Abstract
There is evidence that the CO2-concentrating mechanism in the marine diatom Thalassiosira weissflogii operates as a type of single-cell C4 photosynthesis. In quantitative-PCR assays, we observed 2- to 4-fold up-regulation of two phosphoenolpyruvate carboxylase (PEPC) gene transcripts in Thalassiosira pseudonana cells adapted to low pCO2, but did not detect such regulation in Phaeodactylum tricornutum grown under similar conditions. Transcripts encoding phosphoenolpyruvate carboxykinase did not appear to be regulated by pCO2 in either diatom. In T. pseudonana and T. weissflogii, net CO2 fixation was blocked by 3,3-dichloro-2-(dihydroxyphosphinoyl-methyl)-propenoate (a specific inhibitor of PEPC), but was restored by about 50% and 80%, respectively, by addition of millimolar concentrations of KHCO3. In T. pseudonana, T. weissflogii, and P. tricornutum, rates of net O2 evolution were reduced by an average of 67%, 55%, and 62%, respectively, in the presence of 20 μm quercetin, also an inhibitor of PEPC. Quercetin promoted net CO2 leakage from inhibited cells to levels in excess of the equilibrium CO2 concentration, suggesting that a fraction of the HCO3− taken up is fated to leak back into the medium as CO2 when PEPC activity is blocked. In parallel to these experiments, in vitro assays on crude extracts of T. pseudonana demonstrated mean inhibition of 65% of PEPC activity by quercetin. In the presence of 5 mm 3-mercaptopicolinic acid (3-MPA), a classic inhibitor of phosphoenolpyruvate carboxykinase, photosynthetic O2 evolution was reduced by 90% in T. pseudonana. In T. weissflogii and P. tricornutum, 5 mm 3-MPA totally inhibited net CO2 fixation and O2 evolution. Neither quercetin nor 3-MPA had a significant inhibitory effect on photosynthetic O2 evolution or CO2 uptake in the marine chlorophyte isolates Chlamydomonas sp. or Dunaliella tertiolecta. Our evidence supports the idea that C4-based CO2-concentrating mechanisms are generally distributed in diatoms. This conclusion is discussed within the context of the evolutionary success of diatoms.
Diatoms fix approximately 1015 g of CO2 into organic carbon every year, equivalent to roughly 40% of marine primary production (Granum et al., 2005). Although these phytoplanktons play a pivotal role in mediating the exchange of inorganic carbon between the air-sea interface and the deep ocean (Falkowski and Raven, 1997), our understanding of how they effect this transformation is incomplete. The low affinity of algal Rubisco for CO2 and its propensity to fix O2 instead of CO2 in an oxygenase reaction (photorespiration; Kaplan and Reinhold, 1999) impose a chronic limitation on the rate of CO2 fixation. In seawater, the concentration of inorganic carbon is high (approximately 2 mm), but most of it is HCO3−, whereas only CO2 can serve as the substrate for Rubisco. The concentration of CO2 is around 10 μm (more in upwelling regions and less during blooms; Granum et al., 2005), lower than estimates of the half-saturation constant (KmCO2) for diatom Rubisco, which are in the range of 40 to 60 μm (Badger et al., 1998; Tortell, 2000). Clearly, the concentration of CO2 in seawater should limit the rate of carbon fixation.
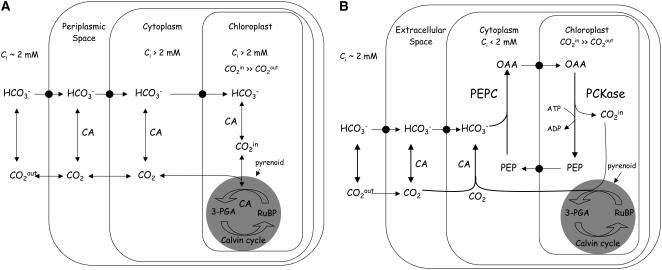
To ameliorate this problem, many eukaryotic phytoplankton species have evolved an energy-dependent CO2-concentrating mechanism (CCM), induced when cells are exposed to low pCO2. For example, in chlorophytes, HCO3− is accumulated in the chloroplast and a high CO2 supply rate to Rubisco is ensured by the rapid dehydration of HCO3− catalyzed by a carbonic anhydrase (CA; EC 4.2.1.1) colocalized with Rubisco (Kaplan and Reinhold, 1999; Fig. 1A). An external CA expressed under low pCO2 maintains a high rate of CO2 supply to the whole cell by catalyzing the dehydration of the large pool of extracellular HCO3− (Moroney et al., 1989).
Figure 1.
Current models of CCM function in C. reinhardtii and other eukaryotic phytoplankton (A) and in marine diatoms (B). Mechanistic details are given in the text.
Available data strongly suggest that the CCM of diatoms is biochemically similar to the CCM of C4 plants (Fig. 1B). Indeed, pioneering work on the path of carbon in marine phytoplankton revealed stark differences between diatoms and green microalgae, with the earliest products of fixation in diatoms typically identified as C4 and amino acids (Beardall and Morris, 1975; Beardall et al., 1976). Instead of being transported into the chloroplast, the HCO3− taken up from the medium is fixed into the four-carbon organic acid oxaloacetate (OAA) by phosphoenolpyruvate (PEP) carboxylase (PEPC; EC 4.1.1.31) in the cytoplasm. In the marine diatom Thalassiosira weissflogii, PEPC activity was shown to increase during growth under low pCO2 or when CA activity was limited by preconditioning at low zinc or by short-term treatment with the CA inhibitor ethoxyzolamide, suggesting that PEPC activity was necessary under low pCO2 or when the cell's ability to express CA was compromised (Reinfelder et al., 2000). A specific inhibitor of PEPC, the PEP analog 3,3-dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate (DECP; Jenkins et al., 1987) was shown to potently inhibit photosynthesis in T. weissflogii. Under high CO2:O2 ratios (which discourage photorespiration), this compound showed no inhibitory effect. It was also ineffective in a marine Chlamydomonas isolate, which utilizes a well-characterized C3 carbon assimilation pathway (Reinfelder et al., 2004). The product of PEPC, OAA, has been shown to support O2 evolution in the chloroplast and both OAA and malate (reduced form of OAA) have been shown to inhibit 14CO2 fixation, but not O2 evolution when applied exogenously to T. weissflogii cells (Reinfelder et al., 2004). Short-term pulse-chase experiments have shown significant initial 14C labeling in malate followed by transfer of label to phosphosugars, such as 3-phosphoglyceric acid in T. weissflogii, which is the characteristic pattern of transfer in C4 plants (Reinfelder et al., 2000; Morel et al., 2002). More recently, short-term 14C-labeling experiments have confirmed the incorporation of inorganic carbon into malate in T. weissflogii, but not in Thalassiosira pseudonana (Roberts et al., 2007). It is possible that the short-term C4 labeling in T. pseudonana might have occurred more rapidly than could be measured in the first 2 s of this study. Alternatively, the difference between these two diatoms could represent genuine intraspecific variations in the type of CCM employed. Whereas evidence for C4 photosynthesis in diatoms is accumulating, it is clear that these organisms maintain one important component of conventional CCMs—that of active uptake of HCO3−, which is essential for rapid photosynthesis in the marine environment (Tortell et al., 1997). Indeed, in terms of primary inorganic acquisition, recent work has shown that diatoms acclimate to low pCO2 by increasing their affinity for both CO2 and HCO3− and that HCO3− transport contributes greater than one-half the carbon required for photosynthesis and growth (Burkhardt et al., 2001; Rost et al., 2003).
In our model for the CCM of diatoms, the OAA is transported by a dedicated dicarboxylic acid transport system into the chloroplast where it is subsequently decarboxylated by a second enzyme, PEP carboxykinase (PCKase; EC 4.1.1.49). The CO2 released by this reaction is subsequently captured through the operation of a typical Calvin cycle in the pyrenoid structure of the chloroplast and the regenerated PEP is translocated back to the cytoplasm (Reinfelder et al., 2000). One mole of ATP is required for decarboxylation of 1 mol of OAA to CO2 and PEP. Reinfelder et al. (2000) found that PCKase copurified with Rubisco in cell fractionation experiments, suggesting chloroplast localization. These authors also measured PCKase activity in crude extracts of T. weissflogii and found it sufficient to support the observed rates of CO2 fixation by Rubisco. In the marine diatom Skeletonema costatum and the kelp Laminaria setchellii, PCKase activity has also been found to localize to the chloroplast, suggesting PCKase may be commonly found in this compartment in marine plants (Cabello-Pasini et al., 2001). The ratio of PEPC to Rubisco activities has been measured in pure diatom cultures and in field samples (Tortell et al., 2006; Cassar and Laws, 2007). The small measured values showing a large excess of Rubisco over PEPC activities have been put forth as evidence against the existence of a C4 pathway in diatoms. We note that in vitro rates are notoriously difficult to relate to in vivo kinetics, partly because they measure maximal potential rates and partly because we do not know the degree of enzymatic saturation in the cell. These data should therefore not be considered conclusive evidence against the presence of C4-based CCMs in diatoms.
As in chlorophytes, CAs play a critical role in the CCM of diatoms (Fig. 1B). Cytoplasmic CA maintains a low concentration of CO2 in this compartment by continuously hydrating it to HCO3−, which is fixed by PEPC. This serves to augment the inward diffusive flux of CO2 from the outside of the cell and to scavenge unfixed CO2 effluxing from the chloroplast. The inward gradient of CO2 is further augmented by extracellular CA, which maintains CO2 at equilibrium with HCO3− at the cell surface and prevents the development of an external CO2 diffusion gradient. Morel et al. (2002) used immunofluorescence techniques to demonstrate that the intracellular CA enzyme is principally cytoplasmic in T. weissflogii and largely absent from the chloroplasts.
Whether the carbon taken up during photosynthesis is stored as an organic or inorganic intermediate is a key difference between C4-based CCMs and more typical CCMs, which accumulate inorganic carbon against a concentration gradient (Kaplan and Reinhold, 1999; Badger and Spalding, 2000). Reinfelder et al. (2004) have addressed this issue and showed in T. weissflogii that only a small fraction of the carbon assimilated by the CCM existed as inorganic carbon when photosynthesis was stopped by treatment with a potent killing solution, which effectively quenched all enzymatic activity, suggesting that inorganic carbon is efficiently trapped into a stable organic compound in the cell. In contrast, in Chlamydomonas suspensions treated with the same killing solution, a large fraction of the carbon was released in inorganic form consistent with the accumulation of HCO3− in the light during photosynthesis.
In this study, we tested several key features of the model C4-CCM shown in Figure 1B. We examined by quantitative-PCR the expression in selected diatom species of the two enzymes PEPC and PCKase thought to be responsible for the carboxylation and decarboxylation of the C4 intermediate. With the aid of a membrane inlet mass spectrometer (MIMS) system, we also explored the effects on photosynthetic gas exchange of inhibitors known to block these enzymes in other organisms. When appropriate, we compared the results obtained with model diatom species to those obtained with chlorophytes.
RESULTS AND DISCUSSION
Regulation of PEPC and PCKase Gene Expression by pCO2
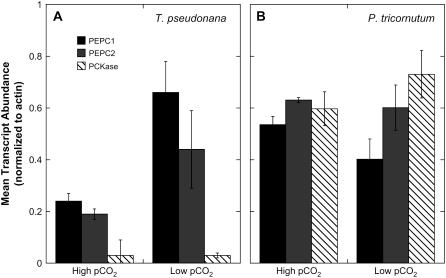
Inspection of the annotated genome sequences of T. pseudonana and Phaeodactylum tricornutum revealed the presence of two genes encoding PEPC and a single PCKase gene in each diatom (http://genome.jgi-psf.org/euk_home.html). To quantify the effect of pCO2 on the abundance of PEPC and PCKase gene transcripts, we conducted quantitative reverse transcription-PCR analyses of RNA isolated from these two organisms. All gene expression levels are reported as mRNA copy number of the gene of interest per copy of the nonregulated actin housekeeper gene. In T. pseudonana, transcripts for each of the two genes apparently encoding PEPC 1 and PEPC 2 were found to be about 20% as abundant as the actin under high pCO2 and increased by a factor of about 3 to about 60% of actin under low pCO2 (Fig. 2A). In contrast, we found no evidence for regulation by pCO2 of the homologous genes in P. tricornutum. However, the absolute abundance of the PEPC transcripts was high in this diatom, remaining between 40% to 60% of actin regardless of pCO2 (Fig. 2B). The abundance of the PCKase transcript did not respond significantly to growth pCO2 in either diatom, although the transcript levels were again constitutively much higher in P. tricornutum than in T. pseudonana (Fig. 2, A and B). Similar responses of PEPC and PCKase gene transcripts to pCO2 in T. pseudonana have recently been reported by Roberts et al. (2007). These authors also demonstrated strong diurnal effects on gene expression, with the large subunit of Rubisco down-regulated at night, whereas the two PEPC isoforms appeared to be mildly up-regulated.
Figure 2.
Effect of growth pCO2 on steady-state levels of PEPC and PCKase transcripts (designated PEPC 1, PEPC 2, and PCKase, respectively) in T. pseudonana (A) and P. tricornutum (B). Annotated coding sequences were obtained from the Joint Genome Institute (http://www.jgi.doe.gov). Procedures for total RNA isolation, cDNA synthesis, and quantitative-PCR amplification and mRNA detection are given in “Materials and Methods.” Values reported represent the means ± sd of three (A) or two (B) separate cultures.
Differing transcriptional responses of the PEPC genes to pCO2 could be related to different modes of regulation operating in the two organisms. Apparently, neither of the PEPC enzymes in either diatom is regulated by posttranslational processes because none contain the characteristic N-terminal Ser residue that typically serves as the site of phosphorylation (data not shown). It thus appears that in T. pseudonana the increase in abundance of PEPC at low pCO2 potentially represents transcriptional up-regulation of PEPC activity, although it must be borne in mind that transcript levels are often an unreliable proxy for the amounts of corresponding functional enzymes (Gibon et al., 2004). The apparent lack of regulation of PCKase transcription in this organism may simply reflect the downstream position of this enzyme in the CCM. Regulation of PEPC as a function of CO2 may result in a relatively constant supply of C4 organic acid, obviating the need to regulate the decarboxylating enzyme. In P. tricornutum, the high abundance of PEPC and PCKase transcripts may correspond to a strategy of maintaining constitutively high capacity to process inorganic carbon.
Sensitivity of Photosynthesis to PCKase Inhibition in Diatoms
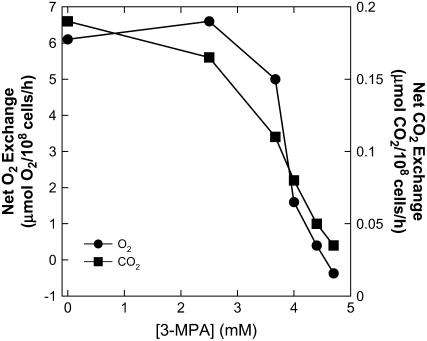
To gain better insight into the importance of PCKase in the CCM of diatoms, we tested the effects of a specific inhibitor, 3-mercaptopicolinic acid (3-MPA), on photosynthesis in low pCO2 cells. This compound has been used as a potent inhibitor of the PCKase enzyme from leaves and isolated chloroplasts of numerous C4 terrestrial and aquatic plants (Ray and Black, 1976, 1977; Rathnam and Edwards, 1977; Reiskind and Bowes, 1991) and in T. weissflogii (Reinfelder et al., 2004) and is usually considered to be specific for this enzyme. In experiments with T. pseudonana, there was little inhibition of photosynthesis until the 3-MPA concentration reached about 4 mm, above which there was a sharp decline in CO2 uptake and O2 evolution (Fig. 3). Similar patterns of inhibition of O2 evolution have been noted in Udotea flabellum, an aquatic C4 macrophyte, although PCKase functions in a carboxylating, rather than decarboxylating, role in this plant (Reiskind and Bowes, 1991). This demonstrates the inhibitory effects of 3-MPA on steady-state photosynthesis in this marine diatom.
Figure 3.
Effect of 3-MPA on steady-state net CO2 uptake and O2 evolution in low pCO2 T. pseudonana cells measured by MIMS. Results are shown from one representative experiment of two separate analyses. Cells (0.5–1.8 × 107 cells mL−1) were concentrated by filtration and resuspended in 1 mL of assay buffer (10 mm HEPES, pH 7.5) containing 3.5% (w/v) NaCl. 3-MPA was added from a stock solution (77 mm) dissolved in 25 mm HEPES (pH 7.5) to a final concentration of 5 mm. Darkened cells were preincubated in the presence of 3-MPA for 5 min. Photosynthesis was initiated by illuminating the cell suspension with white light obtained from a tungsten lamp at an intensity of 300 μmol photons m−2 s−1. The residual dissolved inorganic carbon in the assay buffer (approximately 300 μm) was used as the source of carbon for CO2 fixation.
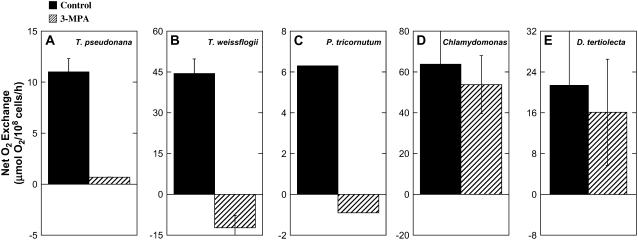
We chose a concentration of 5 mm 3-MPA to further evaluate its effects on photosynthesis in four other phytoplankton species, T. weissflogii, P. tricornutum, Chlamydomonas sp., and Dunaliella tertiolecta (Fig. 4, A–E). In treated suspensions of T. weissflogii and P. tricornutum, we observed total inhibition of net O2 evolution and CO2 uptake and fixation in the light compared to inhibition by an average of 90% in T. pseudonana. Indeed, in T. weissflogii and P. tricornutum, only net respiratory activity was observed. On average, the rates of O2 uptake were equivalent to approximately 20% of the rates of O2 evolution in untreated cells. Contrary to what we observed in the diatoms, we detected no significant effect of 3-MPA on the rates of photosynthesis in either Chlamydomonas sp. or D. tertiolecta suspensions. These results indicate a critical role in CO2 fixation for PCKase in diatoms, but not in chlorophytes. According to our model of the CCM in diatoms (Fig. 1B), inhibition by 3-MPA arises from the blockage of OAA decarboxylation in the chloroplast, which, in turn, starves Rubisco for CO2 in the C3 Calvin cycle.
Figure 4.
Effect of 5 mm 3-MPA on steady-state net O2 evolution in the light by low pCO2 cells of T. pseudonana (A), T. weissflogii (B), P. tricornutum (C), Chlamydomonas sp. (D), and D. tertiolecta (E). Assay conditions were as described for Figure 3. Cells were concentrated to 18 to 23 × 106 mL−1, 1.7 to 2.4 × 106 mL−1, 20 to 30 × 106 mL−1, 5 to 12 × 106 mL−1, and 8.5 × 106 mL−1, respectively, by either filtration or centrifugation (see text for details). Rates of photosynthesis were obtained in the presence/absence of inhibitor in assay medium (10 mm HEPES, pH 7.5, 3.5% NaCl) equilibrated with 400 μm KHCO3 in 300 μmol photons m−2 s−1. Except for P. tricornutum, all experiments were conducted using two to five independently grown cultures conditioned to low pCO2 by harvesting the cells at pH > 8.6. Error bars represent the sd about the mean of two to three independent determinations.
Sensitivity of Photosynthesis and PEPC Activity to DCDP and Quercetin
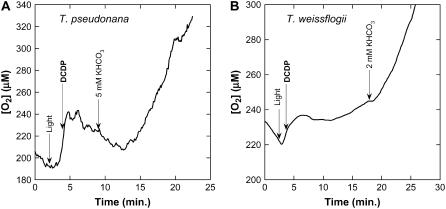
Previous work has shown a strong effect of DCDP, a specific inhibitor of PEPC, on CO2 fixation in T. weissflogii and in C4 land plants that can be largely overcome by adding CO2 to high concentration (Jenkins et al., 1987, 1989; Reinfelder et al., 2004). We revisited this by testing the effects of this compound on T. pseudonana, which had not been previously shown to be sensitive to it. At a concentration of 490 μm, net O2 evolution (as a proxy for CO2 fixation) was totally inhibited in T. pseudonana, but was partially restored (45%) by the addition of 5 mm KHCO3 (Fig. 5A). T. weissflogii was also inhibited by DCDP at this concentration, but this was reversed by about 80% when 2 mm KHCO3 was added (Fig. 5B). Our results with T. weissflogii are in good agreement with Reinfelder et al. (2004) and, taken together, support the conclusion that T. pseudonana has a similar type of CCM to T. weissflogii.
Figure 5.
Effect of DCDP on photosynthesis in T. pseudonana (A) and T. weissflogii (B). Cells were preconcentrated by filtration and resuspended in assay buffer (10 mm HEPES, pH 7.6, 3.5% NaCl) in the chamber of the mass spectrometer and incubated for a few minutes in the dark. Contaminating levels of inorganic carbon (about 400 μm) were used to drive net CO2 fixation. The light was turned on and, after steady-state net O2 evolution was obtained, DCDP was added to a final concentration of 490 μm.
The flavonoid compound quercetin has previously been used as a potent inhibitor of PEPC activity in extracts of both a C4 plant, maize (Zea mays), and a C3 plant, canola (Brassica napus; Pairoba et al., 1996; Moraes and Plaxton, 2000). We explored the potential for this compound to inhibit CCM function in phytoplankton by monitoring the rates of inorganic carbon uptake and O2 evolution in concentrated cell suspensions. Although, as an inhibitor of PEPC, quercetin does not discriminate between the enzymes of C4 and C3 plants, its effect on photosynthesis should be relatively more severe in phytoplankton employing a C4-CCM compared to those utilizing a conventional CCM based on inorganic carbon accumulation.
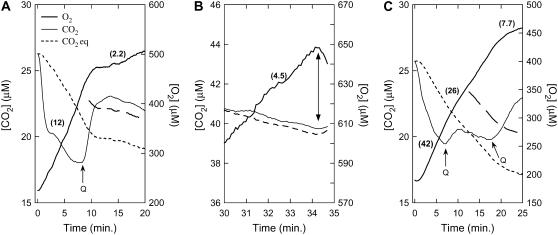
We examined the effect of quercetin on photosynthesis and CO2 exchange in actively photosynthesizing T. pseudonana and T. weissflogii cells (Fig. 6, A and C, respectively; Table I). Within 1 min of illumination (started at time 0), the cells drew [CO2] below the concentration at equilibrium with HCO3−, [CO2 eq], indicating a net CO2 influx into the cells. We believe this influx results from passive CO2 diffusion across the plasmalemma, down a concentration gradient set up by the activities of CA and PEPC in the cytoplasm (Fig. 1B). In T. pseudonana suspensions, addition of 20 μm quercetin inhibited the rate of O2 evolution by 80% to 90% almost immediately, and it subsequently recovered to about 20% of the preinhibited rate (Fig. 6A). The [CO2] in the medium rose rapidly to a value roughly 15% above [CO2 eq] and remained at this level for the ensuing 10 min of the experiment. In T. weissflogii suspensions, after an initial 20 μm quercetin addition, the [CO2] rose in the medium slowly and surpassed [CO2 eq] after about 7 min (Fig. 6C). Addition of a second 20 μm pulse of quercetin resulted in 85% inhibition of net O2 evolution and caused the [CO2] to rise more rapidly and to a higher level than the first pulse. The requirement in this particular experiment for twice the amount of inhibitor to elicit the same response as in T. pseudonana may have been due to the comparatively larger amount of biomass used (2.43 × 109 μm3 cell volume/mL for T. weissflogii versus 0.9 × 109 μm3 cell volume/mL for T. pseudonana). Quantitative analysis of the CO2 fluxes in the presence of the inhibitor (see “Materials and Methods”) revealed that only 65% and 50% of the observed excess of [CO2] over [CO2 eq] could be accounted for by respiratory CO2 release in T. pseudonana and T. weissflogii suspensions, respectively (Fig. 6, A and C, long dashed line). Analyses of several such experiments in both diatoms produced essentially the same result. The most parsimonious explanation for the excess CO2 efflux is that it originates from the pool of cytoplasmic HCO3−. Bicarbonate is still taken up by the cells after quercetin addition as shown by the residual O2 evolution; part of that HCO3− leaks back into the medium as CO2 after dehydration by the cytoplasmic CA. Both the inhibition of photosynthesis resulting from the inhibition of PEPC and the simultaneous CO2 leakage are fully consistent with our model of the diatom CCM (Fig. 1B). Similar to the DCDP experiments (Fig. 5), addition of a high concentration of inorganic carbon partially reversed the inhibitory effect of quercetin, pointing to an inhibition of the cell's ability to acquire carbon, rather than a nonspecific effect on photosynthesis (Fig. 6B). In three separate experiments with quercetin, addition of 2 mm KHCO3 restored the rate of photosynthesis by an average of 42% ± 5%. In T. weissflogii cells inhibited with quercetin, the rate of photosynthesis was restored by about 38% by 2 mm KHCO3 addition (data not shown).
Figure 6.
Effect of quercetin on net O2 and CO2 exchange during steady-state photosynthesis in cell suspensions of T. pseudonana (A and B) and T. weissflogii (C) measured by MIMS. Cells were concentrated 50- to 100-fold by filtration and resuspended in 1 mL of assay buffer (10 mm HEPES, pH 7.6, 3.5% [w/v] NaCl). A 10-μL aliquot of the cell concentrate was sampled for estimates of cell density just prior to placing the suspension in the sample cuvette (thermoregulated to 20°C) attached to the mass spectrometer. Cells were incubated in the dark for approximately 2 min before 20 μm AZ was added to inhibit extracellular CA activity. Dissolved inorganic carbon for photosynthesis was added as KHCO3 to 500 μm. Light (300 μmol photons m−2 s−1) was provided from a tungsten lamp from one side of the cuvette. Photosynthesis was initiated by illuminating the cells. After steady-state CO2 fixation was under way (approximately 10 min), quercetin was added in the light to the indicated concentrations. The long dashed lines represent the maximal potential CO2 concentration above [CO2 eq] that could be supported by the observed rate of respiratory CO2 release. AZ stock solutions were maintained at 10 mm in DMSO. In B, 2 mm KHCO3 was added to the inhibited T. pseudonana suspension (shown in A) approximately 15 min after the initial addition of inhibitor. The double arrow indicates the suspension was darkened. The numbers in parentheses beside the oxygen traces are the rates of net O2 evolution in units of μmol O2/108 cells/h.
Table I.
Effect of quercetin, baicalein, and rutin hydrate on steady-state net O2 evolution in low CO2 cells of T. pseudonana and their associated apparent Ki values for PEPC inhibition
Rates of photosynthesis are reported in units of μmol O2/108 cells/h. *, Values from Pairoba et al. (1996).
| Flavonoid | Net O2 Evolution
|
Ki (μm)* | ||
|---|---|---|---|---|
| Control | 25 μm | 75 μm | ||
| Quercetin | 10.4 ± 1.7 | 3.4 ± 1.3 | n.d. | 2.9 |
| Baicalein | 9.8 ± 1.5 | 1.4 ± 1.6 | n.d. | 6.5 |
| Rutin hydrate | 9.4 | 9.3 | 7.7 | 19.8 |
The observed effects of quercetin on diatoms provide powerful support to the view that PEPC mediates HCO3− fixation in the cytoplasm and that it constitutes the major pathway for carbon fixation in diatoms if indeed quercetin acts by inhibiting PEPC activity. Because quercetin has been shown to inhibit various enzymes and processes in cells (e.g. Lang and Racker, 1974), we performed two types of controls: in vitro assays with cell extracts and in vivo assays with chlorophytes that are known to operate a classic C3 photosynthetic pathway. We also examined the effects of flavonoids other than quercetin on photosynthesis in T. pseudonana.
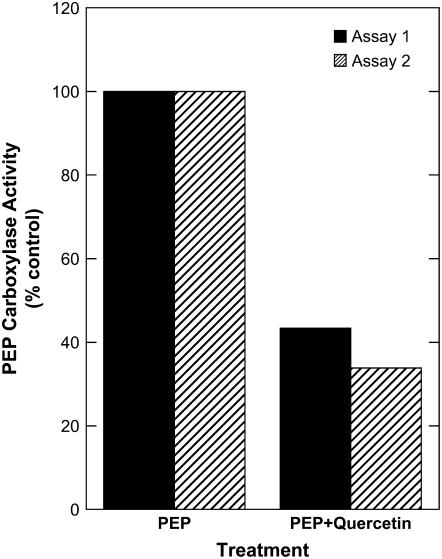
We tested the sensitivity of diatom PEPC to quercetin, with in vitro activity assays in crude extracts of low-CO2-grown T. pseudonana cells. In two separate assays on independent cultures, we detected a mean inhibition of PEPC activity of approximately 65% with 0.2 mm quercetin (Fig. 7). The concentration of quercetin required for inhibition of PEPC activity in crude extracts (0.2 mm) was larger than that necessary for inhibition of in vivo photosynthesis (20–40 μm). This difference is likely due to the much higher concentration of HCO3− present in the assay medium (10 mm) compared to the in vivo gas exchange experiments (0.5–1.5 mm HCO3−) because HCO3− has been shown to decrease the sensitivity of some PEPC isoforms to inhibitors (Parvathi et al., 1998). Although the biochemical mechanism of quercetin inhibition of PEPC is not known with certainty, Pairoba et al. (1996) reported competitive inhibition with respect to PEP in the enzyme from maize leaves.
Figure 7.
Effect of quercetin on apparent PEPC activity in low CO2 cells of T. pseudonana. Cells were ground in a chilled mortar and pestle for two periods of 7 min in extraction buffer (50 mm BICINE, pH 7.5, 1.5 m glycerol, 10 mm MgCl2, 1 mm EDTA, 10 mm NaHCO3, 5 mm dithiothreitol, and 5 mg mL−1 bovine serum albumin). Crude extracts were preincubated at room temperature for 45 min, at which time they were subdivided for treatment. To one vial, 1 μCi of H14CO3− was added and, to a second vial, 1 μCi of H14CO3− was added along with quercetin to a final concentration of 0.2 mm. These reactions were initiated by adding 5 mm PEP followed by a 2-h incubation at 25°C. A third vial was included, which received radiolabel but did not receive PEP. These treatments acted as negative controls. Reactions were terminated by transferring aliquots to vials containing HCl (35%, approximately 9 n). Acidified extracts were evaporated to dryness and 14C incorporated into organic carbon was counted by liquid scintillation.
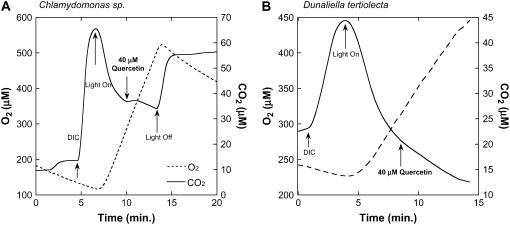
We explored the potential for quercetin to inhibit CCM function in Chlamydomonas sp. and D. tertiolecta. At a concentration of 40 μm quercetin, there was no apparent effect on either CO2 uptake or O2 evolution in either of these phytoplankton species (Fig. 8, A and B). At a concentration of 200 μm quercetin, only a small effect on the rates of photosynthetic gas exchange was detected in Chlamydomonas sp. cells (data not shown). The contrasting effects of quercetin on the diatoms T. pseudonana, T. weissflogii, and P. tricornutum (inhibited by 67%, 55%, and 62%, respectively, at 20 μm quercetin) and the chlorophytes Chlamydomonas sp. and D. tertiolecta (no inhibition) are shown in Table II. The effect of quercetin on photosynthesis and CO2 fluxes observed in diatoms is not seen in C3 phytoplankton. This is consistent with the inhibition of PEPC, which should cripple photosynthesis in organisms with a C4-based CCM but have little or no effect on C3 organisms.
Figure 8.
Effect of quercetin on photosynthetic CO2 and O2 exchange in concentrated cell suspensions of Chlamydomonas sp. (A) and D. tertiolecta (B). Assay conditions were as described for Figure 6. Quercetin was added to a final concentration of 40 μm for both phytoplankton species.
Table II.
Effect of quercetin on the rate of photosynthesis in selected marine phytoplankton
Rates are reported in units of μmol O2/108 cells/h. Chlamydomonas sp. and D. tertiolecta were treated with 40 μm quercetin. All other treatments were 20 μm. Results obtained with T. pseudonana are presented in Table I. Experimental details are given in the legends to Figures 6 and 8. Values are the mean of two to three independent measurements on separate cultures. Error = 1 sd about the mean.
| Phytoplankton Species | Control Rate | +Quercetin |
|---|---|---|
| T. weissflogii | 39 ± 2.1 | 18 ± 12 |
| P. tricornutum | 4.5 ± 0.8 | 1.6 ± 0.7 |
| Chlamydomonas sp. | 53.9 ± 3.4 | 55.5 ± 4.5 |
| D. tertiolecta | 18.1 ± 1.8 | 17.4 ± 0.9 |
We evaluated the effects of two additional flavonoid compounds, baicalein and rutin hydrate, on CCM function in T. pseudonana (Table I). Baicalein appeared to be as effective an inhibitor of photosynthesis as quercetin, inhibiting O2 evolution by about 85% at a concentration of 25 μm. Conversely, rutin hydrate did not inhibit photosynthesis at all at this concentration. In the presence of 75 μm rutin hydrate, only a modest inhibition of about 20% was detected (Table I). These results are wholly consistent with the relative effectiveness of flavonoids as inhibitors of PEPC as documented from the Ki values obtained from in vitro enzymatic assays (Pairoba et al., 1996; Table I). The consistency between the relative effects of flavonoids on photosynthesis in diatoms and their effectiveness as PEPC inhibitors provides further support to the notion that the former is caused by the latter.
C4-CCMs: Biochemical and Ecological Perspectives
Our results provide additional support for the operation of a C4-based CCM in diatoms. These protists appeared some 200 to 300 millions years ago and are now among the most successful photosynthetic autotrophs in freshwater and oceans. What competitive advantages might they have gained by adopting a C4 pathway for inorganic carbon acquisition? Obviously, the O2-insensitive fixation of carbon by PEPC can minimize photorespiratory activity in marine phytoplankton just as well as in terrestrial C4 plants. A C4-based CCM is also particularly efficient for maintaining low CO2 in the cytoplasm while ensuring an efficient rate of fixation by Rubisco. The primary fixation of HCO3− into OAA effectively traps the carbon in a form with low potential for efflux back into the medium. Indeed, the low carbon isotope discrimination factor of diatoms is evidence for a low rate of CO2 leakage during steady-state photosynthesis. In other words, diatoms appear to be among the low pCO2 specialists (Clark and Flynn, 2000) and this may be the key to their global ecological success: A C4-based CCM might make them well adapted to the low pCO2 of the present-day atmosphere and surface waters to which they have contributed through their efficient export of carbon to the deep sea.
Another possible benefit a C4-CCM in diatoms is to integrate efficiently carbon and nitrogen metabolism (Allen et al., 2006). Plants and microalgae carboxylate PEP via PEPC in an anaplerotic reaction to replenish carbon-rich substrates into the TCA cycle to support anabolic metabolism. For example, Guy et al. (1989) and Elrifi and Turpin (1986) demonstrated the diversion of carbon away from the Calvin cycle by the anaplerotic reaction to provide carbon skeletons for amino acid synthesis in nitrogen-limited Selenastrum minutum fed with a transient pulse of NO3− or NH4+. Most regions of the oceans are nitrogen limited and phytoplankton capable of rapidly exploiting new inputs of nitrogen can potentially out-compete other groups. Maintaining high PEPC activity could poise diatoms in a physiological state, whereby newly fixed carbon could either be diverted toward supporting amino acid synthesis during transitions from nitrogen deficiency to nitrogen replenishment or could be used as the primary path of carbon fixation in the chloroplast when nitrogen is not limiting.
CONCLUSION
Taken at face value, our data show a striking difference between the carbon acquisition systems of diatoms and chlorophytes: The former are inhibited by quercetin and 3-MPA, the latter are not. Including chlorophytic phytoplankton in our analysis as C3 controls clearly showed that these compounds do not inhibit photosynthesis generally and that the desired enzymes were indeed properly targeted by them. Moreover, DCDP and 3-MPA, which were both effective in inhibiting photosynthesis in diatoms, are generally accepted to be specific inhibitors of key enzymes in the C4 pathway. Equally telling is the observation that chemically unrelated compounds, whose only connection is that each inhibits a separate enzyme in a functional C4 pathway, all cripple carbon uptake in our model species. Our data fully support the idea that at least some diatoms operate a C4-CCM. Presumably this feature offered an ecological advantage to these organisms during the modern period of declining CO2 and may help in nitrogen-limited regions of contemporary oceans.
MATERIALS AND METHODS
Phytoplankton Cultures
Cultures of the marine diatoms Thalassiosira pseudonana CCMP1335, Thalassiosira weissflogii CCMP1336, and Phaeodactylum tricornutum UTEX642 and the marine chlorophytes Chlamydomonas sp. CCMP222 and Dunaliella tertiolecta CCMP364 were grown in trace metal-buffered medium in polycarbonate bottles under 90 μmol photons m−2 s−1 continuous illumination. Medium was prepared from 0.2-μm-filtered Gulf Stream water supplemented with major nutrients (10 μm PO43−, 100 μm NO3−, 100 μm SiO2), filter-sterilized vitamins, and trace metals (80 nm zinc, 120 nm manganese, 50 nm cobalt, 20 nm copper, and 0.5–1 μm iron) buffered with 100 μm EDTA. For gene expression experiments, T. pseudonana and P. tricornutum cells were grown under two different CO2 regimes. To obtain T. pseudonana cells conditioned to high CO2, cells were transferred into growth medium prebubbled with 1% CO2 gas in air until the pH reached 7.2. As these cultures grew, the pH rose to 7.8, at which point the cultures were harvested. For low CO2 conditioning, T. pseudonana cells were harvested when the pH of the culture medium had reached 8.9. For P. tricornutum, CO2 levels were controlled using filter-sterilized pH buffers. High and low CO2-conditioned cells of P. tricornutum were obtained by including 5 mm Tris buffer at a pH of 7.5 and 8.6, respectively. For PEPC assays, low CO2 T. pseudonana cells were obtained by transferring cells into growth medium buffered to pH 8.7 with 5 mm 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid and growing the culture for nine generations. At the point of harvest, the pH of these cultures had risen slightly to 8.8. Phytoplankton growth in cultures was followed by enumerating cells with a Coulter counter (Beckman-Coulter). Specific growth rates (d−1) were determined by fitting linear regression curves to the natural log of cell number versus time.
MIMS
The exchange of CO2 (m/z = 44) and O2 (m/z = 32) in concentrated phytoplankton suspensions was monitored continuously with MIMS. With the exception of D. tertiolecta, all cells were collected on 3-μm polycarbonate filters (Poretics Corp.) under gentle vacuum and resuspended in 1 mL of assay buffer (10 mm HEPES, pH 7.5; 3.5% [w/v] NaCl). Cells of D. tertiolecta were recalcitrant to filtration, so they were collected by 5-min centrifugation (5,000g) at room temperature and resuspended in 1 mL of assay buffer (as above). For PCKase inhibition experiments, cells were preincubated in the dark for 5 min in the presence of 3-MPA, a specific inhibitor of PCKase. After preincubation, the buffer was supplemented with 400 μm NaHCO3 and the light was turned on. Light for photosynthesis was obtained from a tungsten projector bulb and directed to the cell chamber via a flexible fiber-optic cable at an intensity of 300 μmol photons m−2 s−1 (about 3× growth light). For PEPC inhibition experiments using flavonoid compounds, cells were prepared as above and resuspended in 1 mL of assay buffer (10 mm HEPES, pH 7.6; 3.5% [w/v] NaCl). In these experiments, the membrane-impermeable CA inhibitor acetazolamide (AZ) was added to a final concentration of 20 μm after dissolution in dimethylsulfoxide (DMSO). T. pseudonana and T. weissflogii produce an extracellular CA that equilibrates the CO2 and HCO3− in the bulk medium (data not shown). When this CA is inhibited by addition of AZ, the CO2 concentration in the assay medium, [CO2], can deviate substantially from its equilibrium value, [CO2 eq], as a result of the active uptake and fixation of CO2 and/or HCO3− by the cells. [CO2 eq] can be calculated based on the equilibrium ratio of HCO3− to CO2 at the assay pH and from the HCO3− concentration remaining in the medium corrected for the amount of carbon fixed in photosynthesis. The difference between [CO2] and [CO2 eq] provides a quantitative estimate of the CO2 flux in or out of the cells: flux = kH ([CO2 eq] − [CO2]), where kH, the CO2 hydration rate constant, is 0.03 s−1. The rate of CO2 release from respiration was estimated from the rate of dark O2 uptake measured over a period of 5 min, assuming CO2:O2 stoichiometry of 1:1.
Flavonoids were prepared in equal volumes of DMSO and absolute ethanol. For all inhibitor experiments, nonbiological variations in the 12CO2 signal were corrected by normalizing to the inert gas argon (m/z = 40). Calibration for CO2 was carried out by injecting known quantities of NaHCO3 and monitoring the increase in the CO2 signal until equilibrium was reached in a medium of known pH and temperature. Calibration for O2 was carried out by monitoring the decrease in the O2 signal after addition of sodium dithionite to air-equilibrated distilled water, assuming a concentration of 250 μm O2 at equilibrium with water at 20°C. The cell suspension chamber was thermoregulated to 20°C by a circulating water bath. The lower detection limit for CO2 (designated CO2 zero) was determined by drop-wise addition of 10 m NaOH to distilled water. For accurate estimates of assay cell densities, duplicate 10- to 20-μL aliquots of concentrated cell suspension were sampled just prior to suspension in the MIMS cuvette, diluted 500- to 1,000-fold into 3.5% NaCl and each counted in triplicate in the Coulter counter.
RNA Isolation and cDNA Synthesis
Total RNA was isolated from diatom cells with TRIzol reagent (Invitrogen) following the procedure of McGinn et al. (2003). The yield of total RNA was quantified with the Ribogreen RNA quantitation kit (Molecular Probes) or by measuring the A260. Individual mRNA transcripts were reverse transcribed to cDNA with the SuperScript III kit (Invitrogen) from 2 μg of total RNA. Complementary DNA templates were diluted 5-fold prior to amplification.
Gene Expression Analysis by Quantitative-PCR
Quantitative-PCR double-stranded DNA standards were generated as follows. PCR products were generated for the genes of interest by amplifying cDNA prepared as described above in 25-μL reactions containing 1 μL of diluted cDNA product, 1.5 mm MgCl2, 0.2 mm dNTPs, 12.5 pmol each of forward and reverse primer, and 1 unit of Taq polymerase (Roche). The PCR cycling conditions were as follows: 95°C for 1 min, followed by 35 cycles of 94°C for 15 s, 58°C for 20 s, and 72°C for 30 s. Amplicons were separated on 2% agarose gels and visualized by ethidium bromide staining on a standard UV transilluminator. A single PCR product of the correct size was excised with a sterile razor blade and purified (Qiaex II gel extraction kit; Qiagen). Purified DNA was quantified with a Picogreen double-stranded DNA quantitation kit (Molecular Probes) and copy numbers per unit volume were determined. All quantitative-PCR reactions were carried out on the Stratagene MX3000P thermal cycler instrument. A SYBR Green master mix (Brilliant SYBR Green qPCR Mastermix; Stratagene) was used for quantitative-PCR in 50-μL reactions containing 3 μL of diluted cDNA and 25 pmol each of forward and reverse primer using the following cycling program: 95°C for 10 min followed by 40 cycles of 94°C, 58°C for 20 s, and 72°C for 30 s. Standards corresponding to between 102 to 106 copies per well were amplified on the same 96-well plate as cDNA generated from experimental material. To correct for differences in RNA starting material and variations in cDNA synthesis efficiency (Parker and Armbrust, 2005), the abundance of each transcript was normalized to the abundance of the actin transcript (copies transcript of interest/copy actin), which was not biologically regulated with changes in pCO2 tension (data not shown). Oligonucleotide primers used for quantitative-PCR analyses are given in Table III.
Table III.
Oligonucleotide primers used in quantitative-PCR analyses in this study
| Species | Encoded Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|---|
| T. pseudonana | PEPC 1 | AGATGTATGAACAGTGGCCCTGGT | AGAGGACGCTCGCATCAATTGAAC |
| PEPC 2 | GGAGTTGGCGAGGCTATCAATGAA | GTCGCGCACCAAAACATTCTCA | |
| PCKase | ACTCGAGAAACACAGTGCTAACGC | TCGGGATTGGACCAGGTACTCTTT | |
| Actin | ACTGGATTGGAGATGGATGG | CAAAGCCGTAATCTCCTTCG | |
| P. tricornutum | PEPC 1 | CGAAAGTCTTCGCGCTATTC | ACCATTTCGACCAAATCCAC |
| PEPC 2 | CGGTTTGTTCCCTACTTTCG | TTCGCTTTGATCTGTCGTTG | |
| PCKase | TCAGCCACATTCTCGACTTG | TACGCTTCCCGGTACCATAG | |
| Actin | TGACAGAGCGTGGTTACTCG | ACCATCCATCTCCAAACCAC |
PEPC Activity Assays
PEPC assays were performed according to the method of Cassar and Laws (2007). T. pseudonana cells were collected on a 3-μm polycarbonate filter under gentle vacuum and resuspended in 2 mL of extraction buffer containing 50 mm BICINE (pH 7.5), 1.5 m glycerol, 10 mm MgCl2, 1 mm EDTA, 10 mm NaHCO3, 5 mm dithiothreitol, and 5 mg mL−1 bovine serum albumin. Resuspended cells were placed in a mortar chilled to −20°C and ground manually with a pestle continuously on ice for two consecutive periods of 7 min each. After grinding, the extract was diluted with an additional 2 mL of buffer, mixed thoroughly by pipetting, and placed in a 30-mL glass vial and preincubated on a rotary shaking table at room temperature for 45 min. Immediately after preincubation, three 1.2-mL aliquots of crude extract were transferred into 30-mL glass vials and 3 μCi of NaH14CO3 was added to each. To one vial, 0.2 mm of the PEPC inhibitor quercetin was added just prior to the addition of radiolabel. PEP was added to a final concentration of 5 mm. Negative controls containing no added PEP were also run. After 1 h, the reactions were terminated by addition of 1% (v/v) 9.8 n HCl, swirled gently, and placed in a fume hood to evaporate. Dried extracts were redissolved in 1 mL of deionized water and counted for radioactivity after addition of 10 mL scintillation cocktail (Ultima Gold; Packard Instrument Company).
Acknowledgments
We thank Colin Jenkins (CSIRO, Canberra, Australia) for providing us with the DCDP. We thank Dr. Nicolas Cassar (Princeton) for helpful discussions throughout this work. We also thank two anonymous reviewers for their helpful comments and suggestions.
This work was supported by the National Science Foundation (grant no. 0351499 to F.M.M.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Patrick J. McGinn (pmcginn@princeton.edu).
References
- Allen AE, Vardi A, Bowler C (2006) An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms. Curr Opin Plant Biol 9 264–273 [DOI] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD (1998) The diversity and coevolution of RubisCO, plastids, pyrenoids and chloroplast based CO2 concentrating mechanisms in algae. Can J Bot 76 1052–1071 [Google Scholar]
- Badger MR, Spalding MH (2000) CO2 acquisition, concentration and fixation in cyanobacteria and algae In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 369–397
- Beardall J, Morris I (1975) Effects of environmental factors on photosynthesis patterns in Phaeodactylum tricornutum (Bacillariophyceae). II. Effect of oxygen. J Phycol 11 430–434 [Google Scholar]
- Beardall J, Mukerji D, Glover HE, Morris I (1976) The path of carbon in photosynthesis in marine phytoplankton. J Phycol 12 409–417 [Google Scholar]
- Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D (2001) CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr 46 1378–1391 [Google Scholar]
- Cabello-Pasini A, Swift H, Smith GJ, Alberte RS (2001) Phosphoenolpyruvate carboxykinase from the marine diatom Skeletonema costatum and the phaeophyte Laminaria setchelli. II. Immunological characterization and subcellular localization. Bot Mar 44 199–207 [Google Scholar]
- Cassar N, Laws EA (2007) Potential contribution of β-carboxylases to photosynthetic carbon isotope fractionation in a marine diatom. Phycologia 46 307–314 [Google Scholar]
- Clark DR, Flynn KJ (2000) The relationship between dissolved inorganic carbon concentration and growth rate in marine phytoplankton. Proc R Soc Lond B Biol Sci 267 953–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi IR, Turpin DH (1986) Nitrate and ammonium induced suppression in N-limited Selanastrum minutum. Plant Physiol 81 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, Raven JA (1997) Aquatic Photosynthesis, Ed 1. Blackwell Science, Malden, MA
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M (2004) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes in enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum E, Raven JA, Leegood RC (2005) How do marine diatoms fix 10 billion tones of inorganic carbon every year? Can J Bot 83 898–908 [Google Scholar]
- Guy RD, Vanlerberghe GC, Turpin DH (1989) Significance of phosphoenolpyruvate carboxylase during ammonium assimilation: carbon isotope discrimination in photosynthesis and respiration by the N-limited green alga Selenastrum minutum. Plant Physiol 89 1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins CLD, Furbank RT, Hatch MD (1989) Inorganic carbon diffusion between C4 mesophyll and bundle sheath cells. Plant Physiol 91 1356–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins CLD, Harris RLN, McFadden HG (1987) 3,3-Dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate, a new, specific inhibitor of phosphoenolpyruvate carboxylase. Biochem Int 14 219–226 [Google Scholar]
- Kaplan A, Reinhold L (1999) CO2-concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50 539–570 [DOI] [PubMed] [Google Scholar]
- Lang DR, Racker E (1974) Effects of quercetin and F1 inhibitor on mitochondrial ATPase and energy-linked reactions in submitochondrial particles. Biochim Biophys Acta 333 180–186 [DOI] [PubMed] [Google Scholar]
- McGinn PJ, Price GD, Maleszka R, Badger MR (2003) Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC6803. Plant Physiol 132 218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes TF, Plaxton WC (2000) Purification and characterization of phosphoenolpyruvate carboxylase from Brassica napus (rapeseed) suspension cell cultures. Implications for phosphoenolpyruvate carboxylase regulation during phosphate starvation and the integration of glycolysis with nitrogen assimilation. Eur J Biochem 267 4465–4476 [DOI] [PubMed] [Google Scholar]
- Morel FMM, Cox EH, Kraepiel AML, Lane TW, Milligan AJ, Schaperdoth I, Reinfelder JR, Tortell PD (2002) Acquisition of inorganic carbon by the marine diatoms Thalassiosira weissflogii. Funct Plant Biol 29 301–308 [DOI] [PubMed] [Google Scholar]
- Moroney JV, Husic HD, Tolbert NE, Kitayama M, Manuel LJ, Togasaki RK (1989) Isolation and characterization of a mutant of Chlamydomonas reinhardtii deficient in the CO2 concentrating mechanism. Plant Physiol 89 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairoba CF, Columbo SL, Andreo CS (1996) Flavonoids as inhibitors of NADP-malic enzyme and PEP carboxylase from C4 plants. Biosci Biotechnol Biochem 60 779–783 [DOI] [PubMed] [Google Scholar]
- Parker MS, Armbrust EV (2005) Synergistic effects of light, temperature, and nitrogen source of transcription of genes for carbon and nitrogen metabolism in the centric diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 41 1142–1153 [Google Scholar]
- Parvathi K, Bhagwat AS, Raghavendra AS (1998) Modulation by bicarbonate of catalytic and regulatory properties of C4 phosphoenolpyruvate carboxylase from Amaranthus hypochondriacus: desensitization to malate and glucose 6-phosphate and sensitization to Mg(2+). Plant Cell Physiol 39 1294–1298 [Google Scholar]
- Rathnam CKM, Edwards GE (1977) C4-dicarboxylic acid metabolism in bundle-sheath chloroplasts, mitochondria and strands of Eriochloa borumensis Hack., a phosphoenolpyruvate-carboxykinase type C4 species. Planta 133 135–144 [DOI] [PubMed] [Google Scholar]
- Ray TB, Black CC (1976) Inhibition of oxaloacetate decarboxylation during C4 photosynthesis by 3-mercaptopicolinic acid. J Biol Chem 251 5824–5826 [PubMed] [Google Scholar]
- Ray TB, Black CC (1977) Oxaloacetate as the source of carbon dioxide for photosynthesis in bundle sheath cells of the C4 species Panicum maximum. Plant Physiol 60 193–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinfelder JR, Kraepiel AML, Morel FMM (2000) Unicellular C4 photosynthesis in a marine diatom. Nature 407 996–999 [DOI] [PubMed] [Google Scholar]
- Reinfelder JR, Milligan AJ, Morel FMM (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol 135 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind JB, Bowes G (1991) The role of phosphoenolpyruvate carboxykinase in a marine macroalga with C4-like photosynthetic characteristics. Proc Natl Acad Sci USA 88 2883–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K, Granum E, Leegood RC, Raven JR (2007) C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 145 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sültemeyer D (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48 55–67 [Google Scholar]
- Tortell PD (2000) Evolutionary and ecological perspectives on carbon acquisition in phytoplankton. Limnol Oceanogr 45 744–750 [Google Scholar]
- Tortell PD, Martin CL, Corkum ME (2006) Inorganic carbon uptake and intracellular assimilation by subarctic Pacific phytoplankton assemblages. Limnol Oceanogr 51 2102–2110 [Google Scholar]
- Tortell PD, Reinfelder JR, Morel FMM (1997) Active uptake of bicarbonate by diatoms. Nature 390 243–2449384376 [Google Scholar]