Abstract
Migration and morphology of human melanoma cells (MV3) depend on extracellular pH (pHe) and the activity of the Na+/H+ exchanger NHE1. To distinguish effects of NHE1 activity per se from effects of pHe we compared an NHE1-deficient mutant with rescued and wild-type cells. Time lapse video microscopy was used to investigate migratory and morphological effects caused by pHe and NHE1 activity, and a membrane-bound fluorescein conjugate was employed for ratiometric pH measurements at the outer leaflet of the cell membrane. As long as NHE1 remained inactive due to deficiency or inhibition by cariporide (HOE642) neither migration nor morphology was affected by changes in pHe. Under these conditions pH at the outer leaflet of the plasma membrane was uniform all over the cell surface. The typical pH dependence of MV3 cell migration and morphology could be reconstituted by restoring NHE1 activity. At the same time the proton gradient at the outer leaflet of the plasma membrane with the higher proton concentration at the leading edge and the lower one at the cell rear was re-established as well. Hence, NHE1 activity generates a proton gradient at the cell surface accompanied by the cells' ability to respond to changes in pHe (bulk pH). We conclude that NHE1 activity contributes to the generation of a well-defined cell surface pH by creating a proton gradient at the outer leaflet of the plasma membrane that is needed for (i) the development of a variety of morphologies including a distinct polarity and (ii) migration. A missing proton gradient at the cell surface cannot be compensated for by varying pHe.
The predominantly glycolytic metabolism of tumour cells (Helmlinger et al. 2002) produces protons that need to be removed from the cytosol. This task is carried out by transport proteins such as the Na+/H+ exchanger NHE1 and causes an acidification of the extracellular space inside solid tumours (Tannock & Rotin, 1989). NHE1 activity and acidic extracellular pH (pHe) affect tumour cell migration (Stock et al. 2005), a requirement for metastasis, and they correlate with malignancy. NHE1 activation enhances the invasiveness of human breast carcinoma cells (Reshkin et al. 2000a,b), and an acidic pHe promotes experimental pulmonary metastasis of human melanoma cells (Rofstad et al. 2006). In the slime mold Dictyostelium discoideum, NHE1 expression can be correlated with chemotaxis. Chemotactically competent Dictyostelium cells express NHE1 whereas vegetative cells do not (Patel & Barber, 2005).
Several mechanisms are known by which NHE1 affects migration (Stock & Schwab, 2006). They include the control of gene expression, cytoskeletal anchoring and the generation of cell polarity (Denker & Barber, 2002). The present study focuses on yet another function of NHE1 required for cell migration, namely the ion translocating activity of NHE1. In previous studies (Stock et al. 2005; Stock & Schwab, 2006) we demonstrated that the transport activity of NHE1 is closely linked to the formation and release of integrin-mediated cell adhesions (Jalali et al. 2001; Webb et al. 2003). Focal adhesion contacts, at which integrin heterodimers (Hynes, 2002) interact with components of the extracellular matrix (ECM) such as collagens, laminins or fibronectin (Schwartz et al. 1991; Aguiar et al. 2005) are needed to transduce the mechanical force from the cytoskeleton onto the ECM (Beningo et al. 2001). Extracellular pHe and NHE1 activity influence the strength of cell adhesion and thus affect cell migration on a collagen I matrix (Stock et al. 2005). However, so far it had not been possible to disconnect pHe on the one hand and NHE1 activity on the other hand. In order to distinguish between effects of protons expelled by NHE1 and effects of protons from the bulk solution we compared an NHE1-deficient mutant, its rescued clone and wild-type cells by analysing migration, morphology and pH at the outer leaflet of the plasma membrane. If the protons from the bulk solution had priority over those expelled by NHE1 it should be possible to compensate – at least partially – for a lack of NHE1 activity by increasing the extracellular proton concentration.
Methods
Cells and cell culture
An NHE1-deficient clone, an NHE1-rescued clone and wild-type cells of the human melanoma cell line MV3 (van Muijen et al. 1991) were grown in bicarbonate buffered RPMI 1640 (Sigma, Taufkirchen, Germany) supplemented with 10% (v/v) fetal bovine serum (FBS) at 37°C in a humidified atmosphere of 5% CO2/95% air.
The NHE1-deficient mutant showing reduced NHE1 activity was isolated from the MV3 cell line according to the H+-suicide technique originally described by Pouyssegur et al. (1984) and then adapted to migrating cells by Schwab et al. (2005). An exponentially growing culture was mutagenized applying 2.35 mmol l−1 ethylmethane sulphonate for 16 h. After another 24 h, cells were loaded with Li+ by a 2 h incubation in a Hepes-buffered Ringer solution of which the entire Na+ had been replaced by Li+. In a following step lasting 1 h the Li+ was replaced by choline and the extracellular pH was lowered to pH 5.5 (buffered with 20 mmol l−1 Mes–Tris). This step led to a lethal uptake of H+ ions into cells with NHE activity. Only cells devoid of NHE activity were able to survive. This selection process was repeated four times.
In order to create a rescued cell line, NHE1-deficient cells were transfected with the cDNA of the human NHE1 subcloned into pcDNA3 (HindIII/XbaI). Cells were transfected with the FuGene6 Transfection Reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. The selection of stably transfected cells with 0.6 g l−1 geneticin (G-418-sulphate; PAA Laboratories, Pasching, Austria) was performed for 10 days. Geneticin-resistant cells were cloned by single cell dilution.
Experimental solutions
Cell migration and adhesion were observed in serum-free RPMI 1640 media buffered with NaHCO3. The amount of NaHCO3 to be added to the media was determined by the desired pH value.
pH measurements and one series of migration experiments were performed using Hepes-buffered Ringer solutions containing (mmol l−1): 122.5 NaCl, 5.4 KCl, 0.8 MgCl2, 1.2 CaCl2, 1.0 NaH2PO4.2H2O, 5.0 glucose, 10 Hepes. Various pH values were obtained by adding 1 m NaOH. In order to examine the Na+-dependent recovery of pHi upon intracellular acidification cells were exposed to a Na+-free solution that contained N-methyl-d-glucamine (NMDG) instead of Na+.
When applicable (i) the NHE1 inhibitor HOE642 (Cariporide, final concentration 10 μmol l−1) was added to either the serum-free media or to the Hepes buffered Ringer solutions and (ii) cells were acidified by replacing 15 mmol l−1 NaCl by 15 mmol l−1 sodium propionate (Szatkowski & Thomas, 1989).
Phosphate-buffered saline (PBS) solution was used as a solvent for the antibodies and as washing buffer as well. The PBS contained (mmol l−1): 137 NaCl, 2.7 KCl, 8.1 Na2HPO4, 1.5 KH2PO4.
Preparation of collagen matrices
One hundred microlitres of 10× RPMI− 1640 and 100 μl 10× Hepes buffer (final concentration in the polymerized collagen gels: 10 mmol l−1) were added to 800 μl Collagen G solution (Biochrom AG; Berlin, Germany). The pH of the collagen mixture was always adjusted to pH 7.4 with 1 m NaOH. The bottoms of 25 ml culture flasks (12.5 cm2, Falcon) were covered with 200 μl collagen solution each, and the collagen was allowed to polymerize overnight at 37°C in a humidified atmosphere. Cells were seeded on this collagen matrix and allowed to adapt to different pH values varying from 6.4 to 7.5 for 3 h prior to recording.
Analysing cell migration
The culture flasks were put into heated chambers on stages of inverted microscopes (Axiovert25, Carl Zeiss, Inc. Göttingen, Germany). Cell migration was recorded in 10 min intervals for 5 h at 37°C using video cameras (Models XC-ST70CE and XC-77CE, Hamamatsu/Sony, Japan) and PC-vision frame grabber boards (Hamamatsu, Herrsching, Germany). Acquisition of images was controlled by HiPic and WASABI softwares (Hamamatsu). The circumferences of the cells were labelled applying the AMIRA software (TGS, Template Graphics Sofware, Mercury Communication Systems Inc., Carlsbad, CA, USA). The cell contours then served as the basis for further analysis. Parameters such as structural index (SI), migratory velocity (μm min−1) and translocation (μm) were analysed using self-made JAVA programs and the NIH ImageJ software (http://rsb.info.nih.gov/ij/). Migration was determined as the movement of the cell centre per time unit, the velocity was estimated from the 10 min time intervals applying a three point difference quotient and the cell area was measured as the number of pixels. The structural index (SI) represents the cell shape. Values close to 1 correspond to a spherical cell shape whereas values close to 0 correspond to a spindle or a dendritic cell shape. SI was calculated as follows:
| (1) |
where A is the area covered by the cell and p is the perimeter of A.
Measuring intracellular pH (pHi)
pHi was measured using video imaging techniques and the fluorescent pH indicator BCECF (Molecular Probes, Eugene, OR, USA). Cells were treated as for migration experiments. They were resuspended in one of the Hepes-buffered experimental solutions containing, if applicable, HOE642 and/or 15 mmol l−1 propionic acid. They were plated onto collagen coated coverslips and allowed to adapt for 3 h. Cells were then incubated in the respective control solution containing 2 μmol l−1 BCECF-AM for 5 min. The coverslips were placed on the stage of an inverted microscope (Axiovert 200; Carl Zeiss, Inc.) and continuously superfused with prewarmed (37°C) Hepes-buffered Ringer solutions. The excitation wavelength alternated between 440 nm and 490 nm, respectively, while the emitted fluorescence intensities were monitored at 500 nm using a Photometrics camera (CoolSnapfx, Visitron Systems, Puchheim, Germany). The different wavelengths were generated by a high speed polychromator system (Visichrome, Visitron Systems). Polychromator and data acquisition were controlled by Metafluor software (Visitron Systems). Fluorescence intensities were measured in 35 s intervals and corrected for background fluorescence. At the end of each experiment, the pH measurements were calibrated by successively superfusing the MV3 cells with modified Ringer solutions of pH 7.5, 7.0 and 6.5 containing (mmol l−1): 125 KCl, 1 MgCl2, 1 CaCl2, 20 Hepes and 10 μmol l−1 nigericin (Sigma-Aldrich) (Dordick et al. 1980; Kirschberger et al. 1999). NHE1 activity was assessed by using the NH4+-prepulse technique (Schwab et al. 2005). At first cells were superfused with a Na+-free solution containing NMDG-Cl instead of NaCl. When 40 mmol l−1 NH4Cl was added, the osmolarity of the solution was kept constant by isosmotically substituting NMDG-Cl. The NH4+-pulse leads to an alkalinization of the cytosol. Upon removal of NH4Cl pHi decreases rapidly. pHi recovers when Na+ is added to the superfusate. The initial slope of the change of pHi after re-addition of Na+ in Hepes-buffered solution was taken as a measure of NHE activity.
Measuring pH at the outer leaflet of the plasma membrane
The cell surface pH was measured as previously described (Stock et al. 2007). Initially, cells were treated as those used for pHi or migration experiments. The outer leaflet of the plasma membrane was then labelled with 1 μg ml−1 of the fluorescein-conjugated pH indicator DHPE (1,2-dihexaecanoyl-sn-glycero-3-phospho-ethanolamine, Molecular Probes, OR, USA) in 0.1% dimethylformamide (v/v) in serum-free RPMI 1640 for 15–30 min (Elliott et al. 2003). pH was measured using video imaging techniques as described above. Cells were continuously superfused with prewarmed (37°C) Hepes-buffered Ringer solutions of various pH values. As for BCECF the excitation wavelength for DHPE alternated between 440 nm and 490 nm while the emitted fluorescence intensities were monitored at 500 nm using a Photometrics camera (CoolSnapfx, Visitron Systems). A ratio was calculated from the intensities measured at 440 and 490 nm. Thus, the measurement was virtually independent of the amount of dye excited in a given region of interest and represented the local proton concentration. Fluorescence intensities were measured in 35 s intervals and corrected for background fluorescence by subtracting background intensities of control regions placed right next to the measured regions of interest. At the end of each experiment, the pH measurements were calibrated as described above for the pHi measurements.
In order to quantify a pHem gradient along the direction of movement, cells were divided into four segments of about the same size. Each segment contained four defined regions of interest placed over the plasma membrane. The measured values of the four regions were combined in one single value representing one segment.
Cell adhesion
Wild-type and NHE1-deficient cells were resuspended in serum-free RPMI media of different pH (pH 6.2 to pH 7.5) with or without 10 μmol l−1 HOE642. Cells were then seeded in collagen (4 mg ml−1) coated 24-well plates at a density of 50 000 cells per well. After 60 min the media including the non-adhesive cells were washed with cold PBS buffer. The remaining cells were fixed and counted.
Immunofluorescence
Cells were treated with cold 1% Triton X-100 in PBS for 10 min prior to fixation. Without this pre-permeabilization step NHE1 could hardly be labelled as the epitope was not accessible enough to the antibody. Cells were then fixed by 3.5% paraformaldehyde in PBS. Non-specific binding sites were blocked with 2% BSA(w/v) and 0.2% (w/v) gelatine in PBS. After staining the cells with the immunopurified N1P1 polyclonal antibody (1 : 250) (Sardet et al. 1989, 1990; Lagana et al. 2000) for 1 h and a Cy3-conjugated IgG (1 : 400; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for another hour, the slide preparations were washed in PBS and then covered with Vectashield (Vector Laboratories Inc., Burlingame, CA, USA). Images were taken using an invert microscope (Axiovert 200, Carl Zeiss) and a digital camera (Model 9.0, RT-SE-Spot, Visitron Systems) controlled by MetaVue software.
Statistics
All experiments were repeated three to six times. Data are presented as the mean values ± s.e.m. The data were tested for significance employing Student's unpaired or paired t test or analysis of variance (ANOVA) where applicable. The level of significance was set at P < 0.05 except where stated otherwise.
Results
Function of NHE1 in deficient, rescued and wild-type melanoma cells
Upon intracellular acidification there was virtually no Na+-dependent recovery of the intracellular pH (pHi) in NHE-deficient cells (Fig. 1). pHi recovery of NHE1-transfected and wild-type MV3 cells occurred up to three times as quickly. Treating the cells with propionic acid (15 mmol l−1 for 3 h at pHe 7.0) reduced pHi in NHE-deficient cells to pHi 6.5. Additional application of the NHE1 inhibitor HOE642 caused no further acidification. In contrast, wild-type cells were able to compensate for the acid load in an NHE1-dependent manner. Their pHi was 7.1 in the absence and 6.7 in the presence of HOE642 (Table 1). These findings indicate that NHE1 activity is largely reduced in the selected NHE1-deficient mutant, which is consistent with the below-mentioned results of immunolocalization experiments. In consideration of the fact that there was still a measurable, residual NHE1 activity left in the selected clone we continue to call it the NHE1-deficient mutant as opposed to NHE1 null or NHE−/− mutant.
Figure 1. Na+-dependent recovery of intracellular pH (pHi).
The Na+-dependent recovery of the intracellular pH (pHi) of MV3 cells upon cytosolic acidification (by the NH4+ technique) is significantly reduced in NHE1-deficient cells and can be rescued by transfecting NHE1-deficient cells with NHE1. A, exemplary recordings of single cells. Slopes, i.e. the rates of pHi recovery plotted in B, were derived from the subsidiary lines. B, recovery of pHi depending on the initial pHi right after intracellular acidification (NHE1-deficient, filled circles; rescued, grey triangles; wild-type, open squares; n = at least 80 cells from at least 5 different trials for each cell line containing all 4 (wild-type) or 5 (deficient and rescued) mean values).
Table 1.
Effects of NHE1 stimulation (propionic acid) and additional NHE1 inhibition (HOE642) in NHE1-deficient and in wild-type cells at pHe 7.0
| NHE1-deficient | Wild-type | |||
|---|---|---|---|---|
| Propionic acid | Propionic acid + HOE642 | Propionic acid | Propionic acid + HOE642 | |
| V (μm min−1) | 0.11 ± 0.015 | 0.08 ± 0.009 | 0.07 ± 0.01 | 0.03 ± 0.004 |
| Translocation (μm) | 17.45 ± 3.15 | 9.47 ± 0.96 | 18.5 ± 2.77 | 6.45 ± 0.9 |
| Structural index (SI) | 0.66 ± 0.056 | 0.82 ± 0.03 | 0.11 ± 0.01 | 0.61 ± 0.1 |
| Intracellular pH (pHi) | 6.49 ± 0.07 | 6.48 ± 0.08 | 7.1 ± 0.05 | 6.69 ± 0.04 |
n = at least 30 cells from at least 5 different trials in each case.
Loss of NHE1 activity leads to inhibition of cell migration and adhesion
In Fig. 2 average speed (Fig. 2A) and translocation (Fig. 2B) of NHE1-deficient and rescued cells were compared with those of wild-type cells (see also Table 1 and online supplemental material movies 1–3). Data for the wild-type cells were taken from Stock et al. (2005). At pHe 7.0 migratory speed and translocation over 5 h were significantly lower in NHE1-deficient (movie 1) than in rescued (movie 2) or wild-type cells (movie 3). Moreover, the low migratory activity of NHE1-deficient cells remained unaffected over a wide range of pHe values varying from 6.2 to 7.5. This lack of sensitivity to extracellular protons could be imitated in wild-type cells by inhibiting NHE1 with HOE642. The migratory speed of wild-type cells was then also reduced to less than 0.2 μm min−1 and their translocation to less than 30 μm in 5 h. The remaining, low migratory activity that was found in NHE1-deficient and in HOE642-treated wild-type cells may represent the NHE1-independent migratory activity. Consequently, the specific NHE1-inhibitor HOE642 did not have an additional effect on migratory speed and translocation of NHE1-deficient cells over the entire range of the pH values tested (pH 6.2–pH 7.5). As shown in previous studies (Klein et al. 2000; Schwab et al. 2005; Stock et al. 2005) the presence or absence of HCO3− has only a minor effect on cell migration due to the ability of the anion exchanger AE2 to accept OH− as well. Nevertheless, in order to obtain data that are one-to-one comparable to the below-mentioned results of the pH measurements at the outer leaflet of the plasma membrane, we analysed the migration of deficient, rescued and wild-type cells also in Hepes-buffered Ringer solution (Fig. 2C and D) instead of Hepes-buffered medium. Although the absolute values for the migratory activity were clearly reduced in Ringer solution, the trend in the pHe dependence remained the same and was significant.
Figure 2. Cell migration depends on NHE1 activity and pHe.
A loss of NHE1 activity either by inhibition with cariporide (HOE642) or by mutagenesis impairs migration and reduces the cells' sensitivity to pHe. In both HCO3− buffered medium (A and B) and Hepes-buffered Ringer solution (C and D) the migratory speed (A and C) and the cells' translocation over 5 h (B and D) could be restored for the most part by transfecting the NHE1-deficient cell clone with NHE1. n = at least 30 cells from at least 5 different trials in each case. For the purpose of comparison, data for wild-type cells (grey line, open squares) taken from Stock et al. (2005) were also plotted in A and B.
Our observations suggest that NHE1-activity is a prerequisite for the dependence of migration on protons of the bulk solution.
Like migration the cell morphology remained also nearly unaffected by pHe when NHE1 activity was absent due to NHE1 deficiency or inhibition (Fig. 3). However, upon transfection NHE1-deficient cells regained their ability to develop different, more polarized morphologies in a pH-dependent manner. The lack of NHE1 activity also precluded the formation of numerous cell protrusions that are typically developed upon intracellular acidification with propionic acid (compare Figures 4c, g and l). The present findings show that the forming of different cell morphologies in response to the extracellular proton concentration requires proton export by NHE1.
Figure 3. Cell morphology depends on NHE1 activity and pHe.
As shown by the structural index (SI), NHE1 deficiency or NHE1 inhibition with HOE642 prevent cells from spreading and make them stay rather spherical independently of pHe. This effect can be countered by transfecting the cells with NHE1. n = 30 cells from 5 different trials. For the purpose of comparison data for wild-type cells (grey line, open squares) taken from Stock et al. (2005) were also plotted.
Figure 4. Morphology depending on NHE1 activity.
The morphology of NHE1-deficient cells does not change upon NHE1 inhibition by HOE642 or NHE1 stimulation by cytosolic acidification (e–h). Wild-type and rescued cells react to NHE1 inhibition by getting spherical (b, d, k and m) and to NHE1 stimulation by branching out (c and l). pHe was 7.0. Images represent observations based on at least five different experiments with 5–10 cells per experiment. Scale bar: 20 μm.
Finally, adhesion of NHE1-deficient and wild-type cells to collagen I matrices were compared. In NHE1-deficient cells adhesion was reduced by more than 40% (data not shown). It was not affected by HOE642 and reached a similarly low level as did adhesion of wild-type cells in the presence of HOE642. This observation indicates that NHE1-activity increases adhesiveness.
To conclude, the cells' ability to vary migratory activity, morphology and adhesiveness in response to the extracellular protons present in the bulk solution requires NHE1 activity. As pHi of wild-type, NHE1-deficient and rescued cells did not differ significantly under standard conditions or depending on pHe values varying from pH 6.8–7. (Fig. 5), NHE1 activity per se and/or the protons carried by it seem to be crucial.
Figure 5. Intracellular pH (pHi) depending on extracellular pH (pHe) in wild-type, NHE1-deficient and rescued MV3 cells.
pHi of the three clones does not differ after a 3 h adaptation to different pHe values. N per value is at least 40 cells from at least 4 independent experiments.
NHE1 activity is required for the generation of an extracellular proton gradient at the plasma membrane
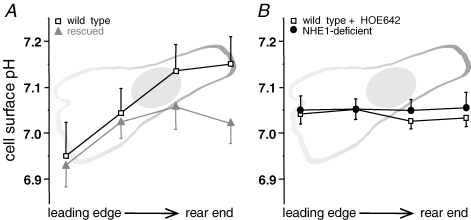
Cells were labelled with the proton-sensitive, fluorescein-coupled DHPE, a pH indicator that is incorporated into the outer leaflet of the plasma membrane. As shown in a previous study (Stock et al. 2007), migrating wild-type cells established a proton gradient at their cell surface corresponding to an inhomogeneous distribution of NHE1. pHem was lower at the leading edge of the lamellipodium than at the rear end (Fig. 6A). Due to the fact that a high percentage of NHE1-deficient cells were rather spherical with a structural index higher than 0.6 (73.25 ± 8.88%, n = 55 cells from 5 independent trials, pHe 7.0), it would not have been possible to determine a gradient in these cells. We therefore picked NHE1-deficient cells that were explicitly polarized. At an extracellular pH (pHe) of 6.84, at which pHi was exactly the same in all of the three cell clones (Fig. 5), there was no gradient in the NHE1-deficient mutant (Fig. 6B, 7.05 ± 0.03 at the leading edge versus 7.05 ± 0.03 at the rear end, n = 10, P = 0.93) and in wild-type cells whose NHE1 had been inhibited by HOE642 (Fig. 6B; pHem 7.04 ± 0.02 at the leading edge and pHem 7.03 ± 0.02 (n = 14, P = 0.76) at the cell rear). However, the gradient could be partially restored by transfecting the deficient cell line with NHE1 (pHem 6.93 ± 0.05 at the leading edge and pHem 7.02 ± 0.05 (n = 6, P = 0.06) at the cell rear). In wild-type cells the difference in pHem between the leading edge (pH 6.95 ± 0.07) and the rear end (pH 7.15 ± 0.06) was about 0.2 pH units (n = 6 cells, P = 0.04). The absence and presence or restoration of the gradient were consistent with the presence and the distribution of NHE1 in the plasma membrane. In NHE1-deficient cells NHE1 could not be detected (Fig. 7A and movie 4). In rescued cells NHE1 accumulated at the leading edge, but otherwise was evenly distributed all over the cell (Fig. 7B and movie 5). The immunolabelling of the lateral membrane and of the membrane at the rear end was weaker than that at the cell front. In wild-type cells NHE1 accumulated at the leading edge of the lamellipodium (Fig. 7C and movie 6). Interestingly, the cell surface pH at the rear end of both the deficient and the rescued clone was rather low compared to that of the wild-type. As for wild-type and rescued cells the different surface pH values at the rear end corresponded to the differences in their NHE1 distribution (Fig. 7B and C).
Figure 6. Proton gradient at the plasma membrane.
A, wild-type (open squares) and NHE1-retransfected (grey triangles) cells show a proton gradient declining along the longitudinal axis from the leading edge to the rear end. B, NHE1-deficient cells (filled circles) and wild-type cells whose NHE1 is inibited by HOE642 (open squares) do not establish a proton gradient at the outer leaflet of the plasma membrane. n = 6–10 cells, 1 cell per experiment, at pHe 6.84 in each case.
Figure 7. Typical immunolocalization of NHE1 underside of an NHE1-deficient (A), a rescued (B) and a wild-type (C) MV3 cell as observed in five independent experiments.
Cells were seeded on collagen I. Leading edges are indicated by asterisks, localization of NHE1 by arrow heads; scale bars: 25 μm. See also movies 4-6.
The absence or presence and the slope of the gradient also correlate with the migratory behaviour and the cells' morphology. Both directed migration and a distinct polarization require the NHE1-dependent gradient. This observation indicates that local pH ranks before bulk pH.
Discussion
The present study aims to distinguish between effects that (i) protons expelled by the Na+/H+ exchanger NHE1 and (ii) protons of the bulk solution have on cell migration. The key observation is that NHE1-deficient cells hardly migrate and that they do not react to protons of the bulk solution (extracellular pH, pHe) by developing morphologically different features such as a polarized morphology. These deficits can be rescued for the most part by transfecting NHE1-deficient cells with NHE1. Wild-type cells whose NHE1 is blocked behave similarly. They do not respond to a varying pHe either. This means that varying pHe affects cell migration and morphology only when NHE1 is active suggesting that the protons exported by NHE1 have priority over those of the bulk solution.
Adhesion on the one hand and migration on the other hand make different demands regarding a proton-modulated cell–matrix interaction. As for cell adhesion an increased extracellular proton concentration can partially compensate for a loss in NHE1 activity (Stock et al. 2005). However, regarding cell migration an increased extracellular proton concentration does not compensate for the missing protons normally provided by NHE1 activity since the protons of the bulk solution affect the integrin–matrix interaction and thus cell adhesion to the same extent all over the cell. In contrast, cell polarity and directed migration require an uneven strength in or a different degree of cell adhesion, and here the NHE1-dependent proton gradient comes into play.
Polarized cells of the wild-type establish an extracellular proton gradient at their surface. The proton concentration decreases from the leading edge to the rear end (Stock et al. 2007). NHE1-deficient cells and wild-type cells whose NHE1 is inhibited are not able to establish this gradient while the rescued cell clone is (Fig. 6). In NHE1-deficient cells structural abnormalities caused either by a possible change in pHi or by an insufficient function of NHE1 as an anchor linking the cytoskeleton to the plasma membrane (Denker & Barber, 2002) might account for the reduced migratory activity and the absence of the proton gradient at the cell surface. However, the large-area pHi did not differ significantly between NHE1-deficient, rescued and wild-type cells and inhibition of the intact NHE1 in wild-type cells led to a reduced migratory activity and a missing gradient as well. These results support the idea that the protons extruded by NHE1 are crucial for the generation of the proton gradient and for cell migration.
In wild-type cells the maximum migratory speed is observed at pHe 7.0; however, the cells are found to branch out the most (minimum structural index) at pHe values between 6.6 and 6.8. This could be due to the strength of the integrin–matrix interaction. It is the strongest at pHe 6.6–6.8 and may therefore hinder the release of focal contacts and consequently the retraction of the cell rear or cell protrusions so that the cells appear to be ‘stuck’ to the matrix (Stock et al. 2005). In addition, the proton gradient at the cell surface is well pronounced at pHe values of 6.84 and higher but its slope decreases as pHe decreases and the gradient even disappears at pHe values of less than 6.6 (Stock et al. 2007). This observation indicates that the presence of the proton gradient correlates with the cells' ability to migrate.
Interestingly, the cell surface pH at the cell rear becomes more acidic in NHE1-deficient cells or in wild-type cells upon NHE1 inhibition (Fig. 6B). At this point we can only speculate that additional proton export mechanisms at the rear end might be activated in order to compensate for the loss in NHE1 activity.
In rescued and wild-type cells the proton concentration at the plasma membrane is significantly lower than that of the bulk solution indicating a buffering function of the glycocalyx. By its chemical buffering capacity and its possible function as a sterical barrier the glycocalyx might form a bordered space different from the bulk solution, particularly with regard to the proton concentration. This idea is supported by two observations. First, the heavily NHE1-dependent extracellular proton gradient is most pronounced in the immediate vicinity of the plasma membrane and less pronounced inside the glycocalyx (Stock et al. 2007). Second, the slope of this gradient decreases as the proton concentration of the bulk solution increases (Stock et al. 2007) which indicates a gradual decrease in the buffering capacity of the glycocalyx due to an increase in proton saturation. Conversely, the gradient has been observed to remain intact at more alkaline pHe values of up to 7.3 (for pHe 7.2 see Stock et al. 2007).
The thickness of the glycocalyx is very variable ranging from 10 nm up to 100 nm (Bailey & Gingell, 1988; Rademacher et al. 1988; Bearer & Friend, 1990; Pries et al. 2000). The binding sites of the adhesion-mediating integrin dimers for ECM proteins stick out of the plasma membrane by about 20 nm (Erlandsen et al. 2001; Takagi et al. 2002) so that they are embedded in the glycocalyx. The interaction between α2β1 integrins and the ECM proteins collagen I and fibronectin depends on the proton concentration where a ΔpH of 0.5 can cause a significant difference in the integrin–matrix bond (Lehenkari & Horton, 1999; Eble & Tuckwell, 2003). Based on the present results we propose that the proton concentration inside the glycocalyx near the binding sites of integrins is affected by both the protons of the bulk solution and those exported from the cytosol to the cell surface by NHE1 activity. The protons extruded by NHE1, however, are those that are essential for the modulation of the cell–matrix interaction.
Admittedly, in addition to the NHE1-mediated, local decrease in the extracellular cell surface pH, the corresponding local increase in the cytosolic pH has to be taken into consideration (Srivastava et al. 2007). Denker & Barber (2002) suggest that NHE1 probably increases the cytoplasmic pH at the leading edge, which could then promote the pH-dependent activation of proteins such as ADF/cofilin and gelsolin (Maciver et al. 1998; Bernstein et al. 2000) that regulate actin polymerization at the cell periphery and hence, membrane protrusion. These authors also found that NHE1 activity is required for maintaining the polarity in fibroblasts migrating on a Matrigel matrix (Denker & Barber, 2002), which is in line with the results of the present study. In MV3 cells the degree of polarization and migratory activity and the slope of the proton gradient at the cell surface decrease as NHE1 activity decreases either in response to an increase in pHe up to 7.5 or due to NHE1 deficiency/inhibition. As a matter of fact NHE1 activity is indispensable for the directed migration of numerous types of cells such as mammalian fibroblasts (Denker & Barber, 2002; Stock et al. 2005), epithelial cells (Klein et al. 2000; Lagana et al. 2000; Reshkin et al. 2000b), neutrophils (Simchowitz & Cragoe, 1986; Ritter et al. 1998), human melanoma cells (Stock et al. 2005) and chemotactically competent Dictyostelium cells (Patel & Barber, 2005).
The present study led us to conclude that the NHE1-modulated, locally different proton concentration at the plasma membrane is one of the requirements for cell migration. It enables the cells to respond to a varying pHe of the bulk solution. Thus the local pH at the cell surface outweighs the pH of the bulk solution.
Acknowledgments
The authors thank Mrs Birgit Gassner for outstanding technical assistance. Thanks go also to Drs H.-J. Lang and J. Pünter at Sanofi Aventis who kindly provided us with HOE642. This work was supported by the fund ‘Innovative Medical Research’ of the University of Münster Medical School (grant number: ST 2 1 06 01) and by the Deutsche Forschungsgemeinschaft (grant number: SCHW 407/10-1).
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.145185/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.145185
References
- Aguiar CB, Lobao-Soares B, Alvarez-Silva M, Trentin AG. Glycosaminoglycans modulate C6 glioma cell adhesion to extracellular matrix components and alter cell proliferation and cell migration. BMC Cell Biol. 2005;6:31. doi: 10.1186/1471-2121-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Gingell D. Contacts of chick fibroblasts on glass: results and limitations of quantitative interferometry. J Cell Sci. 1988;90:215–224. doi: 10.1242/jcs.90.2.215. [DOI] [PubMed] [Google Scholar]
- Bearer EL, Friend DS. Morphology of mammalian sperm membranes during differentiation, maturation, and capacitation. J Electron Microsc Tech. 1990;16:281–297. doi: 10.1002/jemt.1060160403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Painter WB, Chen H, Minamide LS, Abe H, Bamburg JR. Intracellular pH modulation of ADF/cofilin proteins. Cell Motil Cytoskeleton. 2000;47:319–336. doi: 10.1002/1097-0169(200012)47:4<319::AID-CM6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordick RS, Brierley GP, Garlid KD. On the mechanism of A23187-induced potassium efflux in rat liver mitochondria. J Biol Chem. 1980;255:10299–10305. [PubMed] [Google Scholar]
- Eble JA, Tuckwell DS. The α2β1 integrin inhibitor rhodocetin binds to the A-domain of the integrin α2 subunit proximal to the collagen-binding site. Biochem J. 2003;376:77–85. doi: 10.1042/BJ20030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JT, Tona A, Plant AL. Comparison of reagents for shape analysis of fixed cells by automated fluorescence microscopy. Cytometry A. 2003;52:90–100. doi: 10.1002/cyto.a.10025. [DOI] [PubMed] [Google Scholar]
- Erlandsen SL, Greet Bittermann A, White J, Leith A, Marko M. High-resolution CryoFESEM of individual cell adhesion molecules (CAMs) in the glycocalyx of human platelets: detection of P-selectin (CD62P), GPI-IX complex (CD42A/CD42bα,bβ), and integrin GPIIbIIIa (CD41/CD61) by immunogold labeling and stereo imaging. J Histochem Cytochem. 2001;49:809–819. doi: 10.1177/002215540104900702. [DOI] [PubMed] [Google Scholar]
- Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002;8:1284–1291. [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschberger S, Busche R, von Engelhardt W. Surface pH at the basolateral membrane of the caecal mucosa of guinea pig. J Membr Biol. 1999;169:111–122. doi: 10.1007/s002329900523. [DOI] [PubMed] [Google Scholar]
- Klein M, Seeger P, Schuricht B, Alper SL, Schwab A. Polarization of Na+/H+ and Cl−/HCO3− exchangers in migrating renal epithelial cells. J Gen Physiol. 2000;115:599–608. doi: 10.1085/jgp.115.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagana A, Vadnais J, Le PU, Nguyen TN, Laprade R, Nabi IR, Noel J. Regulation of the formation of tumor cell pseudopodia by the Na+/H+ exchanger NHE1. J Cell Sci. 2000;113:3649–3662. doi: 10.1242/jcs.113.20.3649. [DOI] [PubMed] [Google Scholar]
- Lehenkari PP, Horton MA. Single integrin molecule adhesion forces in intact cells measured by atomic force microscopy. Biochem Biophys Res Commun. 1999;259:645–650. doi: 10.1006/bbrc.1999.0827. [DOI] [PubMed] [Google Scholar]
- Maciver SK, Pope BJ, Whytock S, Weeds AG. The effect of two actin depolymerizing factors (ADF/cofilins) on actin filament turnover: pH sensitivity of F-actin binding by human ADF, but not of Acanthamoeba actophorin. Eur J Biochem. 1998;256:388–397. doi: 10.1046/j.1432-1327.1998.2560388.x. [DOI] [PubMed] [Google Scholar]
- Patel H, Barber DL. A developmentally regulated Na-H exchanger in Dictyostelium discoideum is necessary for cell polarity during chemotaxis. J Cell Biol. 2005;169:321–329. doi: 10.1083/jcb.200412145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J, Sardet C, Franchi A, L'Allemain G, Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984;81:4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Albarani V, Guerra L, Tommasino M, Paradiso A, Casavola V. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na+/H+ exchange, motility, and invasion induced by serum deprivation. J Biol Chem. 2000a;275:5361–5369. doi: 10.1074/jbc.275.8.5361. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000b;14:2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- Ritter M, Schratzberger P, Rossmann H, Woll E, Seiler K, Seidler U, Reinisch N, Kahler CM, Zwierzina H, Lang HJ, Lang F, Paulmichl M, Wiedermann CJ. Effect of inhibitors of Na+/H+-exchange and gastric H+/K+ ATPase on cell volume, intracellular pH and migration of human polymorphonuclear leucocytes. Br J Pharmacol. 1998;124:627–638. doi: 10.1038/sj.bjp.0701864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- Sardet C, Counillon L, Franchi A, Pouyssegur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990;247:723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Schwab A, Rossmann H, Klein M, Dieterich P, Gassner B, Neff C, Stock C, Seidler U. Functional role of Na+-HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol. 2005;568:445–458. doi: 10.1113/jphysiol.2005.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Lechene C, Ingber DE. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin α5β1, independent of cell shape. Proc Natl Acad Sci U S A. 1991;88:7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L, Cragoe EJ., Jr Regulation of human neutrophil chemotaxis by intracellular pH. J Biol Chem. 1986;261:6492–6500. [PubMed] [Google Scholar]
- Srivastava J, Barber DL, Jacobson MP. Intracellular pH sensors: design principles and functional significance. Physiology (Bethesda) 2007;22:30–39. doi: 10.1152/physiol.00035.2006. [DOI] [PubMed] [Google Scholar]
- Stock C, Gassner B, Hauck CR, Arnold H, Mally S, Eble JA, Dieterich P, Schwab A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol. 2005;567:225–238. doi: 10.1113/jphysiol.2005.088344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C, Mueller M, Kraehling H, Mally S, Noel J, Eder C, Schwab A. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem. 2007;20:679–686. doi: 10.1159/000107550. [DOI] [PubMed] [Google Scholar]
- Stock C, Schwab A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol (Oxf) 2006;187:149–157. doi: 10.1111/j.1748-1716.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- Szatkowski MS, Thomas RC. The intrinsic intracellular H+ buffering power of snail neurones. J Physiol. 1989;409:89–101. doi: 10.1113/jphysiol.1989.sp017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- van Muijen GN, Jansen KF, Cornelissen IM, Smeets DF, Beck JL, Ruiter DJ. Establishment and characterization of a human melanoma cell line (MV3) which is highly metastatic in nude mice. Int J Cancer. 1991;48:85–91. doi: 10.1002/ijc.2910480116. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Brown CM, Horwitz AF. Illuminating adhesion complexes in migrating cells: moving toward a bright future. Curr Opin Cell Biol. 2003;15:614–620. doi: 10.1016/s0955-0674(03)00105-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.