images were used to measure the angle of root-growing direction against gravity vector using Image Tool Version 3 software from UTHSCSA. Each experiment was conducted at least on three independent occasions. Data from 10 seedlings were averaged per box (one replicate) and used for statistics (one-way ANOVA followed by Tukey's multiple comparison test or t test).
GUS Staining
GUS staining was conducted as described (Lariguet et al., 2003). Seedlings were observed with a binocular loop or an optical microscope and photographed with a digital camera. To better visualize and get an improved contrast between the seedling shape and the background, images were processed with a Photoshop program replacing the background of the original photograph by white or black color.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression of PKS1 in root gravitropism experiments.
Supplemental Figure S2. Protein blot showing the failure of pks1-1 and pks1-2 mutants to accumulate PKS1.
Supplementary Material
Acknowledgments
We thank Soledad Guidolin and Pablo Caparrós for excellent technical support. We thank Bernd Weisshaar (MPIZ, Cologne, Germany) for providing the GABI line number 481C08 generated in the context of the GABI-Kat program.
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (grant nos. BID 1728/OC–AR PICT 11631 and 32492 to J.J.C. and BID 1728/OC–AR PICT 32924 to H.B), the Universidad de Buenos Aires (grant no. G021 to J.J.C), the Swiss National Science Foundation (grant nos. PP00A–103005 and 3100A0–112638 to C.F.), and EMBO and the Roche Foundation (long-term fellowships to I.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jorge J. Casal (casal@ifeva.edu.ar).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Botto JF, Alonso Blanco C, Garzarón I, Sánchez RA, Casal JJ (2003) The Cvi allele of cryptochrome 2 enhances cotyledon unfolding in the absence of blue light in Arabidopsis. Plant Physiol 133 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W, Christie J (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7 204–210 [DOI] [PubMed] [Google Scholar]
- Caspar T, Pickard BG (1989) Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta 177 185–197 [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH (1999) Gravitropism in higher plants. Plant Physiol 120 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll MJ, Kiss JZ (2002) Interactions between gravitropism and phototropism in plants. J Plant Growth Regul 21 89–101 [DOI] [PubMed] [Google Scholar]
- Correll MJ, Kiss JZ (2005) The roles of phytochromes in elongation and gravitropism of roots. Plant Cell Physiol 46 317–323 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Kay SA (2000) Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 12 2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C, Pedmale U, Liscum E (2005) Plant tropisms: providing the power of movement to a sessile organism. Int J Dev Biol 49 665–674 [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J (1999) PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284 1539–1541 [DOI] [PubMed] [Google Scholar]
- Feldman LJ, Briggs WR (1987) Light-regulated gravitropism in seedling roots of maize. Plant Physiol 83 241–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C, Rabenold J, Liscum E (2006) Functional ecology of a blue light photoreceptor: effects of phototropin-1 on root growth enhance drought tolerance in Arabidopsis thaliana. New Phytol 173 91–99 [DOI] [PubMed] [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363 [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278 2120–2123 [DOI] [PubMed] [Google Scholar]
- Janoudi AK, Gordon WR, Wagner D, Quail P, Poff KL (1997) Multiple phytochromes are involved in red-light-induced enhancement of first-positive phototropism in Arabidopsis thaliana. Plant Physiol 113 975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J, Correll M, Mullen J, Hangarter R, Edelmann R (2003. a) Root phototropism: how light and gravity interact in shaping plant form. Gravit Space Biol Bull 16 55–60 [PubMed] [Google Scholar]
- Kiss JZ, Mullen JL, Correll MJ, Hangarter RP (2003. b) Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiol 131 1411–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Boccalandro HE, Alonso JM, Ecker JR, Chory J, Casal JJ, Fankhauser C (2003) A growth regulatory loop that provides homeostasis to phytochrome A signaling. Plant Cell 15 2966–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, De Carbonnel M, Alonso JM, Ecker JR, Liscum E, et al (2006) Phytochrome kinase substrate 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA 103 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1996) Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol 112 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP (1993) Genetic evidence that the red-absorbing form of phytochrome-B modulates gravitropism in Arabidopsis thaliana. Plant Physiol 103 15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli D, Briggs W (1984) Fiber optics in plants. Sci Am 251 90–98 [Google Scholar]
- Mandoli DF, Ford GA, Waldron LJ, Nemson JA, Briggs WR (1990) Some spectral properties of several soil types: implications for photomorphogenesis. Plant Cell Environ 13 287–294 [Google Scholar]
- Molas M, Kiss J, Correll M (2006) Gene profiling of the red light signalling pathways in roots. J Exp Bot 57 3217–3229 [DOI] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286 961–964 [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J (1993) Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol 102 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura Y (1994) Modulation of root growth by physical stimuli. In EM Meyerowitz, CR Sommerville, eds, Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 665–684
- Parks BM, Quail PH, Hangarter RP (1996) Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol 110 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Hangarter RP, Sharrock RA, Nagy F, Schäfer E (1996) The light-induced reduction of the gravitropic growth-orientation of seedlings of Arabidopsis thaliana (L.) Heynh. is a photomorphogenic response mediated synergistically by the far-red-absorbing forms of phytochromes A and B. Planta 199 511–514 [DOI] [PubMed] [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53 247–259 [DOI] [PubMed] [Google Scholar]
- Ruppel N, Hangarter R, Kiss J (2001) Red-light-induced positive phototropism in Arabidopsis roots. Planta 212 424–430 [DOI] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell 12 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs W (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M, Zacherl M, Luff L, Rudiger W (1997) Exposure of oat seedlings to blue light results in amplified phosphorylation of the putative photoreceptor for phototropism and in higher sensitivity of the plants to phototropic stimulation. Plant Physiol 115 493–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Chen R, Masson PH (1999) ARG1 (altered response to gravity) encodes a DnaJ-like protein that potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA 96 1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Quail PH (1995) Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J 7 413–427 [DOI] [PubMed] [Google Scholar]
- Stowe-Evans E, Luesse D, Liscum E (2001) The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via photoreversible phytochrome A-dependent modulation of auxin responsiveness. Plant Physiol 126 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M (2005) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA 102 13705–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, Zhao L, Sack FD (2000) Interaction of root gravitropism and phototropism in Arabidopsis wild-type and starchless mutants. Plant Physiol 122 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.