Abstract
We analyzed expression of candidate genes encoding cell surface or secreted proteins in normal kidney and kidney cancer. This screen identified a bone morphogenetic protein (BMP) antagonist, SOSTDC1 (sclerostin domain–containing-1) as down-regulated in kidney tumors. To confirm screening results, we probed cDNA dot blots with SOSTDC1. The SOSTDC1 message was decreased in 20/20 kidney tumors compared with normal kidney tissue. Immunohistochemistry confirmed significant decrease of SOSTDC1 protein in clear cell renal carcinomas relative to normal proximal renal tubule cells (p < 0.001). Expression of SOSTDC1 was not decreased in papillary and chromophobe kidney tumors. SOSTDC1 was abundantly expressed in podocytes, distal tubules, and transitional epithelia of the normal kidney. Transfection experiments demonstrated that SOSTDC1 is secreted and binds to neighboring cells and/or the extracellular matrix. SOSTDC1 suppresses both BMP-7–induced phosphorylation of R-Smads-1, -5, and -8 and Wnt-3a signaling. Restoration of SOSTDC1 in renal clear carcinoma cells profoundly suppresses proliferation. Collectively, these results demonstrate that SOSTDC1 is expressed in the human kidney and decreased in renal clear cell carcinoma. Because SOSTDC1 suppresses proliferation of renal carcinoma cells, restoration of SOSTDC1 signaling may represent a novel target in treatment of renal clear cell carcinoma.
INTRODUCTION
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGF-β) superfamily that function as extracellular signaling proteins (Urist, 1965; Wozney, 1992; Reddi, 2001). BMPs are pleiotropic whole body morphogens involved in proliferation, differentiation, and maintenance of diverse tissue types (ten Dijke et al., 1994; Yamashita et al., 1995). BMP signaling is primarily mediated though binding to a heterotetrameric complex of cognate type I and type II receptors (BMPRs). On ligand binding, the serine/threonine kinase on the type II receptors phosphorylates the type I receptors, triggering intracellular Smad signal transduction cascades. Phosphorylation of the BMP-specific intracellular Smads (Smad-1, -5, and -8) allows them to bind the partnering co-Smad, Smad4 (Yamashita et al., 1995; Kawabata et al., 1998; Miyazono et al., 2000, 2001; Moustakas et al., 2001; Chen et al., 2004). This complex of Smads and other DNA-binding proteins undergoes nuclear translocation and triggers transcriptional activation of target genes, including cell cycle regulators or apoptosis mediators such as p21/Cip1/Waf1, bax, and p53 (Chen et al., 2002; Pouliot and Labrie, 2002; Fukuda et al., 2006).
Regulation of BMP signaling is accomplished in a tissue-specific manner via BMP antagonists (Yanagita, 2005, 2006). Classical BMP antagonists bind BMPs in the extracellular space, preventing the association of BMPs with their receptors. BMP antagonists may also prevent the activity of BMPs by fostering their retention in the endoplasmic reticulum (ER; Guidato and Itasaki, 2007). BMP antagonists have been postulated to act as the key on/off switches that spatially and temporally regulate local BMP activity. Thus, BMP antagonists may represent local targets to control specific tumors.
In addition to their effects on BMP pathways, BMP antagonists have also been reported to impinge on Wnt signaling, For example, sclerostin exerts its inhibitory effects on bone formation in mice primarily through inhibition of Wnt signaling (van Bezooijen et al., 2007); in Xenopus, the BMP antagonist Wise inhibits or stimulates Wnt signaling in a manner dependent on context (Itasaki et al., 2003) and cellular localization (Guidato and Itasaki, 2007).
Several genes have been implicated in the development of renal clear cell carcinoma. These include VHL, an ubiquitin ligase and tumor suppressor, as well as c-Met and BHD (Linehan et al., 2005; Brugarolas, 2007). To identify novel genes whose expression is altered in renal cancer, we probed arrays of secreted and cell surface cDNAs with cDNA libraries prepared from tumor tissue and matched normal tissue from 20 kidney cancer patients. This screen identified SOSTDC1, a BMP antagonist, as a candidate gene with reduced expression in renal cancer.
SOSTDC1 is orthologous to a recently characterized murine antagonist of BMPs-2, -4, and -7 termed ectodin/USAG-1 (Yanagita et al., 2004). In other species, this protein is known as USAG-1 (Rattus norvegius; Yanagita et al., 2004), ectodin (Mus musculus; Laurikkala et al., 2003; Kassai et al., 2005), and wise (Xenopus laevis; Itasaki et al., 2003). Recent mouse knockouts of ectodin/USAG-1 have shown that this molecule is important in tooth formation and modulation of the renoprotective effects of BMP-7 (Kassai et al., 2005; Yanagita et al., 2006). The human protein is 98% identical to the rodent protein, and like most members of the BMP and BMP antagonist families, is predicted to contain a C-terminal cystine-knot motif (Avsian-Kretchmer and Hsueh, 2004). However, the role of SOSTDC1 in human tissues or human cancers has not been reported.
In this report, we demonstrate that SOSTDC1 is highly expressed in cells of the normal human kidney, but is significantly decreased in clear cell renal carcinoma.
MATERIALS AND METHODS
Recombinant Proteins and Reagents
Recombinant human BMP-7 (rhBMP-7), noggin-Fc chimera, recombinant mouse Wnt-3a (rmWnt-3a), and DKK1 were purchased from R&D Systems (Minneapolis, MN). Mammalian expression vectors encoding the open reading frame (ORF) for BMP-7 (pReceiver-M2-BMP7), dickkopf-1 (DKK1; pReceiver-M2-DKK1), noggin (pReceiver-M02-noggin), and Wnt-3a (pReceiver-M02-Wnt3a) were purchased from GeneCopoeia, (Germantown, MD). The TCF/LEF luciferase reporter construct Super 8X TOPFlash and the corresponding mutant control Super 8X FOPFlash vectors were obtained from the Addgene Repository (Cambridge, MA), where they had been deposited by Dr. Randall Moon (Veeman et al., 2003).
The following commercial antibodies were obtained: anti-phospho-Smad-1,-5,-8 (Cell Signaling Technologies, Danvers, MA), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Research Diagnostics International, Flanders, NJ), and mouse monoclonal anti-FLAG (Sigma Aldrich, St. Louis, MO).
Production of anti-hSOSTDC1 Antisera
Production and affinity purification of an anti-SOSTDC1 peptide polyclonal rabbit anti-sera directed against the 18 C-terminal amino acids of the SOSTDC1 sequence was performed by Quality Control Biochemicals Custom Services (Hopkinton, MA).
Cells and Culture Conditions
Human embryonic kidney cells, HEK293, were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (Invitrogen). The following kidney cancer cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD): 786-O (CRL-1932, renal clear cell adenocarcinoma), ACHN (CRL-1611, renal cell adenocarcinoma, pleural effusion), 769-P (CRL-1933, derived from primary renal clear cell adenocarcinoma), A-704 (HTB-45, adenocarcinoma), and Caki-1 (HTB-46, clear cell carcinoma skin metastasis). All kidney cancer cell lines were grown in McCoy's 5A basal media (Invitrogen) supplemented with 10% FBS, 1% penicillin/streptomycin, and 200 mM l-glutamine. Renal epithelial proximal tubule cells (RPTECs) were purchased from Cambrex (Hopkinton, MA) and were grown in Renal Epithelial Basal Medium (REBM) with supplements (Cambrex).
Mammalian Vector Construction and Preparation of Recombinant, Human SOSTDC1
The ORF encoding the SOSTDC1 protein (NCBI: NM_015464) without the final stop codon was amplified from cDNA by PCR and cloned into the BamHI/Asp718 restriction sites of the pFLAG-CMV5a vector (Invitrogen). The resulting recombinant human SOSTDC1 (rhSOSTDC1) has the FLAG (DYKDDDDK) epitope added to its C-terminus.
HEK293 cells were transiently transfected with the pFLAG-CMV5A SOSTDC1 vector using LipofectAMINE reagent (Invitrogen). Conditioned media was harvested after 2 d and purified using an anti-FLAG M2 affinity column (Sigma).
Tissue Samples and Immunohistochemistry
All tissue samples were used with approval from the Institutional Review Board at Wake Forest University. Paraffin blocks from the pathology archives were sectioned at 5-μm intervals. After heat-induced antigen retrieval with antigen unmasking solution (AUS, Vector Labs, Burlingame, CA), slides were stained with anti-SOSTDC1 polyclonal sera followed by affinity-purified goat anti-rabbit-IgG-HRP (Jackson ImmunoResearch, West Grove, PA) and diaminobenzidine (DAB) horseradish peroxidase substrate. The slides were counterstained with hematoxylin, viewed under brightfield conditions, and photographed at the same magnification.
Intracellular SOSTDC1 Relative Quantification
SOSTDC1 staining was quantified in Image J (http://rsb.info.nih.gov/ij/; NIH freeware (Rasband, 1997–2007) as mean pixel intensity. Brightfield digital images of the peroxidase-stained tissues were collected at equal exposure conditions. To avoid bias of cell selection, an identical grid consisting of nine equal squares was superimposed over every image digitally. Only tumor cells crossing lines of the grid were included in analysis. Analysis in Image J was completed via the “measure line” function as follows: a 5-pixel straight line selection was drawn in the perinuclear region of each candidate cell. Then, the “Measure” command was chosen from the Analyze menu. The mean pixel intensity value was averaged for 30 separate cells and reported as a percentage of maximal density. Mean pixel intensity was also quantified using Adobe Photoshop (San Jose, CA; Lehr et al., 1997), which gave virtually identical results.
Statistical Analysis
Statistical analysis was performed by the Biostatistics core of the Comprehensive Cancer Center of Wake Forest University. A one-way ANOVA was fit to compare the mean percent maximal density of the five groups. Pairwise comparisons were then performed between groups using a conservative p value of 0.005 for significance (using a Bonferroni adjustment to allow for all 10 pairwise comparisons between groups to be made). SDs for the outcome were compared across the five groups to assure that they were comparable.
cDNA Microarray and RNA Dot Blot
Microarray analyses were performed on tissues from matched normal and tumor tissues from 20 kidney cancer patients and cDNAs from other normal and tumor tissue types. Labeled SOSTDC1 probes were hybridized to the array, and resulting signals were scanned and quantified. To independently verify these results, the BD Clontech Cancer Profiling Array I, also having 20 matched normal and tumor kidney tissues, was probed with radiolabeled SOSTDC1. The majority of these tissues (∼70%) are from patients with renal cell carcinoma, clear type.
rhSOSTDC1 Transient Transfection and Immunofluorescence
pFLAG-CMV5a-SOSTDC1 was transiently transfected into HEK293 cells via LipofectAMINE reagent. Twenty-four hours after transfection, cells were washed once in room temperature phosphate-buffered saline (PBS), and then fixed in 100% methanol at 4°C for 15 min. Cells were labeled with anti-FLAG mouse mAb (Sigma Aldrich) conjugated to rhodamine. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, CalBiochem, EMD Biosciences, San Diego, CA), and cells were visualized through fluorescent microscopy.
Stimulation of Phosphorylated Smad Production with BMP-7 and Phosphorylated Smad Immunoblot
Renal cancer cells were plated in phenol red-free McCoy's 5A with 5% FBS and 1% penicillin/streptomycin and allowed to attach overnight. Medium was replaced with phenol-red-free McCoy's 5A (Promocell GmBH, Heidelberg, Germany) containing 0.5% FBS and 1% penicillin/streptomycin with additions of rhBMP-7 (40 ng/ml), rhnoggin-Fc chimera (150 ng/ml), and/or rhSOSTDC1 (150 ng/ml). Cells were placed at 37°C for either 1 or 3 h. At harvest, cells were washed with PBS, and 150 μl of SDS-PAGE loading buffer was added (0.5 M Tris, pH 6.8, 20% glycerol, 10% SDS, and 5% β-mercaptoethanol). Lysates were electrophoresed on 10% SDS-PAGE gels and transferred to nitrocellulose, and phosphorylated Smad (p-Smad) was detected by Western blot analysis according to the instructions provided by the antibody supplier. p-Smad-1, -5, and -8 were visualized with PicoWest chemiluminescent substrate (Pierce Biotechnology, Rockford, IL). GAPDH was used as a loading control.
Wnt Signaling Luciferase Assay
Transfections were performed using Fugene6 (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. For each condition, 300,000 HEK293 cells were transfected with 1.76 μg of total plasmid DNA. Each group was some combination of: 0.25 μg TOPFlash reporter plasmid, 0.01 μg of internal control pRL-Tk, 0.5 μg pReceiver-M02-Wnt3a expression vector, 1 μg pFLAG-CMV5a-SOSTDC1, or 1 μg pReceiver-M02-DKK1. Empty pFLAG-CMV5a vector was used to maintain a consistent level of plasmid DNA in each transfection. Luciferase activity was measured 24 h after transfection using a Dual Luciferase Assay Kit according to the manufacturer's instructions (Promega, Madison, WI).
Transient Transfection of SOSTDC1 in Kidney Cancer Cell Lines
pFLAG-CMV5a (empty) and pFLAG-CMV5a-SOSTDC1 were transiently transfected into 769-P renal cancer cell lines using LipofectAMINE 2000 (Invitrogen) via manufacturer's instructions. Twenty-four hours after transfection, FBS was added to the transfected cell cultures to a final concentration of 5%. To evaluate the success of transient SOSTDC1 overexpression, cells were harvested for real-time RT PCR 36 h after transfection.
Real-Time RT PCR Evaluation of SOSTDC1 Transfected Cells
Approximately 2.5 × 106 transfected cells were trypsinized, washed twice with PBS, and then suspended in Trizol solution (Invitrogen) for preparation of total RNA. RNA was treated with RQ1 RNase-free DNase (Promega) and then purified with the Absolutely RNA MiniPrep kit (Stratagene, La Jolla, CA). Reverse transcriptase reactions were performed with the Taqman Reverse transcription kit (Applied Biosystems, Foster City, CA) per kit instructions. Resulting cDNA was used in real-time PCR reactions on the ABI Prism 7000 Sequence Detection System machine with SYBR Green PCR Master Mix (Applied Biosystems) according to manufacturer's instructions. SOSTDC1 primers used in the real-time analysis were as follows: Forward, 5′-TGGATTGGAGGAGGCTATGGAACA-3′; Reverse, 5′-ACTTGCAGGCAGTGACTACTGTGA-3′. Primers to the housekeeping gene GAPDH were used to standardize for total RNA levels: Forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; Reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
Effect of SOSTDC1, noggin, and DKK1 on Proliferation
50,000 cells of the 769-P cell line were plated per well of a 24-well plate. Cells were transfected with 0.4 μg of either pFLAG-CMV5a empty vector control, pFLAG-CMV5a-SOSTDC1, pReceiver-M02-Noggin, or pReceiver-M02-DKK1 with LipofectAMINE 2000 as described above. Twenty-four hours after transfection, FBS was added to the transfected cell cultures to a final concentration of 5%. Cells were allowed to recover from transfection for an additional 2 h before trypsinization and reseeding at 5000 cells per well in an E-plate for use on the ACEA RT-CES system (ACEA Biosciences, San Diego, CA). This system allows real-time monitoring of cultures grown in a 96-well plate format. Each type of transfected cell was replated in its own conditioned medium.
RESULTS
Down-Regulation of SOSTDC1 in Renal Tumors
To identify novel genes whose expression is altered in cancer, we probed cDNA arrays of secreted and cell surface genes with cDNA libraries prepared from tumor tissue and matched normal tissue from 20 renal cell carcinoma patients. High SOSTDC1 expression was observed in normal kidney tissue, but mean SOSTDC1 expression decreased by more than one SD in renal tumors (data not shown). To confirm this finding, a Cancer Profiling Array (Clontech, Mountain View, CA) containing 500 cDNAs from a variety of tissues, tumors, and cancer cell lines was probed with radiolabeled SOSTDC1 oligonucleotide. As seen in Figure 1, SOSTDC1 was down-regulated in all 20 of matched normal and tumor kidney sample pairs on this array. Of note, these matched samples were from a different set of kidney cancer patients than the 20 patients included in the microarray analysis. These results suggest that SOSTDC1 mRNA is down-regulated in a majority of kidney cancers.
Figure 1.
Down-regulation of human SOSTDC1 mRNA in kidney tumors. cDNA of matched normal and tumor kidney tissue from the same patient was hybridized with radiolabeled DNA oligonucleotides to human SOSTDC1. N, normal (lane 1); T, tumor (lane 2). SOSTDC1 cDNA decreases in normal versus tumor tissue in 20 of 20 patients; 14 of 20 (70%) of tumors show virtually no detectable SOSTDC1 mRNA.
Expression of SOSTDC1 within the Normal Kidney and in Kidney Cancer
To test whether expression of the SOSTDC1 protein was similarly affected, we prepared an anti-SOSTDC1 antibody and performed immunohistochemical analyses. We first evaluated the distribution of SOSTDC1 protein within the normal human kidney. Immunochemistry (IHC) revealed the presence of intracellular SOSTDC1 within most epithelial cells of the kidney, including proximal and distal tubules and transitional urothelium (Figure 2, top panels). A strong SOSTDC1 signal was observed within the distal tubules, collecting ducts, and podocytes of the glomerulus, with more moderate staining in the cytoplasm of proximal tubule epithelial cells. Quantification of cytoplasmic SOSTDC1 staining intensity in each of these cell types demonstrated that an increase in expression of SOSTDC1 was generally correlated with progression through the nephron and into the collecting ducts and urothelium (Table 1). These results are consistent with the strong distal tubule expression observed for USAG-1, the murine homolog of SOSTDC1 (Yanagita et al., 2004).
Figure 2.
Immunohistochemical analysis of SOSTDC1 expression in normal kidney and renal cancer subtypes. Representative images of IHC staining with anti-SOSTDC1 antiserum are shown. All images were obtained at equal magnification and exposure. Left panel, procedural background control (without anti-SOSTDC1 primary antibodies); right panel, the same tissue stained with anti-SOSTDC1 antiserum. Top row, normal kidney. G, glomerulus; arrowhead, distal tubule; arrow, proximal tubule. Bottom rows, renal epithelial or urothelial cancers. RCC; renal cell carcinoma; TCC; transitional cell carcinoma.
Table 1.
Quantification of intracellular anti-SOSTDC1 by immunohistochemical staining
| Normal cell type | Staining intensity (% of maximum) |
|---|---|
| Podocyte | 45±6 |
| Proximal tubule | 25±4 |
| Distal tubule | 49±4 |
| Collecting duct | 53±4 |
| Renal urothelium | 48±3 |
| Bladder urothelium | 61±5 |
Staining intensity was analyzed as described in Materials and Methods. Values are means ± SD.
We then assessed the expression of SOSTDC1 in a variety of kidney tumor types. Over 75% of kidney tumors are of the clear cell type. Papillary and chromophobe types are also seen. These represent ∼15 and 5% of all kidney cancers, respectively, and have a more favorable prognosis than clear cell (Iliopoulos, 2006). We investigated SOSTDC1 protein expression in these kidney tumor types and compared them were normal controls. In addition, we examined expression of SOSTDC1 in transitional cell carcinoma of the bladder. Representative images are shown in Figure 2, and results are quantified in Figure 3.
Figure 3.
Quantification of SOSTDC1 staining. Relative SOSTDC1 staining of normal and tumor tissues was quantified as described in Materials and Methods in 3–9 individuals per group; means and SDs are shown. RCC-clear cell has significantly less SOSTDC1 than the other tissues. * p < 0.001.
As seen in Figure 2, SOSTDC1 expression is markedly diminished in clear cell renal carcinoma (RCC-clear cell) when compared with normal kidney. In papillary (RCC-papillary), transitional cell carcinoma (TCC), and chromophobe types (data not shown), SOSTDC1 staining was intracellular and of higher signal intensity than clear cell renal carcinoma. Results of SOSTDC1 protein quantification in various renal cell carcinomas are shown in Figure 3. Because multiple comparisons were performed, we required a conservative p value <0.005 for significance (see Materials and Methods). ANOVA analysis demonstrated a large group difference, p < 0.001. RCC-clear cell had significantly lower SOSTDC1 immunostaining than any other group, including normal renal urothelium (p < 0.001 for all comparisons). Additionally, no RCC-clear cell sample had higher staining than its likely cell of origin, the proximal tubule cell (also p < 0.001; see also Table 1). Normal renal urothelium and bladder TCC groups were not significantly different from one another (p = 0.137), but were significantly higher than the proximal tubule and RCC-clear cell groups. The RCC-Papillary group was significantly higher than RCC-clear cell (p < 0.001), but not significantly different from proximal tubules (p = 0.03) or bladder TCC (p = .008) based on the Bonferroni correction. Comparison of standard deviations across the five groups indicated that variability was comparable between groups. Thus, immunohistochemical analysis is concordant with SOSTDC1 mRNA analysis and indicates that SOSTDC1 is profoundly decreased in RCC-clear cell tumors.
SOSTDC1 Is Secreted and Antagonizes Signaling of BMP-7 in Kidney Cancer Cells
We next assessed functional consequences of SOSTDC1 expression. As classic BMP antagonists function extracellularly, we first tested whether the localization of SOSTDC1 was compatible with an extracellular mode of action. cDNA for C-terminally FLAG-tagged SOSTDC1 was cloned into an expression vector and transiently transfected into HEK293 cells. rhSOSTDC1-FLAG was detected via immunofluorescence after binding of anti-FLAG-rhodamine antibodies to fixed cells (Figure 4). This experiment revealed the presence of SOSTDC1 predominantly in the secretory apparatus of transfected, highly expressing cells (Figure 4, A–D). We transfected cells at low efficiency for this experiment so that each highly expressing transfectant was relatively isolated, enabling visualization of extracellular SOSTDC1-specific signal on the outside of low-expressing cells (Figure 4, B and E). Greatest staining intensity was seen closest to highly expressing cells, with staining intensity decreasing with distance (Figure 4, A and D vs. B and E). Western blotting also demonstrated the presence of SOSTDC1 in the media of cultured cells (data not shown). These results demonstrate that SOSTDC1 is secreted and binds to the extracellular matrix and/or cell surface, consistent with an ability to function in an autocrine or paracrine manner.
Figure 4.
Normal human SOSTDC1 is secreted and binds to matrix or cell surface proteins around neighboring cells. HEK293 cells were transfected with SOSTDC1-FLAG, and expression was analyzed using rhodamine-conjugated anti-FLAG antibody (mouse anti-FLAG-rh). Nuclei were stained with DAPI. (A–C) Color fluorescent image. (D–F) Corresponding black and white channel of same images. All images were obtained at the same magnification and exposure. (A and D) A highly expressing cell (arrow) shows strong signal in the cytoplasm, especially the endoplasmic reticulum and Golgi apparatus. (B and E) Secreted SOSTDC1 (small arrowheads) accumulates around both expressing and nonexpressing cells. (C and F) Empty vector control transfected cells show no background rhodamine fluorescence. N, nucleus.
We next tested whether SOSTDC1 acts as a functional antagonist of BMP-7 signaling. BMP-7 binds to BMPRs, leading to receptor activation and phosphorylation of intracellular regulatory Smads (R-Smads; Smad-1, -5, and -8). We predicted that if SOSTDC1 functions as an extracellular BMP antagonist, it should bind to BMP-7, prevent the BMP-receptor interaction, and stop BMP-7–induced Smad phosphorylation. To test this, cells were treated with BMP-7 in presence and absence of SOSTDC1, and phosphorylation of the BMP-specific R-Smads was measured.
As shown in Figure 5, treatment with rhBMP-7 alone caused a rapid and robust increase in phosphorylation of R-Smads (p-Smad) in cultured kidney cancer (786-O) cells. A similar rapid increase in p-Smad levels was also observed in other kidney cell lines, including ACHN, 769-P, A-704, and Caki-1, as well as normal RPTEC cells (data not shown). To test the ability of rhSOSTDC1 to antagonize this effect, rhSOSTDC1 was added to the cells simultaneously with rhBMP-7. Noggin, a classical antagonist of BMP-7, was used as a positive control (Avsian-Kretchmer and Hsueh, 2004; Kawabata et al., 1998). At both 1 and 3 h, rhSOSTDC1 antagonized the BMP-7 induced production of p-Smad at least as efficiently as noggin (Figure 5). Thus, SOSTDC1 effectively antagonizes BMP-7 signaling.
Figure 5.
rhSOSTDC1 antagonizes the production of phosphorylated Smad in response to BMP-7 signaling. 786-O cells were treated BMP-7, SOSTDC1, or noggin as indicated and Smad phosphorylation was analyzed by Western blotting. SOSTDC1 inhibited the production of p-Smad-1, -5, and -8 by 3.5-fold. GAPDH was used as a loading control. Note: for clarity, lanes from the same blot have been rearranged. Similar results were observed in the 769-P and A-704 renal adenocarcinoma cell lines (data not shown).
SOSTDC1 Suppresses Wnt Signaling in Kidney Cells
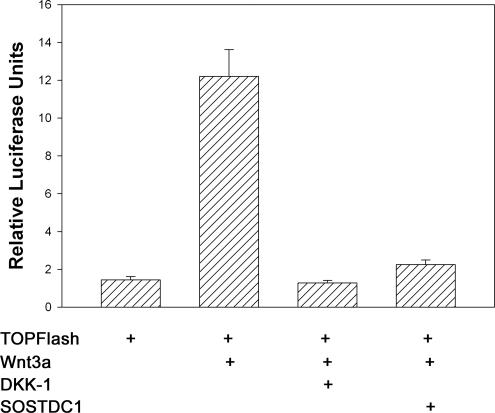
Because Wise, an ortholog of SOSTDC1, can both stimulate and inhibit the Wnt pathway (Itasaki et al., 2003), we asked whether SOSTDC1 would affect Wnt signaling in human kidney cells. Cells were transfected with Super 8X TOPFlash, a construct containing eight TCF/LEF transcription factor binding sites upstream of the luciferase gene. Cells were cotransfected with Wnt-3a (to activate the classical Wnt/β-catenin/TCF signaling pathway) with and without cotransfection with SOSTDC1 expression vector. Cotransfection with DKK1, a known Wnt inhibitor, was used as a control. As seen in Figure 6, Wnt-3a increased expression of the TOPFlash luciferase, and this response was blocked by DKK1. SOSTDC1 inhibited Wnt signaling to an extent comparable to DKK1. Thus, SOSTDC1 suppresses Wnt-3a signaling as well as BMP signaling in human kidney cells.
Figure 6.
SOSTDC1 antagonizes Wnt-3a signaling in renal cells. HEK293 cells were transiently transfected with Super 8X TOPFlash luciferase reporter construct plus Wnt-3a and antagonist expression constructs as outlined in Materials and Methods. After 24 h, resulting luciferase activity was measured for each transfection group and reported as relative luciferase units. Means and SEs of triplicate experiments are shown.
SOSTDC1 Suppresses Proliferation of RCC-Clear Cell Cultures
Clear cell tumors are the most prevalent histopathologic type of kidney cancer. Because these tumors showed the greatest change in SOSTDC1 protein expression (Figures 2 and 3), we asked what functional effects SOSTDC1 might have on these cells. To address this question, the renal cancer clear cell line 769-P was transiently transfected with the pFLAG-CMV5a-SOSTDC1 expression plasmid, and the effect of SOSTDC1 on proliferation measured. As shown in Figure 7, SOSTDC1 strikingly inhibited proliferation of 769-P cells: SOSTDC1 was cytostatic to these cells, whereas cells transfected with empty vector proliferated normally. Because SOSTDC1 inhibits BMP and Wnt signaling (Figures 5 and 6), we asked whether other inhibitors of these pathways would exert similar effects. Cells were transfected with an expression vector for noggin, an inhibitor of the BMP pathway, or a vector encoding DKK1, an inhibitor of the Wnt pathway, and effects on proliferation were measured. As seen in Figure 7, inhibition of either of these pathways also inhibited proliferation, although the effect was more modest than that seen with SOSTDC1.
Figure 7.
Overexpression of SOSTDC1 suppresses proliferation of 769-P RCC-clear cell cultures. 769-P cells were transiently transfected with pFLAG-CMV5a-SOSTDC1 (○), pFLAG-CMV5a empty vector control (●), pReceiver-M02-noggin (△), or pReceiver-M02-DKK1 (▼). After transfection recovery, cells were reseeded onto the 96-well ACEA E-plate and loaded into the ACEA RT-CES system for continuous cell growth monitoring. All treatment groups were seeded in triplicate; values are means and standard deviations for each group at selected times for a representative experiment.
DISCUSSION
Differential expression of genes in normal and cancer tissue has been used to study processes involved in malignant change, to discover new tumor markers, and to identify new targets in tumor therapy. Motivated by these goals, we sought to identify secretory or cell surface proteins altered in kidney cancer. Several genes have been implicated in the development of renal clear cell carcinoma, including VHL, a ubiquitin ligase and tumor suppressor, as well as c-Met, BHD, and sFRP1 (Linehan et al., 2005; Brugarolas, 2007; Gumz et al., 2007); however current molecular understanding of the disease is incomplete. To identify novel genes whose expression is altered in renal cancer, we probed arrays of secreted and cell surface cDNAs with cDNA libraries prepared from tumor tissue and matched normal tissue from 20 kidney cancer patients. This screen identified SOSTDC1, a BMP antagonist, as a candidate gene with reduced expression in renal cancer.
Studies have previously linked BMPs, the target of BMP antagonists, as well as BMP receptors, to cancer. For example, aberrant BMP or BMPR expression has been noted in cancers, including osteosarcomas, breast, kidney, colon, and prostate (Miyazaki et al., 2004; Hsu et al., 2005; Alarmo et al., 2006). Smad4, the main intracellular target of both BMP and TGF-β signaling, is a known tumor suppressor in pancreatic and intestinal cancer (Alberici et al., 2006).
SOSTDC1 is a BMP antagonist, as evidenced both by its sequence (Vitt et al., 2001; Avsian-Kretchmer and Hsueh, 2004) and function (Figure 5). Although BMP antagonists have not been previously implicated in kidney cancer, they have been implicated in malignant processes in other tissue types. For example, noggin (a BMP antagonist) suppresses growth of implanted prostate cancer cells (Feeley et al., 2006). DAN, another BMP antagonist discovered in v-mos–transformed cells, inhibits neoplastic transformation (Chen et al., 2002). However, the activities of BMP antagonists are complex and may vary. For example the BMP antagonist gremlin 1 is expressed by stromal cells associated with esophageal, pancreatic, and other cancers (not including the kidney) and may promote tumor cell proliferation (Sneddon et al., 2006). Our data demonstrate that in renal clear cell cancer, decreases in SOSTDC1 mRNA and protein are associated with malignant change. These data are the first link between the BMP antagonist SOSTDC1 and the process of carcinogenesis.
A number of intersections between BMP signaling and regulatory pathways important in carcinogenesis have been recently identified. These include such key cancer pathways as Wnt/β-catenin, PI3-K/PTEN, and MAPK/ERK (Aubin et al., 2004; He et al., 2004; Moustakas and Heldin, 2005; Pardali et al., 2005). Further, BMP signaling directly impinges on the cell cycle regulatory proteins p21 and Rb: BMP signaling regulates p21/Cip1/Waf1 expression in prostate cancer (Haudenschild et al., 2004) and thyroid carcinomas (Franzen and Heldin, 2001) and inhibits phosphorylation of Rb protein in breast cancer (Ghosh-Choudhury et al., 2000).
We found that in human kidney cells, SOSTDC1 inhibits both BMP-7 (Figure 5) and Wnt-3a (Figure 6) signaling. Similarly, previous reports have indicated that orthologues of SOSTDC1 can affect both BMP and Wnt pathways in complex ways that are dependent on context and cellular localization. USAG-1, the murine ortholog of SOSTDC1, binds to BMPs-2, -4, and -7 and antagonizes BMP activity in Xenopus embryos (Yanagita et al., 2004). Wise, another ortholog of SOSTDC1 identified in chick and Xenopus, can both activate and antagonize Wnt signaling during Xenopus development (Itasaki et al., 2003; Guidato and Itasaki, 2007).
Recent work has directly implicated the Wnt pathway in kidney cancer. WTX, a negative regulator of Wnt signaling, was identified as a new tumor suppressor in Wilms' tumor, a pediatric form of kidney cancer (Major et al., 2007). In a separate study using genomic profiling of adult renal cell carcinoma tumors, loss of sFRP1, a secreted form of the frizzled receptor that acts as an inhibitor of the Wnt pathway, was identified in 15 of 15 renal cell carcinoma patients (Gumz et al., 2007). Our results indicate that SOSTDC1, a BMP antagonist that can also negatively regulate the Wnt pathway (Figure 6), is similarly down-regulated in kidney cancer. Collectively, these results suggest that upregulation of Wnt signaling via reduction of sFRP1, SOSTDC1, or perhaps other as yet unidentified mechanisms may make a critical contribution to the development of kidney cancer.
We observed that SOSTDC1 exerts an antiproliferative effect on clear cell renal carcinoma cells (Figure 7). Similarly, sFRP1 was also reported to negatively affect cell proliferation (Gumz et al., 2007). Considering that SOSTDC1 is down-regulated in virtually all of the 20 cancer specimens we analyzed (Figure 1), we speculate that the pervasive decrease in SOSTDC1 in kidney cancers may arise because of the requirement of the malignant cell to overcome this antiproliferative activity of SOSTDC1. Our evidence further suggests that inhibition of both BMP and Wnt pathways may underlie the antiproliferative effect of SOSTDC1. Thus, the BMP antagonist noggin as well as the Wnt antagonist DKK1 also inhibited proliferation in RCC cells (Figure 7). The ability of SOSTDC1 to simultaneously antagonize both Wnt and BMP signaling may explain its enhanced antiproliferative activity when compared with noggin and DKK alone (Figure 7).
In this article, we identify SOSTDC1 as a BMP antagonist that is down-regulated in renal cancer, particularly clear cell carcinoma, the most common kidney cancer. In contrast, expression of SOSTDC1 was not decreased in papillary and chromophobe kidney tumors, which have a more favorable prognosis than clear cell carcinoma. We show a pattern of extracellular secretion in human tissue sections. We observe that reestablishment of SOSTDC1 expression in clear cell renal cancer cells profoundly inhibits proliferation of these cells, suggesting that SOSTDC1 is involved in regulating the proliferative capacity of these cancer cells. Finally, we show that SOSTDC1 expression leads to inhibition of both BMP and Wnt signaling. The observations that SOSTDC1 is significantly down-regulated in clear cell renal carcinoma and that overexpression of SOSTDC1 inhibits proliferation of clear cell carcinoma cells suggest that modulation of SOSTDC1 may represent a novel therapeutic approach for renal cancers.
ACKNOWLEDGMENTS
K.R.B. is supported by the Department of Defense Breast Cancer Research Program under award number W81XWH-05-1-0287 and S.V.T. by National Institutes of Health Grant R21 CA119181. The authors also gratefully acknowledge support from the Ben Mynatt family and the Brown Foundation. Views, opinions, and endorsements by the author(s) do not reflect those of the U.S. Army or the Department of Defense.
Abbreviations used:
- BMP
bone morphogenetic protein
- R-Smad
regulatory Smad
- RPTEC
renal proximal tubule epithelial cells
- SOSTDC1
SclerOSTin domain-containing-1
- TGF-β
transforming growth factor-β
- USAG-1
uterine sensitization-association gene-1
- Wnt
wingless-type MMTV integration site family
- DKK1
Dickkopf-1
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0433) on November 21, 2007.
REFERENCES
- Alarmo E. L., Rauta J., Kauraniemi P., Karhu R., Kuukasjarvi T., Kallioniemi A. Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosomes Cancer. 2006;45:411–419. doi: 10.1002/gcc.20307. [DOI] [PubMed] [Google Scholar]
- Alberici P., Jagmohan-Changur S., De Pater E., Van Der Valk M., Smits R., Hohenstein P., Fodde R. Smad4 haploinsufficiency in mouse models for intestinal cancer. Oncogene. 2006;25:1841–1851. doi: 10.1038/sj.onc.1209226. [DOI] [PubMed] [Google Scholar]
- Aubin J., Davy A., Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–1494. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avsian-Kretchmer O., Hsueh A. J. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol. Endocrinol. 2004;18:1–12. doi: 10.1210/me.2003-0227. [DOI] [PubMed] [Google Scholar]
- Brugarolas J. Renal-cell carcinoma—molecular pathways and therapies. N. Engl. J. Med. 2007;356:185–187. doi: 10.1056/NEJMe068263. [DOI] [PubMed] [Google Scholar]
- Chen B., Athanasiou M., Gu Q., Blair D. G. Drm/Gremlin transcriptionally activates p21(Cip1) via a novel mechanism and inhibits neoplastic transformation. Biochem. Biophys. Res. Commun. 2002;295:1135–1141. doi: 10.1016/s0006-291x(02)00828-8. [DOI] [PubMed] [Google Scholar]
- Chen D., Zhao M., Mundy G. R. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Feeley B. T., Krenek L., Liu N., Hsu W. K., Gamradt S. C., Schwarz E. M., Huard J., Lieberman J. R. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006;38:154–166. doi: 10.1016/j.bone.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Franzen A., Heldin N. E. BMP-7-induced cell cycle arrest of anaplastic thyroid carcinoma cells via p21(CIP1) and p27(KIP1). Biochem. Biophys. Res. Commun. 2001;285:773–781. doi: 10.1006/bbrc.2001.5212. [DOI] [PubMed] [Google Scholar]
- Fukuda N., Saitoh M., Kobayashi N., Miyazono K. Execution of BMP-4-induced apoptosis by p53-dependent ER dysfunction in myeloma and B-cell hybridoma cells. Oncogene. 2006;25:3509–3517. doi: 10.1038/sj.onc.1209393. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N., Woodruff K., Qi W., Celeste A., Abboud S. L., Ghosh Choudhury G. Bone morphogenetic protein-2 blocks MDA MB 231 human breast cancer cell proliferation by inhibiting cyclin-dependent kinase-mediated retinoblastoma protein phosphorylation. Biochem. Biophys. Res. Commun. 2000;272:705–711. doi: 10.1006/bbrc.2000.2844. [DOI] [PubMed] [Google Scholar]
- Guidato S., Itasaki N. Wise retained in the endoplasmic reticulum inhibits Wnt signaling by reducing cell surface LRP6. Dev. Biol. 2007;310:250–263. doi: 10.1016/j.ydbio.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Gumz M. L., et al. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin. Cancer Res. 2007;13:4740–4749. doi: 10.1158/1078-0432.CCR-07-0143. [DOI] [PubMed] [Google Scholar]
- Haudenschild D. R., Palmer S. M., Moseley T. A., You Z., Reddi A. H. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res. 2004;64:8276–8284. doi: 10.1158/0008-5472.CAN-04-2251. [DOI] [PubMed] [Google Scholar]
- He X. C., et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hsu M. Y., Rovinsky S., Penmatcha S., Herlyn M., Muirhead D. Bone morphogenetic proteins in melanoma: angel or devil? Cancer Metastasis Rev. 2005;24:251–263. doi: 10.1007/s10555-005-1575-y. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O. Molecular biology of renal cell cancer and the identification of therapeutic targets. J. Clin. Oncol. 2006;24:5593–5600. doi: 10.1200/JCO.2006.08.8948. [DOI] [PubMed] [Google Scholar]
- Itasaki N., Jones C. M., Mercurio S., Rowe A., Domingos P. M., Smith J. C., Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- Kassai Y., Munne P., Hotta Y., Penttila E., Kavanagh K., Ohbayashi N., Takada S., Thesleff I., Jernvall J., Itoh N. Regulation of mammalian tooth cusp patterning by ectodin. Science. 2005;309:2067–2070. doi: 10.1126/science.1116848. [DOI] [PubMed] [Google Scholar]
- Kawabata M., Imamura T., Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Laurikkala J., Kassai Y., Pakkasjarvi L., Thesleff I., Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev. Biol. 2003;264:91–105. doi: 10.1016/j.ydbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Lehr H. A., Mankoff D. A., Corwin D., Santeusanio G., Gown A. M. Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. J. Histochem. Cytochem. 1997;45:1559–1565. doi: 10.1177/002215549704501112. [DOI] [PubMed] [Google Scholar]
- Linehan W. M., Grubb R. L., Coleman J. A., Zbar B., Walther M. M. The genetic basis of cancer of kidney cancer: implications for gene-specific clinical management. BJU Int. 2005;95(Suppl 2):2–7. doi: 10.1111/j.1464-410X.2005.05189.x. [DOI] [PubMed] [Google Scholar]
- Major M. B., et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- Miyazaki H., Watabe T., Kitamura T., Miyazono K. BMP signals inhibit proliferation and in vivo tumor growth of androgen-insensitive prostate carcinoma cells. Oncogene. 2004;23:9326–9335. doi: 10.1038/sj.onc.1208127. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Kusanagi K., Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J. Cell. Physiol. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- Miyazono K., ten Dijke P., Heldin C. H. TGF-beta signaling by Smad proteins. Adv. Immunol. 2000;75:115–157. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Heldin C. H. Non-Smad TGF-beta signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Souchelnytskyi S., Heldin C. H. Smad regulation in TGF-beta signal transduction. J. Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Pardali K., Kowanetz M., Heldin C. H., Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1). J. Cell. Physiol. 2005;204:260–272. doi: 10.1002/jcp.20304. [DOI] [PubMed] [Google Scholar]
- Pouliot F., Labrie C. Role of Smad1 and Smad4 proteins in the induction of p21WAF1,Cip1 during bone morphogenetic protein-induced growth arrest in human breast cancer cells. J. Endocrinol. 2002;172:187–198. doi: 10.1677/joe.0.1720187. [DOI] [PubMed] [Google Scholar]
- Rasband W. S. ImageJ. Bethesda, MD: National Institutes of Health; 1997–2007. [Google Scholar]
- Reddi A. H. Bone morphogenetic proteins: from basic science to clinical applications. J. Bone Joint Surg. Am. 2001;83-A(Suppl 1):S1–S6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- Sneddon J. B., et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc. Natl. Acad. Sci. USA. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Sampath T. K., Reddi A. H., Estevez M., Riddle D. L., Ichijo H., Heldin C. H., Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Urist M. R. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- van Bezooijen R. L., et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J. Bone Miner. Res. 2007;22:19–28. doi: 10.1359/jbmr.061002. [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., Moon R. T. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Vitt U. A., Hsu S. Y., Hsueh A. J. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol. Endocrinol. 2001;15:681–694. doi: 10.1210/mend.15.5.0639. [DOI] [PubMed] [Google Scholar]
- Wozney J. M. The bone morphogenetic protein family and osteogenesis. Reprod. Dev. 1992;32:160–167. doi: 10.1002/mrd.1080320212. [DOI] [PubMed] [Google Scholar]
- Yamashita H., ten Dijke P., Huylebroeck D., Sampath T. K., Andries M., Smith J. C., Heldin C. H., Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 2005;16:309–317. doi: 10.1016/j.cytogfr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Yanagita M. Modulator of bone morphogenetic protein activity in the progression of kidney diseases. Kidney Int. 2006;70:989–993. doi: 10.1038/sj.ki.5001731. [DOI] [PubMed] [Google Scholar]
- Yanagita M., Oka M., Watabe T., Iguchi H., Niida A., Takahashi S., Akiyama T., Miyazono K., Yanagisawa M., Sakurai T. USAG-1, a bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem. Biophys. Res. Commun. 2004;316:490–500. doi: 10.1016/j.bbrc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Yanagita M., et al. Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J. Clin. Invest. 2006;116:70–79. doi: 10.1172/JCI25445. [DOI] [PMC free article] [PubMed] [Google Scholar]