Abstract
The signaling pathways governing pathophysiologically important autophagic (ACD) and necrotic (NCD) cell death are not entirely known. In the Dictyostelium eukaryote model, which benefits from both unique analytical and genetic advantages and absence of potentially interfering apoptotic machinery, the differentiation factor DIF leads from starvation-induced autophagy to ACD, or, if atg1 is inactivated, to NCD. Here, through random insertional mutagenesis, we found that inactivation of the iplA gene, the only gene encoding an inositol 1,4,5-trisphosphate receptor (IP3R) in this organism, prevented ACD. The IP3R is a ligand-gated channel governing Ca2+ efflux from endoplasmic reticulum stores to the cytosol. Accordingly, Ca2+-related drugs also affected DIF signaling leading to ACD. Thus, in this system, a main pathway signaling ACD requires IP3R and further Ca2+-dependent steps. This is one of the first insights in the molecular understanding of a signaling pathway leading to autophagic cell death.
INTRODUCTION
Apoptosis, or type I cell death, has long been considered the major type of programmed cell death in animal cells. However, other cell death types exist and occur in particular when the apoptosis machinery is disrupted. In animal cells, the main nonapoptotic types of cell death, autophagic and necrotic cell death (type II and type III cell death, respectively) are still incompletely defined, whereas their pathophysiological importance is increasingly appreciated (Festjens et al., 2006; Zong and Thompson, 2006; Golstein and Kroemer, 2007; Gozuacik and Kimchi, 2007). To better understand the molecular mechanisms governing these nonapoptotic cell death types, mammalian model systems were used, and they brought novel insights in the field (Shimizu et al., 2004; Yu et al., 2004; Høyer-Hansen et al., 2005; Pyo et al., 2005). However, data from these models also revealed the difficulty of unambiguously dissecting nonapoptotic cell death mechanisms from the apoptosis machinery. For example, Bcl-2 family members are well-characterized mediators of apoptosis, but they also regulate nonapoptotic programmed cell death requiring autophagy genes (Shimizu et al., 2004). Bcl-2 and Bcl-XL bind to and inhibit Beclin-1, the mammalian orthologue of yeast Atg6, an essential mediator of autophagy (Pattingre et al., 2005; Maiuri et al., 2007). Moreover, Caspase-8 has been shown to inhibit autophagic death dependently of Atg7 and Beclin-1 (Yu et al., 2004). Conversely, calpain-cleaved Atg5, another protein required for autophagy, interferes with apoptosis by binding to Bcl-XL (Yousefi et al., 2006). Thus, these cell death types share components, and one may regulate and modify the activity of the other, complicating their study. An experimental system from which main apoptosis-governing proteins are absent would thus be a significant asset to investigate nonapoptotic cell death types.
The protist Dictyostelium discoideum benefits from favorable experimental and genetic properties, including a small, sequenced, and haploid genome (Eichinger et al., 2005; Kessin, 2006; Kuspa and Loomis, 2006) (http://dictybase.org/). It undergoes developmental cell death upon differentiation into stalk cells (Whittingham and Raper, 1960). Under in vitro monolayer conditions (Kay, 1987) mimicking this development, wild-type Dictyostelium cells underwent vacuolar cell death (Cornillon et al., 1994; Levraud et al., 2003). Vacuolization could be suppressed (Kosta et al., 2004) by targeted mutagenesis of the autophagy gene atg1 (Otto et al., 2004), showing a link between vacuolization and autophagy. The corresponding cell death was referred to as autophagic cell death (ACD). Importantly, in Dictyostelium cells, there are no main members of the apoptosis machinery that could interfere with nonapoptotic cell death: there are no caspase-family members (except one paracaspase gene that is not involved in autophagic or necrotic cell death), no Bcl-2 family member and no BH3 (Bcl-2 family domain)-bearing molecule (Roisin-Bouffay et al., 2004; Lam et al., 2007).
Triggering ACD in monolayers required at least two distinct stimuli. The first stimulus was starvation, which triggered autophagy as manifested by the formation of autophagosomes revealed by electron microscopy (de Chastellier and Ryter, 1977; unpublished data). However, starvation and the resulting autophagy did not by itself lead to ACD. A second stimulus was required for this, namely, DIF-1 (DIF throughout), a major differentiation factor in Dictyostelium. DIF is a small dichlorinated molecule (Morris et al., 1987), which when added to starved cells undergoing autophagy led to their vacuolization and death (Kay, 1987; Cornillon et al., 1994; Levraud et al., 2003). DIF is normally made by Dictyostelium prespore cells, upon starvation. It is not made by Dictyostelium strain HMX44, which thus requires exogenous DIF to vacuolize and die, enabling one to distinguish easily the role of starvation (other than leading to DIF synthesis) and that of DIF. Other Dictyostelium strains make some DIF when starved in monolayer tests. However, this is made in relatively small amounts so that addition of exogenous DIF still leads to markedly more cell death than controls with only starvation-induced endogenous DIF. Importantly, exogenous DIF has no detectable effect on cells that do not undergo starvation. These data enabled one to dissect out DIF-induced ACD from starvation proper.
Inactivation of the autophagy gene, atg1, also revealed another type of death in Dictyostelium (Kosta et al., 2004). The atg1-null cells, when starved and subjected to DIF, underwent in succession reactive oxygen species (ROS) production, ATP depletion, and early membrane rupture reflecting necrotic cell death (NCD) (Laporte et al., 2007). DIF may induce NCD through a direct or indirect uncoupler-like effect on mitochondria of starved cells. However, it was not clear through which intracellular pathways this was achieved.
Mechanisms leading to nonapoptotic death are still poorly understood in animal cells. We took advantage of the powerful genetic tools and the absence of main actors of apoptosis in Dictyostelium to study signaling pathways involved in these cell death types. As reported here, through random insertional mutagenesis (Kuspa and Loomis, 1992) and selection for resistance to death we obtained a Dictyostelium mutant that did not vacuolize and did not undergo ACD. The disrupted gene was iplA, the only gene encoding inositol 1,4,5-trisphosphate (IP3) receptor (IP3R). This governs Ca2+ fluxes from the endoplasmic reticulum (ER) stores into the cytosol (Patterson et al., 2004; Choe and Ehrlich, 2006). Although the iplA mutation suppressed ACD, it inhibited NCD only inconsistently. Response to exogenous DIF, analysis of the iplA phenotype, and additional use of Ca2+-related drugs allowed us to define an iplA-dependent DIF pathway required for ACD. Thus, in this model system, DIF leads through IP3R, Ca2+ fluxes, and Ca2+-related proteins to autophagic cell death.
MATERIALS AND METHODS
Cells, Cell Culture, Induction of Cell Death, and Microscopy
The parental Dictyostelium strains used in this report were HMX44A (for derivation, see Levraud et al., 2003), HMX44A.atg1-1 (an HMX44A derivative mutated by insertion for the autophagy gene atg1; Kosta et al., 2004), DH1, DH1.atg1-1 (a DH1 derivative mutated for atg1; Otto et al., 2004), and the thymidine auxotroph JH10. The mutants obtained in the work reported here were HMX44A.atg1-3 (another HMX44A derivative mutated by deletion for the autophagy gene atg1; see below), HMX44A.atg1-3.iplA−(ins) [iplA−(ins), an insertional disruption of the iplA gene], HMX44A.iplA−(ins), JH10.iplA−(ins), DH1.iplA−(ins), DH1.iplA−(del), and DH1.atg1-1.iplA−(del) [iplA−(del), a deletion mutation of the iplA gene]. Strains bearing the iplA−(ins) mutation were grown in HL5-modified medium (Cornillon et al., 1994) supplemented with 10 μg/ml blasticidin (Invitrogen, Carlsbad, CA). The thymidine auxotroph JH10 cells were grown in HL5 supplemented with 100 μg/ml thymidine (Sigma-Aldrich, St. Louis, MO). Others strains were grown in HL5-modified medium alone.
To induce vacuolar death in JH10, DH1, and HMX44A cells (Cornillon et al., 1994; Levraud et al., 2003), or necrotic cell death in strains mutated for the autophagy gene atg1 (Kosta et al., 2004; Laporte et al., 2007), cells were starved in the presence of cAMP, and then they were treated with the differentiation factor DIF-1 as described. After the indicated period, cells in the Lab-Tek chambers were examined using an Axiovert 200 microscope (Carl Zeiss, Jena, Germany) (differential interference contrast [DIC], 63× or phase contrast, 100×, oil immersion). Fluorescence-activated cell sorter determination of 2′-7′-dichlorofluorescin diacetate (DC-FDA) fluorescence and regrowth assays were as described previously (Levraud et al., 2003; Laporte et al., 2007). Each figure with histograms is a single experiment, most often assayed in duplicate, and representative of at least three such experiments.
Reagents
Cyclosporin A (CsA), thapsigargin (Tg), and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) were purchased from Sigma-Aldrich. Stock solutions of CsA (50 mg/ml) were prepared in dimethyl sulfoxide, Tg (3 mM) in pure ethanol, and BAPTA (100 mM) in 0.3 N sodium bicarbonate NaHCO3. Each reagent was added to starved cells in Lab-Tek chambers at the indicated final concentrations, at the same time as DIF or 30 min before DIF. After the indicated duration of incubation, cells were analyzed by microscopy and/or flow cytometry.
Random Insertional Mutagenesis and Developmental Screening
For each transfection, 2 × 107 exponentially growing vegetative JH10 cells were electroporated with 10 μg of BamHI-linearized pUCBsrΔBamHI vector in ice-cold electroporation buffer (10 mM Na2HPO4/KH2PO4, pH 6.1, and 50 mM sucrose) in the presence of 12 U of the DpnII restriction enzyme (New England Biolabs, Ispwich, MA; reference 1992) using a Bio-Rad Gene Pulser (1 kV; 3 μF; expected pulse time 0.6–1.1 ms). Cells were incubated in HL5 medium at 22°C for 24 h, and then 10 μg/ml blasticidin was added to select transformants during a further period of culture of 5–10 d. After selection, 12 × 106 transformants were subjected to cell death induction in monolayer in 75-cm2 flasks (Falcon; BD Biosciences Discovery Labware, Bedford, MA) (as described above, upscaled to 20-ml final volume). After 48 h, DIF-containing Soerensen buffer was removed and replaced with HL5 medium to enable growth of surviving cells. When enough cells had been obtained, a second similar round of cell death induction and selection was performed. After 48 h of incubation in DIF, the resulting surviving cells were plated on Klebsiella aerogenes bacterial lawns on SM/5 plates and incubated for 3–5 d at 22°C. Development of each clone as a separate plaque on the bacterial lawns was examined with a binocular photomicroscope (Carl Zeiss). Clones with abnormal stalks were recovered from plates and grown in HL5 medium supplemented with antibiotics (100 μg/ml ampicillin and 300 μg/ml streptomycin; Sigma-Aldrich) to remove remaining bacteria. This procedure led to the isolation of the 25A mutant (Figure 1A).
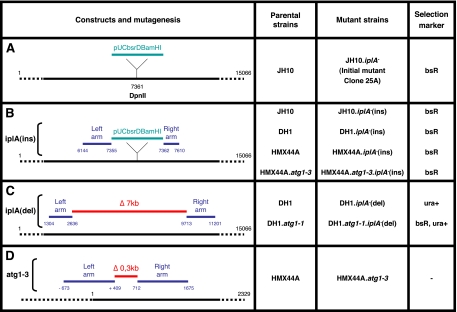
Figure 1.
iplA and atg1 constructs and mutants in distinct Dictyostelium strains. (A) Localization of the pUCbsrDBamHI insertion in the iplA gene (accession no. AJ277590) in the initial JH10 mutant clone 25A. Plasmid rescue and identification of flanking sequences on each side of pUCbsrDBamHI showed its insertion in the DpnII site at position 7361 of the iplA gene. pUCbsrDBamHI contained a blasticidin resistance marker. (B) Rescued plasmid, called iplA(ins) vector, used to mutagenize the iplA gene by homologous recombination, and its insertion point. The right and left arms flanking pUCbsrDBamHI were used to target the iplA gene, leading to its disruption by insertion. Left and right arms correspond to positions 6144-7355 and 7362-7610 of the iplA gene, respectively. Compared with the published AX4 genome sequence, there is a small deletion of six nucleotides upstream of the insertion, due either to a sequence difference between strains, or to an event occurring during preparation of the construct (which we nevertheless consider an insertion construct). This plasmid was used to obtain simple iplA− mutants in JH10, DH1, and HMX44A and by insertion in HMX44A.atg1-3 (see D), a double atg1− iplA− mutant in HMX44A. (C) Construct, called iplA(del) vector, used to introduce a deletion in the iplA gene. Left and right arms amplified by PCR correspond to positions 1304-2636 and 9713-11201 of the iplA gene, respectively, enabling to delete ∼7 kb of the iplA gene. This vector, which contained an ura selection marker, was used to obtain in DH1 a simple iplA− and a double atg1− iplA− mutant. (D) Construct, called atg1-3 vector, used to introduce a deletion in the atg1 gene. Left and right arms amplified by PCR correspond to the positions −673 to +409 and 712-1675 of the atg1 gene (accession no. AY191011), respectively, resulting in deletion of ∼0.3 kb of the atg1 gene. The floxed blasticidin resistance cassette in the insert was removed using the Cre expression vector pDEX-NLS-Cre (Faix et al., 2004). The resulting HMX44A.atg1-3 cells were mutated for the atg1 gene and sensitive to blasticidin. They were used in (B) to obtain a double atg1− iplA− mutant.
Plasmid Rescue from 25A Mutant Cells and Identification of the iplA Gene
Genomic DNA from 25A mutant cells was digested with different enzymes and analyzed by Southern blot by using a BsdR probe (a 450-base pair XhoI–BglII fragment of pUCBsRBamHI). ClaI digestion yielded a 9.7-kb fragment encompassing the pUCBsrΔBamHI vector (4.6 kb) and its genomic flanking sequences (5.1 kb). For rescue and recircularization of this plasmid with its flanking sequences, genomic DNA from 25A mutant cells was digested with ClaI, and an aliquot of this DNA was ligated and transfected into electrocompetent SURE bacteria (Stratagene, La Jolla, CA). Transformants were selected on LB plates containing 100 μg/ml ampicillin. This rescued plasmid, called iplA(ins) vector (Figure 1B), was purified from amplified transformants and sequenced using the reverse primers in pUCBsrΔBamHI vector (bsr5, 5′-GCATTAGATGTAAAACAGCCAAAG–3′; PR161, 5′-GGCTCGTATGTTGTGTGG-3′; and PR214, 5′-GCTATGACCATGATTACGAA-3′). The corresponding sequences were used for BLASTn search on the National Center for Biotechnology Information site (www.ncbi.nlm.nih.gov/BLAST/, limited to the Dictyostelium genome), identifying iplA sequences.
Preparation and Validation of Further iplA Mutants, Including Double Mutants
To obtain a deletion mutant in iplA, we prepared a homologous recombination construct bearing 5′ and 3′ arms made separately by PCR from genomic DNA. Restriction sites were generated at the ends of PCR products by inclusion of appropriate sequences in the primers. For the iplA deletion (7087 base pairs), the 5′ arm (1295 base pairs) was from nucleotide (nt) 1341 to nt 2635, and the 3′ arm (1226 base pairs) from nt 9722 to nt 10947 of the iplA gene. Both arms were ligated into pGEM-T Easy (Promega, Madison, WI) with BamHI. After linearization of the plasmid with BamHI, the cohesive ends were filled in and dephosphorylated. The pyr5-6 cassette (3700 base pairs) was removed from pJB1 with ScaI and ClaI, filled in, and cloned between both arms by blunt end ligation to give the iplA(del) vector (Figure 1C). Cells from various strains were electroporated with either the iplA(ins) vector linearized by ClaI or the iplA(del) vector digested by SacII-AccI, and resulting transformants were selected in HL5-modified medium containing blasticidin or in SIH medium (Formedium; deprived of uracil), respectively. Resistant cells were cloned by limiting dilution in microplates. Putative iplA−(ins) clones were screened by PCR by using primers bsr5 (5′-CGCCAACCCAAGTTTTTTTAAACC-3′) and iplA13 (5′-CCAATTACAGCGGAATGACA-3′), and putative iplA−(del) clones by using primers ura4 (5′-CTGGGGTACCTATAGACCTC-3′) and iplA7 (5′-GGCTTTAGATGACCAAGGTA-3′). The corresponding mutation was then confirmed by Southern blot.
HMX44A.atg1-3 Mutant Cells
The homologous recombination construct for the deletion allele atg1-2 was described previously (Kosta et al., 2004) (Figure 1D). Subsequently this construct was linearized with BamHI, the ends were filled in and dephosphorylated. The floxed blasticidin resistance cassette was removed from the targeting vector pLRBLP (Faix et al., 2004) and ligated to the above-mentioned sequence. The resulting deletion construct was linearized and used for electroporation of the HMX44A cells. Transformants were selected with blasticidin (10 μg/ml) in HL5 medium for 10 d, and then they were cloned and screened by PCR for homologous recombination of the deletion construct with the endogenous atg1 locus. One homologous recombinant clone was picked, expanded, and electroporated with the Cre expression vector pDEX-NLS-cre (Faix et al., 2004). Transformants were selected with neomycin 20 μg/ml in HL5 medium for 2 wk, cloned, and the clonal cell lines were subsequently picked in replica onto two different plates. In one plate, blasticidin was added (10 μg/ml). Clones the replica of which died in the presence of blasticidin were chosen, expanded, and allowed to grow in HL5 medium without any selection. These blasticidin-sensitive cells were cloned and replicated one more time. In one plate neomycin was added. The clones the duplicates of which died in the presence of neomycin were selected. These cells were now free of blasticidin- and neomycin-resistance cassettes, and this was confirmed by PCRs and Southern blot hybridizations. The blasticidin cassette was removed, but a 73-nt sequence remained that included the translational stop cassette and a single loxP site. Additionally, there was a deletion of 302 base pairs in the atg1 gene.
Measurement of ATP Levels
Quantification of ATP in cell populations was performed with the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega). For each sample, 3 × 105 cells/ml were centrifuged 5 min at 1500 rpm. Cells were resuspended in 100 μl of 2-(N-morpholino)ethanesulfonic acid and transferred into wells of opaque-walled 96-well plates (Nalge Nunc, Rochester, NY; reference 236105). Cells were lysed by adding 100 μl of the CellTiter-Glo reagent and mixing on a shaker for 2 min. The plates were incubated at room temperature for 10 min. ATP levels present in the cellular extracts were measured by luminometry and expressed on a per cell basis.
RESULTS
A Genetic Screen for Altered Stalk Development Yielded a Mutant with an Insertion in the iplA Gene
Dictyostelium cell populations were random mutagenized (Kuspa and Loomis, 1992), enriched for mutants resistant to cell death by two rounds of DIF-induced cell death in monolayers, and spread on bacterial lawns, where each wild-type cell can multiply and develop into fruiting bodies. This allowed us to visually screen these clones, reject those where development did not take place at all, and select those where development took place, but the stalks were abnormal thus possibly made of death-resistant mutant cells.
Using this approach in Dictyostelium strain JH10, we isolated a mutant clone called 25A. Clone 25A showed fruiting bodies generally smaller than those of wild-type JH10. On induction of cell death in monolayers, 25A mutant cells showed a defect in autophagic cell death with a clear-cut reduction in the proportion of vacuolated cells. This gene was identified by plasmid rescue, and its targeted mutagenesis in JH10 and DH1 cells led to the same phenotype qualitatively, demonstrating that the mutation in this gene accounts for the defect in autophagic cell death. In the rescued plasmid, the genomic sequences flanking the inserted plasmid pUCbsrDBamHI were entirely sequenced and compared with the sequence of the Dictyostelium genome (Eichinger et al., 2005). The 5′ and 3′ flanking sequences corresponded to segments 6144-7355 and 7362-7610, respectively, of the iplA gene (accession no. AJ277590). The mutagenizing pUCbsrDBamHI plasmid had inserted in the DpnII site at position 7361 of this gene (Figure 1A). The iplA gene (totaling 15,066 nucleotides, including 3 introns), encodes a 3177-amino acid protein that is the only IP3R in Dictyostelium. This IP3R governs Ca2+ fluxes from the ER to the cytosol (Traynor et al., 2000; Schaloske et al., 2005).
IP3R Is Required for Autophagic Cell Death
The rescued plasmid with flanking sequences corresponding to the iplA gene, called thereafter iplA−(ins) vector, was used to inactivate this gene by homologous recombination in three genetic backgrounds: JH10, DH1, and HMX44A (Figure 1B). The corresponding mutant cells were designated JH10.iplA−(ins), DH1.iplA−(ins), and HMX44A.iplA−(ins). The insertional mutation at position 7361 of the iplA gene in each of these cases was validated by PCR and Southern blots. These mutants and the corresponding wild-type cells were tested for ACD and NCD upon starvation and addition of DIF in monolayer experiments. The iplA mutation, thapsigargin, BAPTA and cyclosporin A, which had marked effects on DH1 cells as reported below, had only intermediate effects on JH10 cells and little detectable effects on HMX44A cells. These results suggest that there are differences between the HMX44A and DH1 backgrounds as to Ca2+ fluxes or Ca2+ requirements, through alternative or redundant pathways leading from DIF to death. We will describe in detail below only the results obtained in the more revealing DH1 background.
Starved DH1 wild-type cells showed massive vacuolization after 24 h in the presence of DIF. In sharp contrast, similarly treated DH1.iplA−(ins) cells did not vacuolize (Figure 2A), were highly mobile in particular at the edge of cell clusters, and resembled emergent paddle cells (Levraud et al., 2003). After 48 h in DIF, the clusters of DH1.iplA−(ins) cells were completely dissociated, and the resulting single cells still did not vacuolize and showed paddle cell morphology (Figure 2A). Even at later times, DH1.iplA−(ins) cells usually did not vacuolize, and they ended up dying after several days in starvation medium similar to the control without DIF. Altogether, in 14 of 14 experiments checking vacuolization by phase-contrast microscopy there was marked DIF-induced vacuolization of control DH1 cells, and in 12 of these 14 experiments there was no detectable vacuolization of DH1.iplA− cells. Whereas in the presence of DIF DH1 cells synthesized cellulose shells, reflecting the induction of the cellulose synthesis machinery (Blanton et al., 2000), DH1.iplA−(ins) cells did not (Figure 2B), suggesting that iplA may act upstream of this induction.
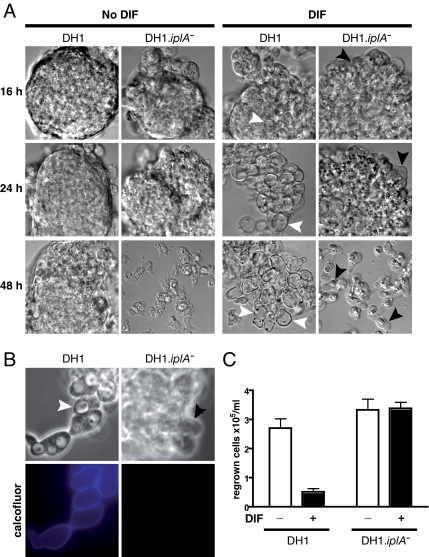
Figure 2.
iplA mutant cells showed almost complete inhibition of DIF-induced autophagic cell death. (A) Vacuolization was inhibited in DH1.iplA−(ins) mutant cells subjected to DIF. Wild-type and DH1.iplA−(ins) mutant cells were starved for 8 h, and then they were washed and further incubated in the presence of 100 nM DIF (DIF; right) or in its absence (No DIF; left). DIC microscopy pictures at 16, 24, or 48 h after the initial 8-h starvation period. Vacuoles (white arrowheads) were visible in DH1 wild-type cells subjected to DIF. However, there were almost no vacuoles in the absence of DIF or in mutant iplA− cells subjected to DIF. The latter group showed paddle cells (Levraud et al., 2003) (black arrowheads) occurring most often at the periphery of cell aggregates. (B) Cellulose shells did not occur in DH1.iplA−(ins) mutant cells in response to DIF. After 16 h of incubation in DIF, DH1 wt, and DH1.iplA−(ins) mutant cells were labeled with calcofluor. For each cell type, pictures taken by phase-contrast (top) or fluorescence (bottom) microscopy corresponded to the same microscopic field. Arrowheads as in A. Only DH1 wild-type, not DH1.iplA−(ins) mutant cells were vacuolized and calcofluor positive. (C) Clonogenic cell death was markedly less in DH1.iplA−(ins) mutant cells than in DH1 wild type cells in response to DIF. After 24 h of incubation with or without DIF, HL5 rich medium was added, and cells were counted after a further 48-h incubation period.
Wild-type and iplA− mutant cells were subjected to regrowth tests to quantify DIF-induced ACD in terms of multiplication of surviving cells (Figure 2C). As expected, compared with control without DIF the number of DH1 wild-type cells was much less after incubation with DIF, reflecting cell death triggered by starvation and DIF. In contrast, the number of DH1.iplA−(ins) cells was the same whether treated with DIF or not (Figure 2C), showing resistance of these mutant cells to DIF-induced death. In six of six experiments, there was marked DIF-induced death of control DH1 cells, but no significant DIF-induced death of DH1.iplA− cells. Altogether, in DH1 cells iplA inactivation led to apparent arrest of DIF-induced events at the paddle cell stage. IP3R was required for subsequent autophagic vacuolization and death.
IP3R Is Required for Necrotic Cell Death in a Variable Fraction of the Cells
In cells mutated for the atg1 autophagy gene, starvation and DIF led to absence of vacuolization and to NCD (Kosta et al., 2004; Laporte et al., 2007). To determine whether a mutation of the iplA gene could affect, not only ACD, but also NCD, we carried out double targeted mutagenesis (on atg1 and iplA) in each of the DH1 and HMX44A backgrounds. Four atg1− mutant cells were obtained (Figure 1, B and C) and checked for NCD. The iplA− mutant cells HMX44A.atg1-3.iplA−(ins) and DH1.atg1-1.iplA−(del) were compared with their respective iplA+ counterparts HMX44A.atg1-3 et DH1.atg1-1. Again, only results obtained in the DH1 background will be shown.
Both iplA+ and iplA− cells starved in the absence of DIF were round and refringent (Figure 3A). After addition of DIF, compared with iplA+ controls (Laporte et al., 2007), mutant iplA− cells tended to show less DC-FDA–positive cells (thus less ROS-producing cells, as discussed in detail previously; Laporte et al., 2007) (Figure 3B) and less ATP depletion (Figure 3C) at 20 min, a mixture of cells with perinuclear condensation and round and refringent cells at 60 min (Figure 3A) and with time less cells with plasma membrane rupture in two independent iplA− clones (Figure 3D). However, compared with its clearcut and well-reproducible effect on ACD shown above, the iplA mutation only partially impaired NCD, and the degree of impairment was variable from one experiment to the next. Of 18 experiments, compared with the percentage of cells with plasma membrane rupture in control cells, that of iplA− cells was lower than one third in five experiments, but higher than half in 11 experiments. We looked for but could not identify experimental parameters accounting for this variability (data not shown). We speculate that under limiting conditions, even moderately elevated Ca2+ levels due to IP3R might “nonspecifically” aggravate starvation-induced mitochondrial sensitization to DIF. Cells may need or not need IP3R and the corresponding extra Ca2+ for optimal mitochondrial uncoupling, which may reflect a preexisting heterogeneity between developing Dictyostelium cells with regards to cytosolic Ca2+ concentrations (Cubitt et al., 1995; Azhar et al., 1996).
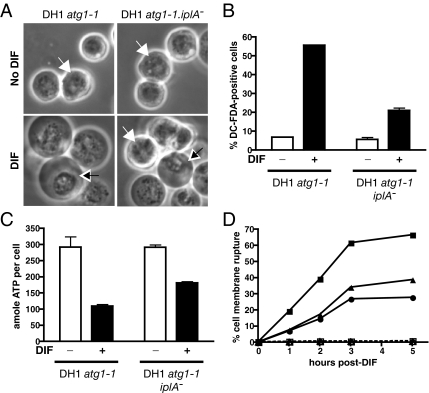
Figure 3.
iplA mutant cells showed partial inhibition of DIF-induced necrotic cell death. (A) Some DH1.atg1-1.iplA−(del) cells did not show necrotic cell death morphological features in response to DIF. DH1.atg1-1 and DH1.atg1-1.iplA−(del) cells were starved for 16 h, and then they were washed and further incubated in the presence of 100 nM DIF (DIF, bottom) or in its absence (No DIF, top). Phase contrast microscopy pictures taken 60 min after addition of DIF. White arrows, cells showing signs of necrotic cell death; black arrows, cells not showing such signs. (B) The percentage of DC-FDA–positive cells was less in iplA− than in iplA+ cells. Starved DH1.atg1-1 and DH1.atg1-1.iplA−(del) cells were incubated with DC-FDA, a fluorescent ROS-detecting probe, with or without DIF for 20–30 min, and fluorescent DC-FDA–positive cells were quantified by flow cytometry. (C) ATP depletion was less in iplA− than in iplA+ cells. Starved DH1.atg1-1 and DH1.atg1-1.iplA−(del) cells were incubated with or without DIF for 20–30 min, and quantification of ATP (in attomoles per cell) in each cell population was performed using the CellTiter-Glo Luminescent Cell Viability Assay kit. (D) The percentage of cells with DIF-induced plasma membrane rupture was less in iplA− than in iplA+ cells. Cell membrane rupture was detected and quantified as a function of time by flow cytometry in starved DH1.atg1-1 (squares) and two independent clones of DH1.atg1-1.iplA−(del) (circles; triangles) cells incubated either without (dotted line) or with DIF (continuous lines). The same percentage of cells (and presumably the same cells) show perinuclear clustering, DC-FDA fluorescence and later plasma membrane rupture.
Altogether, the iplA− mutation impaired ACD, showing that IP3R was required for this death, and impaired NCD only partially. These results contributed to define a DIF-triggered pathway involving IP3R strictly required for ACD, but only partially required for NCD.
Thapsigargin Induces and BAPTA and Cyclosporin A Impair Autophagic Not Necrotic Cell Death
The experiments mentioned above showed that IP3R was required for at least ACD in Dictyostelium DH1 cells, strongly suggesting a key involvement of Ca2+. Tg, an inhibitor of sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) pumps at the ER membrane, would prevent Ca2+ flux from the cytosol into the ER, resulting in an increase in cytosolic Ca2+ concentration as shown in particular in Dictyostelium (Tanaka et al., 1998). Although starved DH1 cells by themselves showed no vacuolization (Figure 4A, top left), a high proportion of vacuolated cells could be observed 24 h after addition of Tg (Figure 4A, bottom left), similar to that observed after addition of DIF (Figure 4B, top left). Also, similar to DIF, addition of Tg led to death of DH1 cells as evaluated in a regrowth test (Figure 4C, left). Tg or DIF triggered much less vacuolization or death in iplA− cells (Figure 4A, bottom right, C, right). Also, in the presence of 1 mM Ca2+ chelator BAPTA, DIF-triggered DH1 cells showed far less vacuoles (Figure 4B, left). BAPTA also partially inhibited DIF-induced cell death as shown in a regrowth test (Figure 4C, left). In contrast, in starved DH1.atg1-1 cells Tg was not able to trigger NCD without DIF (Figure 4D), and the Ca2+ chelator BAPTA did not alter perinuclear condensation and membrane rupture (data not shown). Altogether, the Tg and BAPTA results indicated that given cytosolic Ca2+ concentration thresholds may be required (in line with the IP3R results) and sufficient for further vacuolization and ACD events, but not or less so for NCD.
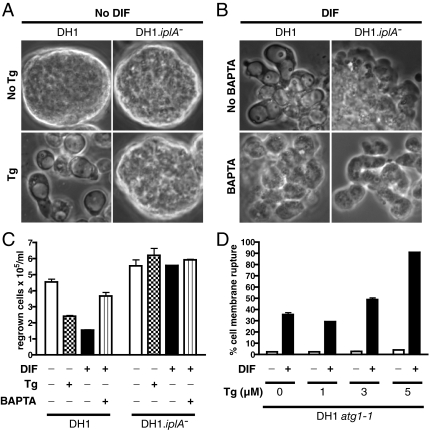
Figure 4.
Effect of thapsigargin or BAPTA on autophagic or necrotic cell death. (A) Tg could induce vacuolization without exogenous DIF in DH1 but not in DH1.iplA−(ins) mutant cells. DH1 and DH1.iplA−(ins) mutant cells were starved for 8 h, washed, and further incubated for 24 h either with Tg 5 μM (bottom) or without Tg (top). (B) The Ca2+-chelator BAPTA inhibited vacuolization induced by DIF in DH1 cells. DH1 and DH1.iplA−(ins) mutant cells were starved for 8 h, washed, and further incubated for 24 h with DIF and either BAPTA 1 mM (bottom) or no BAPTA (top). DH1.iplA−(ins) mutant cells subjected to the same treatments served as nonvacuolizing controls. Phase-contrast pictures. (C) Clonogenic death in DH1 WT cells was induced or inhibited by Tg or BAPTA, respectively. Starved DH1 and DH1.iplA−(ins) mutant cells were subjected for 24 h to different combinations of DIF, Tg, and BAPTA, and then they were compared regarding their clonogenic ability. (D) Tg could increase the percentage of cells with plasma membrane rupture induced by DIF. Starved DH1.atg1-1 cells were incubated with or without DIF and with increasing concentrations of Tg. After 2.5 h, the percentage of cells with plasma membrane rupture was quantified by flow cytometry.
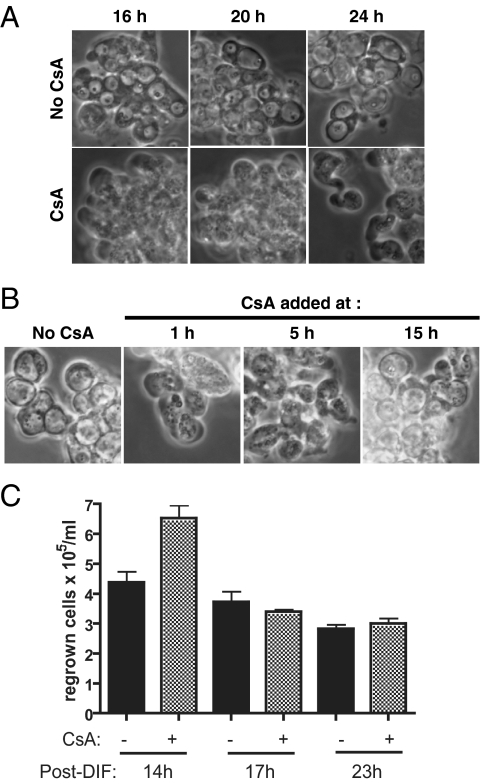
The results mentioned above were in line with an IP3R-mediated release from ER stores into the cytosol of Ca2+, which in starved cells might then be necessary for ACD. A prediction is that impairing downstream Ca2+-dependent steps may have the same effect as IP3R inactivation. A main Ca2+-binding protein is calmodulin. Ca2+/calmodulin activates kinases and the protein phosphatase calcineurin, which couples Ca2+ signals to protein dephosphorylation. Calcineurin can be inhibited by the small protein cyclophilin bound by the drug CsA. CsA indeed markedly delayed DIF-induced vacuolization in DH1 cells, but it did not modify the aspect and time of appearance of paddle cells (Figure 5A). This showed that the delay in vacuolization was due to a CsA-sensitive event occurring at or after the paddle cell stage, or on a pathway independent of paddle cell formation. Inhibition by CsA of vacuolization but not paddle cell formation was due to a late effect of CsA, because vacuolization was impaired when CsA was added as late as 5 h after DIF (Figure 5B). This confirmed similar earlier observations (Horn and Gross, 1996) and indicated that a required CsA-sensitive event took place on the autophagic cell death pathway several hours after addition of DIF. Regrowth experiments showed that CsA delayed not only vacuolization, but also ACD, because CsA rescued some DH1 cells from DIF-induced ACD when rich HL5 medium was added at 14h but not 17h after DIF (Figure 5C). Also, importantly CsA did not delay or inhibit NCD in DH1.atg1-1 cells (data not shown; if anything, CsA tended to increase NCD). Thus, although CsA did not impair NCD, it markedly delayed at a postpaddle stage vacuolization and death on the ACD pathway. These and previous results suggested that a DIF/IP3R pathway governed ACD but only partially NCD. A CsA-inhibitable moiety, likely calcineurin, was more downstream on the path to ACD.
Figure 5.
Cyclosporin A markedly delayed autophagic cell death. (A) Cyclosporin A delayed DIF-induced vacuolization. Starved DH1 wt cells were incubated with DIF in the absence (top) or presence (bottom) of 1 μg/ml CsA. Phase-contrast microscopy pictures after 16, 20, or 24 h of incubation with DIF. (B) Addition of cyclosporin A even 5 h after DIF delayed vacuolization. CsA (1 μg/ml) was added to DH1 wt cells 1, 5, or 15 h after addition of DIF. Phase-contrast microscopy pictures taken 22 h after addition of DIF. (C) Cyclosporin A delayed clonogenic death of DH1 wt cells. CsA (1 μg/ml) was added or not to starved DH1 wt cells, together with DIF. Supernatants were replaced with HL5 rich medium at various times after addition of DIF. Some cells were still protected by CsA at 14 h post-DIF, but not at later times.
DISCUSSION
In animal cells, the involvement of IP3R in some cases of apoptosis is well documented (Khan et al., 1996; Sugawara et al., 1997; Blackshaw et al., 2000; Assefa et al., 2004; Oakes et al., 2005; White et al., 2005). However, there are only a few demonstrations of involvement of IP3R in nonapoptotic cell death. Importantly, in C. elegans mutations of the ER Ca2+ release channels unc-68 (ryanodine receptor) or itr-1 (IP3 receptor) inhibited necrotic-like neuronal death (Xu et al., 2001). In the protist Dictyostelium, we showed by random mutagenesis that a mutation of the iplA gene encoding the only IP3 receptor in this organism (Traynor et al., 2000) inhibited ACD, suggesting that Ca2+ flux from ER to cytosol through the activation of IP3R was necessary to signal this caspase-independent cell death. Traynor et al. (2000) previously inactivated by targeted mutagenesis in the AX2 strain the iplA gene through a deletion of ∼7 kb. The mutant cells showed less Ca2+ influx in response to cAMP, with only minor consequences on development (including, interestingly, smaller fruiting bodies), signal transduction, reorganization of actin cytoskeleton or chemotaxis. Schaloske et al. (2005) subsequently showed that entry of extracellular Ca2+ and Ca2+ release from internal stores in response to cAMP were much lower in the very same mutant than in wild-type AX2 cells. These data showed that the IP3R is important to ensure Ca2+ flux in response to stimulation in Dictyostelium cells.
Ca2+ fluxes governed by IP3R (from the ER Ca2+ stores to the cytoplasm) and SERCAs (from the cytoplasm to the ER) would condition cytoplasmic Ca2+ levels. A role for Ca2+ flux in ACD was supported not only by the genetic arguments above but also by pharmacological manipulations of intracellular Ca2+ levels: 1) Tg, used to increase cytosolic Ca2+, could lead to ACD in agreement with previous observations (Kubohara and Okamoto, 1994; Schaap et al., 1996; Kubohara et al., 2007). Of note, we used starvation buffers not supplemented with Ca2+ (see Materials and Methods). This suggested that extracellular Ca2+ influx was not necessary for ACD and NCD, but also made it more difficult to detect Ca2+ flux (Nebl and Fisher, 1997; Schlatterer et al., 2004). 2) BAPTA, a potent Ca2+ chelator, inhibited ACD, in further support of a requirement for Ca2+ in this pathway. 3) CsA had an effect similar to that of the iplA mutant in terms of inhibition of ACD. CsA is a classical inhibitor of the Ca2+/calmodulin-dependent phosphatase calcineurin, but it could also operate otherwise, for example, by interfering (as in animal cells) with mitochondrial cyclophilin D. We favor in this case an effect on calcineurin, because decreased expression of the regulatory B subunit of calcineurin led to the formation of short stalks and incomplete vacuolization of stalk cells in Dictyostelium AX2 strain (Boeckeler et al., 2006). Because IP3 is the major ligand of IP3R, it is tempting to speculate that DIF may activate the opening of these calcium channels through induction of IP3 production. Of note, a third signal essential for ACD in this system is cAMP. We cannot exclude that both cAMP and DIF contribute to the triggering of the IP3R pathway (Europe-Finner and Newell, 1987; van Haastert, 1989).
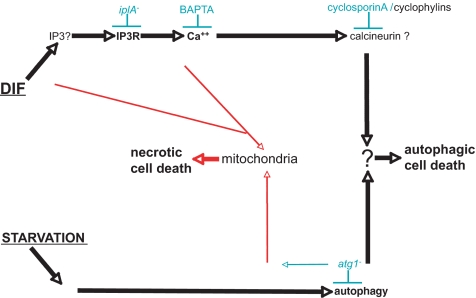
Strikingly, the same developmental and cell death phenotypes (when these tests were performed) were independently found in IP3R knockouts (Traynor et al., 2000; this study), calcineurin knockdown (Boeckeler et al., 2006), and addition of cyclosporin A (Horn and Gross, 1996; this study). These consistent observations reinforced the notion of a Ca2+-dependent pathway for ACD in Dictyostelium, and they strongly suggested that similar mechanisms operated in vivo and in vitro. They also suggested that IP3R, Ca2+ and calcineurin acted in the same pathway, upstream of a critical postpaddle or paddle-independent step(s) toward vacuolization and ACD (Figure 6).
Figure 6.
Schematic representation of pathways leading to cell death in Dictyostelium cells (see References). Common and autophagy pathways are in black, necrotic cell death pathways in red. Experimentally, these pathways were triggered by starvation (not producing enough DIF) and addition of exogenous DIF. Bottom, starvation leading to autophagy, preparing to induction by DIF of ACD. When atg1 is mutated, this pathway seems to lead to alterations of mitochondria preparing them to induction by DIF of NCD. Top, DIF leading (possibly by inducing IP3 production) to IP3R activation and subsequent Ca2+ release from ER to cytosol. In atg1+ cells, cytosolic Ca2+ could then activate calmodulin/calcineurin leading to ACD. In atg1− cells, Ca2+ could facilitate DIF-induced mitochondrial uncoupling and activation of NCD, whereas in a sizable proportion of atg1− cells DIF could directly alter mitochondria.
As shown here, IP3R/Ca2+ fluxes played a key role in DIF signaling leading to ACD, whereas previous evidence indicated that this death pathway (interpreted as differentiation to stalk cells) involved induction of gene expression. Tg and BAPTA induced and inhibited the expression of prestalk-specific genes (Schaap et al., 1996; Kubohara et al., 2007), respectively, which correlated with their effects on ACD. Moreover, a bZIP transcription factor mutant, dimA−, did not differentiate to stalk cells in monolayer assays, like the iplA− mutant, and also showed little or no prestalk-specific gene expression in response to DIF (Thompson et al., 2004). The iplA− mutation prevented cellulose shell synthesis around cells subjected to DIF, consistent with control by IP3R and Ca2+ flux of the expression of genes governing cellulose synthesis. Moreover, addition of DIF triggered nuclear accumulation of DimA, and also of DimB, a second bZIP transcription factor, within a few minutes in AX2 and AX4 strains (Huang et al., 2006; Zhukovskaya et al., 2006). Interestingly, DimB was required for vacuolar cell death and repression of nonvacuolar cell death in response to DIF (Huang et al., 2006), like Atg1. Together, these data support the idea that DIF could trigger ACD by activating transcription factors through the IP3R/Ca2+ pathway.
Although our data demonstrated that ACD required IP3R in Dictyostelium, in mammalian cells a knockdown of IP3R isoforms with RNA interference sufficed to induce autophagy (Criollo et al., 2007) and a rise in the free cytosolic calcium through ATP stimulation that causes IP3 production was a potent inducer of macroautophagy (Høyer-Hansen et al., 2007). These discordant results may relate to the possibility that IP3R activation and inhibition are able to induce autophagy through different signaling pathways (Høyer-Hansen and Jaattela, 2007) and/or to the observation that the antiapoptotic proteins Bcl-2 and Bcl-XL can interact physically with IP3R (Chen et al., 2004; White et al., 2005) and Beclin-1, an essential protein of autophagy (Pattingre et al., 2005; Maiuri et al., 2007). A tight regulation via IP3R between the apoptosis and autophagy machineries might exist in mammalian cells, further emphasizing the interest of the Dictyostelium apoptosis-less model to study autophagic cell death.
On incubation with DIF, a mutation of the atg1 autophagy gene suppressed vacuolization but revealed NCD, suggesting that vacuolization is related to starvation-induced autophagy and also that the atg1 gene plays a crucial role to switch from DIF-induced ACD to NCD. Such a mutation sensitized mitochondria to subsequent uncoupling by DIF leading to NCD (Laporte et al., 2007). Not only atg1− mutant cells but also cells mutated for another autophagy gene (atg5) showed necrotic cell death (unpublished data). BAPTA and CsA inhibited ACD not NCD, whereas the iplA mutation inhibited also NCD, however less consistently than ACD, suggesting the existence of at least two DIF-originating pathways (Figure 6). In starved atg1− cells, DIF could affect mitochondria either directly (consistent with the previous report by Shaulsky and Loomis, 1995) or through IP3R (consistent with spatial proximity between ER and mitochondria favoring Ca2+ exchanges; Rizzuto et al., 2004; Csordas et al., 2006; Rizzuto and Pozzan, 2006), leading to mitochondrial uncoupling and NCD. In starved atg1+ cells, a DIF-originating pathway went through IP3R and probably calcineurin, because it could be blocked by CsA. Its convergence with autophagy at an unknown subcellular location led to vacuolization and ACD (Figure 6).
ACKNOWLEDGMENTS
We thank J. Ewbank, L. Leserman, C. Giusti, E. Tresse, and Mathieu Fallet (Centre d'Immunologie) for discussions and help; the Dicty stock center (http://dictybase.org/StockCenter/StockCenter.html) and depository for the pJB1 vector from P. Devreotes and J. Borleis (Johns Hopkins University); and for the pLRBLP and pDEX-NLS-Cre vectors from J. Faix (Hannover, Germany), and R. H. Kessin (Columbia University) for initial DH1.atg1-1 cells. We thank Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique for institutional support, and for specific grants Ligue Nationale Contre le Cancer, Agence Nationale pour la Recherche (DictyDeath ANR-05-BLAN-0333-01), the European Community (FP6 STREP TransDeath LSHG-CT-2004-511983), the Ministèrepour la Recherche (ACI BCMS174), Cancéropôle PACA, and Association pour la Recherche sur le Cancer.
Abbreviations used:
- ACD
autophagic cell death
- CsA
cyclosporin A
- DIF
differentiation-inducing factor
- IP3R
inositol 1,4,5-trisphosphate receptor
- NCD
necrotic cell death
- Tg
thapsigargin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-08-0823) on December 12, 2007.
REFERENCES
- Assefa Z., Bultynck G., Szlufcik K., Nadif Kasri N., Vermassen E., Goris J., Missiaen L., Callewaert G., Parys J. B., De Smedt H. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J. Biol. Chem. 2004;279:43227–43236. doi: 10.1074/jbc.M403872200. [DOI] [PubMed] [Google Scholar]
- Azhar M., Manogaran P. S., Kennady P. K., Pande G., Nanjundiah V. A Ca2+-dependent early functional heterogeneity in amoebae of Dictyostelium discoideum, revealed by flow cytometry. Exp. Cell Res. 1996;227:344–351. doi: 10.1006/excr.1996.0283. [DOI] [PubMed] [Google Scholar]
- Blackshaw S., Sawa A., Sharp A. H., Ross C. A., Snyder S. H., Khan A. A. Type 3 inositol 1,4,5-trisphosphate receptor modulates cell death. FASEB J. 2000;14:1375–1379. doi: 10.1096/fj.14.10.1375. [DOI] [PubMed] [Google Scholar]
- Blanton R. L., Fuller D., Iranfar N., Grimson M. J., Loomis W. F. The cellulose synthase gene of Dictyostelium. Proc. Natl. Acad. Sci. USA. 2000;97:2391–2396. doi: 10.1073/pnas.040565697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckeler K., Tischendorf G., Mutzel R., Weissenmayer B. Aberrant stalk development and breakdown of tip dominance in Dictyostelium cell lines with RNAi-silenced expression of calcineurin B. BMC Dev. Biol. 2006;6:12. doi: 10.1186/1471-213X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe C. U., Ehrlich B. E. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006;2006:re15. doi: 10.1126/stke.3632006re15. [DOI] [PubMed] [Google Scholar]
- Cornillon S., Foa C., Davoust J., Buonavista N., Gross J. D., Golstein P. Programmed cell death in Dictyostelium. J. Cell Sci. 1994;107:2691–2704. doi: 10.1242/jcs.107.10.2691. [DOI] [PubMed] [Google Scholar]
- Criollo A., et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- Csordas G., Renken C., Varnai P., Walter L., Weaver D., Buttle K. F., Balla T., Mannella C. A., Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt A. B., Firtel R. A., Fischer G., Jaffe L. F., Miller A. L. Patterns of free calcium in multicellular stages of Dictyostelium expressing jellyfish apoaequorin. Development. 1995;121:2291–2301. doi: 10.1242/dev.121.8.2291. [DOI] [PubMed] [Google Scholar]
- de Chastellier C., Ryter A. Changes of the cell surface and of the digestive apparatus of Dictyostelium discoïdeum during the starvation period triggering aggregation. J. Cell Biol. 1977;75:218–236. doi: 10.1083/jcb.75.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger L., et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Cyclic AMP stimulates accumulation of inositol trisphosphate in Dictyostelium. J. Cell Sci. 1987;87:221–229. doi: 10.1242/jcs.87.2.221. [DOI] [PubMed] [Google Scholar]
- Faix J., Kreppel L., Shaulsky G., Schleicher M., Kimmel A. R. A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 2004;32:e143. doi: 10.1093/nar/gnh136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festjens N., Vanden Berghe T., Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signaling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Golstein P., Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem. Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gozuacik D., Kimchi A. Autophagy and cell death. Curr. Top. Dev. Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- Horn F., Gross J. A role for calcineurin in Dictyostelium discoideum development. Differentiation. 1996;60:269–275. doi: 10.1046/j.1432-0436.1996.6050269.x. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M., Bastholm L., Mathiasen I. S., Elling F., Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M., et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M., Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Huang E., Blagg S. L., Keller T., Katoh M., Shaulsky G., Thompson C. R. bZIP transcription factor interactions regulate DIF responses in Dictyostelium. Development. 2006;133:449–458. doi: 10.1242/dev.02240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R. R. Cell differentiation in monolayers and the investigation of slime mold morphogens. Methods Cell Biol. 1987;28:433–448. doi: 10.1016/s0091-679x(08)61661-1. [DOI] [PubMed] [Google Scholar]
- Kessin R. H. The secret lives of Dictyostelium. Methods Mol. Biol. 2006;346:3–14. doi: 10.1385/1-59745-144-4:3. [DOI] [PubMed] [Google Scholar]
- Khan A. A., Soloski M. J., Sharp A. H., Schilling G., Sabatini D. M., Li S. H., Ross C. A., Snyder S. H. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- Kosta A., Roisin-Bouffay C., Luciani M. F., Otto G. P., Kessin R. H., Golstein P. Autophagy gene disruption reveals a non-vacuolar cell death pathway in Dictyostelium. J. Biol. Chem. 2004;279:48404–48409. doi: 10.1074/jbc.M408924200. [DOI] [PubMed] [Google Scholar]
- Kubohara Y., Arai A., Gokan N., Hosaka K. Pharmacological evidence that stalk cell differentiation involves increases in the intracellular Ca(2+) and H(+) concentrations in Dictyostelium discoideum. Dev. Growth Differ. 2007;49:253–264. doi: 10.1111/j.1440-169X.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- Kubohara Y., Okamoto K. Cytoplasmic Ca2+ and H+ concentrations determine cell fate in Dictyostelium discoideum. FASEB J. 1994;8:869–874. doi: 10.1096/fasebj.8.11.8070636. [DOI] [PubMed] [Google Scholar]
- Kuspa A., Loomis W. F. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc. Natl. Acad. Sci. USA. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuspa A., Loomis W. F. The Genome of Dictyostelium discoideum. Methods Mol. Biol. 2006;346:15–30. doi: 10.1385/1-59745-144-4:15. [DOI] [PubMed] [Google Scholar]
- Lam D., Levraud J.-P., Luciani M.-F., Golstein P. Autophagic or necrotic cell death in the absence of caspase and bcl-2 family members. Biochem. Biophys. Res. Commun. 2007;363:536–541. doi: 10.1016/j.bbrc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Laporte C., Kosta A., Klein G., Aubry L., Lam D., Tresse E., Luciani M. F., Golstein P. A necrotic cell death model in a protist. Cell Death Differ. 2007;14:266–274. doi: 10.1038/sj.cdd.4401994. [DOI] [PubMed] [Google Scholar]
- Levraud J.-P., Adam M., Luciani M.-F., De Chastellier C., Blanton R. L., Golstein P. Dictyostelium cell death: early emergence and demise of highly polarized paddle cells. J. Cell Biol. 2003;160:1105–1114. doi: 10.1083/jcb.200212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri M. C., et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Masento M. S., Jermyn K. A., Kay R. R. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature. 1987;328:811–814. doi: 10.1038/328811a0. [DOI] [PubMed] [Google Scholar]
- Nebl T., Fisher P. R. Intracellular Ca2+ signals in Dictyostelium chemotaxis are mediated exclusively by Ca2+ influx. J. Cell Sci. 1997;110:2845–2853. doi: 10.1242/jcs.110.22.2845. [DOI] [PubMed] [Google Scholar]
- Oakes S. A., Scorrano L., Opferman J. T., Bassik M. C., Nishino M., Pozzan T., Korsmeyer S. J. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto G. P., Wu M. Y., Kazgan N., Anderson O. R., Kessin R. H. Dictyostelium macroautophagy mutants vary in the severity of their developmental defects. J. Biol. Chem. 2004;279:15621–15629. doi: 10.1074/jbc.M311139200. [DOI] [PubMed] [Google Scholar]
- Patterson R. L., Boehning D., Snyder S. H. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Pattingre S., Tassa A., Qu X., Garuti R., Liang X. H., Mizushima N., Packer M., Schneider M. D., Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Pyo J. O., et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J. Biol. Chem. 2005;289:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Duchen M. R., Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci. STKE. 2004;2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Roisin-Bouffay C., Luciani M. F., Klein G., Levraud J. P., Adam M., Golstein P. Developmental cell death in Dictyostelium does not require paracaspase. J. Biol. Chem. 2004;279:11489–11494. doi: 10.1074/jbc.M312741200. [DOI] [PubMed] [Google Scholar]
- Schaap P., Nebl T., Fisher P. R. A slow sustained increase in cytosolic Ca2+ levels mediates stalk gene induction by differentiation inducing factor in Dictyostelium. EMBO J. 1996;15:5177–5183. [PMC free article] [PubMed] [Google Scholar]
- Schaloske R. H., Lusche D. F., Bezares-Roder K., Happle K., Malchow D., Schlatterer C. Ca2+ regulation in the absence of the iplA gene product in Dictyostelium discoideum. BMC Cell Biol. 2005;6:13. doi: 10.1186/1471-2121-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatterer C., Happle K., Lusche D. F., Sonnemann J. Cytosolic [Ca2+] transients in Dictyostelium discoideum depend on the filling state of internal stores and on an active sarco/endoplasmic reticulum calcium ATPase (SERCA) Ca2+ pump. J. Biol. Chem. 2004;279:18407–18414. doi: 10.1074/jbc.M307096200. [DOI] [PubMed] [Google Scholar]
- Shaulsky G., Loomis W. F. Mitochondrial DNA replication but no nuclear DNA replication during development of Dictyostelium. Proc. Natl. Acad. Sci. USA. 1995;92:5660–5663. doi: 10.1073/pnas.92.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Kanaseki T., Mizushima N., Mizuta T., Arakawa-Kobayashi S., Thompson C. B., Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Sugawara H., Kurosaki M., Takata M., Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Itakura R., Amagai A., Maeda Y. The signals for starvation response are transduced through elevated [Ca2+](i) in Dictyostelium cells. Exp. Cell Res. 1998;240:340–348. doi: 10.1006/excr.1998.3947. [DOI] [PubMed] [Google Scholar]
- Thompson C. R., Fu Q., Buhay C., Kay R. R., Shaulsky G. A bZIP/bRLZ transcription factor required for DIF signaling in Dictyostelium. Development. 2004;131:513–523. doi: 10.1242/dev.00939. [DOI] [PubMed] [Google Scholar]
- Traynor D., Milne J. L., Insall R. H., Kay R. R. Ca(2+) signaling is not required for chemotaxis in Dictyostelium. EMBO J. 2000;19:4846–4854. doi: 10.1093/emboj/19.17.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haastert P.J.M. Determination of inositol 1,4,5-trisphosphate levels in Dictyostelium by isotope dilution assay. Anal. Biochem. 1989;177:115–119. doi: 10.1016/0003-2697(89)90024-9. [DOI] [PubMed] [Google Scholar]
- White C., Li C., Yang J., Petrenko N. B., Madesh M., Thompson C. B., Foskett J. K. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat. Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham W. F., Raper K. B. Non-viability of stalk cells in Dictyostelium. Proc. Natl. Acad. Sci. USA. 1960;46:642–649. doi: 10.1073/pnas.46.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Tavernarakis N., Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron. 2001;31:957–971. doi: 10.1016/s0896-6273(01)00432-9. [DOI] [PubMed] [Google Scholar]
- Yousefi S., Perozzo R., Schmid I., Ziemiecki A., Schaffner T., Scapozza L., Brunner T., Simon H. U. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Yu L., Alva A., Su H., Dutt P., Freundt E., Welsh S., Baehrecke E. H., Lenardo M. J. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Zhukovskaya N. V., Fukuzawa M., Yamada Y., Araki T., Williams J. G. The Dictyostelium bZIP transcription factor DimB regulates prestalk-specific gene expression. Development. 2006;133:439–448. doi: 10.1242/dev.02190. [DOI] [PubMed] [Google Scholar]
- Zong W. X., Thompson C. B. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]