Abstract
γ-Aminobutyric acid type A (GABA-A) receptors are a major mediator of inhibitory neurotransmission in the mammalian central nervous system, and the site of action of a number of clinically important drugs. These receptors exist as a family of subtypes with distinct temporal and spatial patterns of expression and distinct properties that presumably underlie a precise role for each subtype. The newest member of this gene family is the θ subunit. The deduced polypeptide sequence is 627 amino acids long and has highest sequence identity (50.5%) with the β1 subunit. Within the rat striatum, this subunit coassembles with α2, β1, and γ1, suggesting that γ-aminobutyric acid type A receptors consisting of arrangements other than αβ + γ, δ, or ɛ do exist. Expression of α2β1γ1θ in transfected mammalian cells leads to the formation of receptors with a 4-fold decrease in the affinity for γ-aminobutyric acid compared with α2β1γ1. This subunit has a unique distribution, with studies so far suggesting significant expression within monoaminergic neurons of both human and monkey brain.
In the mammalian central nervous system, inhibitory neurotransmission is mediated primarily by the γ-aminobutyric acid (GABA), which acts on GABA type A (GABA-A) receptors, ligand-gated ion channels acting over a rapid time frame. Over the past 10 years, it has become clear that a family of GABA-A receptor subtypes exists, generated through the coassembly of polypeptides selected from α1–α6, β1–β3, γ1–γ3, δ, ɛ, and π to form what is most likely a pentameric macromolecule (1–6). The subunits show distinct patterns of temporal and spatial expression, so that GABA-A receptor subtypes have a defined localization presumably reflecting their physiological role (7–11).
In this article, we report the identification and characterization of a further member of the GABA-A receptor gene family that we have termed theta (θ).
MATERIALS AND METHODS
Cloning of θ Subunit cDNA.
Full-length cDNA was cloned starting from sequence information in GenBank entry U47334. PCR was performed under standard conditions, on human whole-brain cDNA (CLONTECH) with oligonucleotide primers specific to the 5′ and 3′ ends of the U47334 sequence. A single PCR product of approximately 1,600 bp was obtained. The 5′ and 3′ ends of the coding region corresponding to full-length U47334 sequence were obtained by 5′ and 3′ anchored PCR using human brain Marathon cDNA (CLONTECH). Full-length cDNA (GenBank accession no. AF144648) was generated by PCR using a primer derived from sequences surrounding the initiating methionine incorporating a consensus Kozak sequence (12), and a primer based on the 3′ untranslated region of the anchored PCR product. The PCR product (1,958 bp) was sequenced completely on both strands by primer walking by using dye terminator chemistry and an Applied Biosystems model 373A sequencer.
Epitope-tagged θ subunit was constructed that contained nucleotides −224 to +99 (i.e., the 5′ untranslated region, the signal peptide, 6 amino acids of the mature protein) of bovine GABA-A receptor α1 cDNA, a sequence encoding the c-myc epitope tag (EQKLISEEDL), a cloning site encoding the amino acids Asn-Ser-Gly, and DNA encoding amino acids 22–627 of the GABA-A receptor θ gene product. c-myc-tagged human GABA-A receptor β2 was generated in the same way to contain amino acids 38–474 of the subunit.
c-Myc Cell Surface ELISA.
Briefly, HEK 293 cells were seeded at 1 × 105 cells per well in a 24-well tissue culture plate. After 24 h, each well was transiently transfected with a total of 1 μg of DNA by using calcium phosphate precipitation (13). Two days after transfection, the cells were aspirated, and aspirated cells were incubated at room temperature with 2 ml of PBS containing 4% (wt/vol) skimmed milk powder (4% MPBS) for 30 min. The cells were then aspirated and incubated at room temperature with 500 μl of 4% MPBS containing the anti-c-myc monoclonal antibody 9E10 (American Type Culture Collection product CRL-1729) for 1 h. The cells were aspirated and washed once with 2 ml of 4% MPBS, and then incubated as before with 500 μl of 4% MPBS containing horseradish peroxidase-conjugated anti-mouse IgG (Promega) for 1 h. The cells were aspirated and washed three times with 4% MPBS, three times with PBS, and the reaction product was developed with 500 μl of K-Blue substrate (Neogen, Lexington, KY). Aliquots of 200 μl were transferred to a 96-well ELISA plate, and the A620 was determined.
Generation of Polyclonal Sera to the θ Subunit.
Antibodies to the human GABA-A receptor θ subunit were generated by using a glutathione S-transferase (GST) fusion protein consisting of residues 353–595 of the putative cytoplasmic loop region of the θ subunit. DNA encoding this region was cloned into the bacterial expression vector pGEX-2T (Pharmacia), transformed into Escherichia coli DH10B cells (Life Technologies), and expression and purification of the fusion protein was carried out by using the Pharmacia protocols. The purified GST fusion protein was used to immunize rabbits for the subsequent generation of antiserum.
Localization of the θ Subunit mRNA in Monkey Brain by in Situ Hybridization.
The sequences used for the two antisense oligonucleotides (equivalent to nucleotides 1,035–1,079 and 1,532–1,576 of human θ cDNA sequence) showed no significant homology to any other nucleotide sequence in GenBank as of September 1996. Oligonucleotides were purified by preparative polyacrylamide gel electrophoresis and 3′ end labeled with deoxyadenosine 5′-[[35S]thio]triphosphate as described (4) to give specific radioactivities of 1.2–2.3 × 109 cpm/μg. Monkey brains were removed and fresh-frozen in 1-cm blocks. Sections (12 μm) were taken, fixed for in situ hybridization, and processed as described (4, 13). Autoradiographs were analyzed by using a MCID computerized image analysis system (Image Research, Ontario, Canada).
Localization of θ Subunit in Monkey and Human Brain by Immunocytochemistry.
Sections from monkey brains were prepared as described (4). To enhance the immunoreactivity, sections were subjected to antigen retrieval techniques, as described by Shi et al. (14). Sections were then incubated overnight at +4°C in the anti-θ-subunit rabbit polyclonal antibody diluted 1:1000 in blocking buffer (5% normal goat serum in PBS). Immunoreactivity was visualized with the Vector elite system (Vector Laboratories). Sections were counterstained in Gill’s hematoxylin (Menarini, U.K.), dehydrated, and mounted. Postmortem human brainstems (fixed in 10% formalin) were processed in an identical manner. Antibody specificity was determined by preabsorption of the antibody with its cognate peptide, resulting in an loss of the observed immunoreactivity (data not shown). Sections used for immunofluorescent colocalization of θ subunit and tyrosine hydroxylase (TH) were pretreated in the same manner; θ subunit immunoreactivity was detected by using first a biotinylated anti-rabbit immunoglobulin antiserum diluted 1:200 (Vector Laboratories) followed by FITC-conjugated streptavidin (Sigma). The second rabbit polyclonal serum, anti-TH, was again visualized by using biotinylated anti-rabbit immunoglobulin antiserum, reacted with Cy3-conjugated streptavidin (Sigma). Sections were counterstained with Hoescht 33258 (0.5 μg/ml). To avoid any cross-reactivity of the detection systems, sections were placed in boiling distilled water for 5 min before the application of the second primary antibody and its subsequent detection.
Immunoprecipitation of GABA-A Receptors from Rat Brain.
Receptors were solubilized from rat brain, and immunoprecipitation experiments performed as described (15). The production of antiserum to the θ subunit is described above, and antisera to other GABA-A receptor subunits have been described (15). Briefly, protein-A agarose beads were first coated with antibody by incubating 50 μl of antiserum with 100 μl of packed protein A-agarose in a total volume of 1 ml of Tris-buffered saline (TBS) for 1 h at room temperature. The antibody–protein-A complex was then washed three times in TBS and bound to the receptor by overnight incubation at 4°C with 0.5 ml of detergent-solubilized membranes (0.1 M KCl/5 mM MgCl2/1% Triton X-100/0.5% deoxycholate/1 mM PMSF/0.1 M Tris⋅HCl, pH 8.2). The beads were then washed three times in TBS/0.1% Tween 20 and resuspended in 10 mM KH2PO4/100 mM KCl, pH 7.4.
Control immunoprecipitation was determined as radioligand binding sites immunoprecipitated by the same amount of antiserum raised against an irrelevant antigen (5-HT3 receptor) and was typically less than 50 specific dpm. Where coprecipitations were carried out, 50 μl of each subunit specific antiserum was separately incubated with 100 μl of protein beads, washed, and then combined before the addition of soluble receptor.
Binding of [3H]muscimol (8 nM) was carried out on beads and supernatants in 10 mM KH2PO4/100 mM KCl, pH 7.4, in a total volume of 0.5 ml. After incubation at 4°C for 1 h, the reaction was terminated by filtration through Whatman GF/C filters, followed by three 1-ml washing with 10 mM KH2PO4/100 mM KCl, pH 7.4, and radioactivity was measured by scintillation counting. Nonspecific binding was determined with 100 μM GABA.
Expression of GABA-A Receptors in Xenopus Oocytes and Transiently Transfected HEK 293 Cells.
Oocytes from adult female Xenopus laevis were isolated and injected with different combinations of subunit cDNAs (20 ng/μl), and electrophysiological analyses were performed as described (4). In all experiments, drugs were applied in the perfusate until the peak of the response was observed. Allosteric modulators were preapplied for 40 sec before coapplication of GABA at an effective concentration, 50% (EC20), concentration.
Experiments were performed on HEK 293 cells transiently transfected with human cDNA combinations α2β1γ1, and α2β1γ1θ (6 μg of cDNA total per coverslip) by using calcium phosphate precipitation (13). Electrophysiological analyses were performed as described (4). GABA was applied from a double-barrel pipette assembly approximately 300 μm in diameter positioned ∼300 μm from the cell. Solution equilibration times were on the order of 15 msec. Increasing GABA concentrations were applied for 2-sec pulses with a 30-sec interval between applications. Noncumulative concentration–response curves to GABA were constructed. Curves were fitted by using a nonlinear least square-fitting program to the equation f(x) = Bmax/[1 + (EC50/x)n], where x is the drug concentration, EC50 is the concentration of drug eliciting a half-maximal response, and n is the Hill coefficient. EC50 values were analyzed by using an unpaired Student’s t test. Allosteric potentiation of GABA receptors was measured relative to a GABA EC20 and antagonists were investigated by using a GABA EC50, each determined for each cell to account for differences in GABA affinity. Receptor desensitization was measured by applying a 10-sec application of a maximum GABA concentration and recording peak to steady-state ratio at the end of the 10-sec pulse.
RESULTS
U47334 Encodes a Partial Sequence of a GABA-A Receptor Subunit.
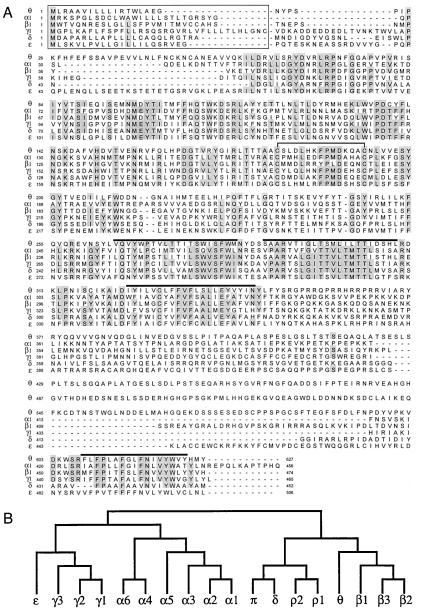
Searching GenBank nucleotide database with the coding region of GABA-A receptor subunit identified the sequence U47334, submitted as an exon-trapped sequence from chromosome Xq28 annotated as “similar to chicken γ aminobutyric acid receptor beta4 subunit” (16). This is clustered with the GABA-A receptor ɛ and α3 genes (17). Full-length cDNA had a deduced ORF of 627 amino acids (Fig. 1A) that has all of the predicted features characteristic of ligand gated ion channel subunits: signal peptide, two cysteines separated by 13 amino acids, and four putative transmembrane (TM) domains, the third and fourth separated by a large cytoplasmic loop. There are two putative N-glycosylation sites, Asn-122 and Asn-317. Fig. 1A also shows an alignment of the θ subunit with other members of the gene family. The regions of significant sequence homology are the extracellular domain and the putative TM domains. The putative cytoplasmic loop between TM3 and TM4 shows no homology with other proteins, no obvious structural features, and no convincing consensus phosphorylation sites. Fig. 1b shows that θ is most similar to the β subunits. However, it exhibits only 50.5% sequence identity with β1, whereas the amino acid sequence identity of β1, β2, and β3 is around 80%, suggesting that it is not a β subunit. Additionally, it did not substitute functionally for a β subunit (see below). As such, it is more appropriately classified as a new subunit class, rather than β4.
Figure 1.
Human GABA-A receptor θ subunit. (A) Alignment of the deduced amino acid sequences of the human GABA-A receptor α1(18), β1(18), γ1 (19), δ (unpublished results), ɛ (4), and θ subunits. Numbering of amino acids is given by assigning the initiating methionine as 1. Positions where amino acid residues are conserved in four or more sequences are shaded. Putative signal peptides (20) are boxed, putative transmembrane domains are overlined, and the two cysteine residues conserved in the ligand-gated ion channel family are joined by a solid line. (B) Dendrogram of the deduced amino acid sequences of the GABA-A receptor (and ρ1–ρ2 of GABA-C) family. The analysis was performed with clustalw (GCG).
Localization of the θ Subunit in Monkey and Human Brain.
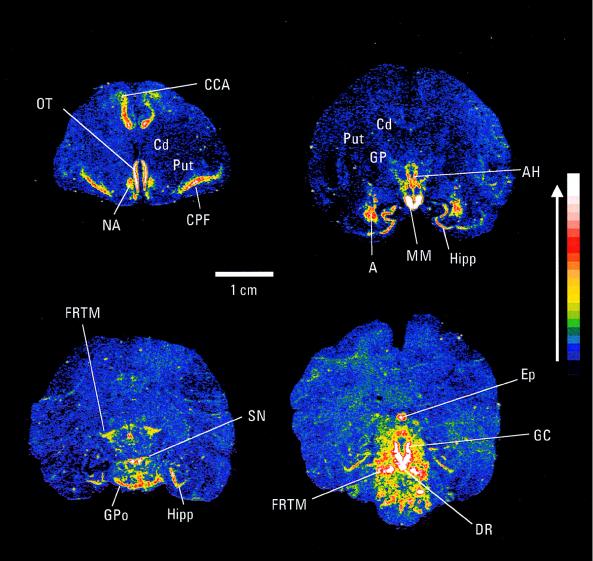
The localization of the θ subunit in squirrel monkey brain was determined by in situ hybridization and by immunocytochemistry. θ mRNA had a discrete distribution (Fig. 2). Cortical expression was restricted to the cingulate and piriform regions. Significant expression was present in hypothalamic regions including the anterior hypothalamus and the mammiliary body. Relatively strong expression of θ mRNA was present in the amygdala, and expression was also observed within the hippocampus. Very strong expression was seen within the substantia nigra, although interestingly the caudate putamen showed no significant labeling. Regions of the hindbrain, including the pons, dorsal raphe, and the central grey, were also strongly labeled. There was no significant hybridization signal in the cerebellum (data not shown).
Figure 2.
In situ hybridization autoradiograms of monkey brain sections showing expression of θ subunit mRNA. The signal intensity is indicated, with white representing the strongest signal. CCA, anterior cingulate cortex; OT, olfactory tract; NA, nucleus accumbens; Cd, caudate; Put, putamen; CPF, piriform cortex; GP, globus pallidus; AH, anterior hypothalamus; Hipp, hippocampus; MM, mamillary body; A, amygdala; FRTM, reticular formation; GPo, pons; DR, dorsal raphe nucleus; GC, central grey; Ep, pineal gland.
Immunoreactivity for the θ subunit was detected in both monkey and human brain with a polyclonal serum raised against recombinant polypeptide of the putative cytoplasmic region of the θ subunit (Fig. 3). Confirmatory data (not shown) has also been generated with a polyclonal antiserum raised against a synthetic peptide corresponding to amino acids 492–510 of the θ subunit. The data shown for monkey brain are derived from a single animal. The same overall pattern of distribution for θ subunit immunoreactivity has also been observed in brain tissue from a second squirrel monkey brain and also from two cynomolgus monkey brains.
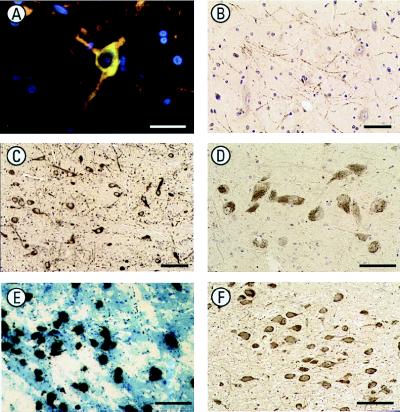
Figure 3.
Immunohistochemical localization of the GABA-A receptor θ subunit in monkey and human brain. (A) The expression of immunoreactivity (green) for the GABA-A receptor θ subunit in the dopaminergic neurons of the periventricular hypothalamus of monkey brain was confirmed by colocalization with TH immunoreactivity (red). Colocalization is observed as yellow/orange. Nuclei are counterstained with Hoechst 33258 (blue). (Bar = 30 μm.) (B) θ subunit immunoreactive processes in the monkey globus pallidus are similar in pattern to those demonstrated by TH, further suggesting expression by nigra–striatal projection neurons. (Bar = 50 μm.) (C) θ subunit immunoreactivity is expressed by the neurons of the primate substantia nigra pars compacta, suggesting expression in dopaminergic neurons. (Bar = 10 μm.) (D) θ subunit immunoreactivity in melanized neurons of the human substantia nigra pars compacta, again suggesting expression in dopaminergic neurons. (Bar = 50 μm.) (E) High-resolution autoradiography in situ hybridization experiments show expression of θ subunit mRNA as silver grains over melanized neurons of the human locus coeruleus. (Bar = 100 μm.) (F) θ subunit immunoreactivity in human locus coeruleus melanized neurons (probably noradrenergic). (Bar = 100 μm.)
Immunolabeling of neurons in various monkey hypothalamic regions and cortical pyramidal neurons, particularly the deeper layers, was observed. Significant labeling of the monkey brainstem was found, including neurons in the substantia nigra pars compacta (Fig. 3C), and associated neurites of the pars reticulata, ventral and lateral tegmental areas, the locus coeruleus, reticular pontine nuclei, and the dorsal raphe. A pattern of labeling consistent with terminals within the monkey caudate putamen was observed with only occasional neuronal soma. TH-positive fibers, which constitute nigra–striatal projections, show a characteristic pattern as they pass through the globus pallidus; this pattern is mimicked by θ subunit immunoreactivity (Fig. 3B). This apparent expression of the θ subunit by TH containing neurons and processes was confirmed with combination immunofluorescence. For example, colocalization of θ subunit immunoreactivity and TH immunoreactivity was observed in dopaminergic neurons of the monkey periventricular hypothalamus (Fig. 3A). The expression of the θ subunit was further substantiated in sections of postmortem human brainstem; expression was observed in both the putative dopaminergic neurons of the substantia nigra pars compacta (Fig. 3D) and putative noradrenergic neurons of the locus coeruleus, by using high-resolution in situ hybridization autoradiography (Fig. 3E) and immunolabeling (Fig. 3F).
Subunit Composition of Native θ Containing GABA-A Receptors.
Polyclonal rabbit antisera raised against the putative cytoplasmic loop domain of the θ subunit were used to characterize native GABA-A receptors in rat brain that contain this subunit. Immunoprecipitation of [3H]muscimol binding sites (muscimol is a high-affinity ligand for the GABA binding site of GABA-A receptors) from solubilized rat brain membranes was used to quantitate θ-containing receptors. The assumption in this experiment is that θ-containing receptors have a high-affinity [3H]muscimol binding site, and this turned out to be the case. The striatum had the highest level of θ-subunit-containing receptors (22 ± 4% of all [3H]muscimol binding sites in striatum, 16 ± 2% of binding sites in hypothalamus, 6 ± 4% in hippocampus, and no significant binding in cortex and cerebellum). Receptors immunoprecipitated with the θ subunit antiserum did not bind benzodiazepine site ligands ([3H]Ro 15–1788, [3H]Ro 15–4513, [3H]flunitrazepam, or [3H]CGS-8216) with affinities high enough to be detected in a radioligand binding assay (data not shown). Assembly of the θ subunit with other GABA-A receptor subunits was investigated by quantitative co-precipitation of solubilized receptors with combinations of subunit-specific antisera. As shown in Table 1, the θ subunit can coassemble with α2, β1, and γ1. Because, within the detection limits of the approach, all θ immunoprecipitated receptors appear to contain γ1 and β1 subunits (theoretical sum of binding sites immunoprecipitated is equal to the highest antiserum individually), the preferred combination in vivo (at least in the striatum) is α2β1γ1θ. Within the detection levels of this experimental approach, in rat striatum, there was no significant coassembly of the θ subunit with α1, α3, α4, α5, α6, γ2, γ3, δ, or ɛ. However, the possibility that there are small populations of receptors present where θ is coassembled with these subunits cannot be discounted.
Table 1.
Immunoprecipitation of [3H]muscimol binding from rat striatum by using combinations of antisera
| Antiserum | % [3H]muscimol binding sites immunoprecipitated
|
Theoretical sum if subunits present in different | |
|---|---|---|---|
| +control IgG | +θ | ||
| α1 | 40 ± 3 | 56 ± 5 | 57 |
| α2 | 21 ± 4 | 17 ± 2 | 38 |
| α3 | 10 ± 2 | 24 ± 5 | 27 |
| β1 | 28 ± 4 | 34 ± 4 | 47 |
| γ1 | 22 ± 4 | 23 ± 1 | 39 |
| γ2 | 35 ± 5 | 58 ± 3 | 52 |
| θ | 17 ± 1 | ||
Data shown are the mean ± SEM of three determinations. No significant immunoprecipitation from striatum was observed if α6, δ, or ɛ subunit antiserum was used. The presence of very small amounts of receptors containing α4, α5, and γ3 subunits was detected with the subunit-specific antisera but the amounts were too low for accurate quantitation by this approach.
Assembly of θ-Containing Receptors in Vitro.
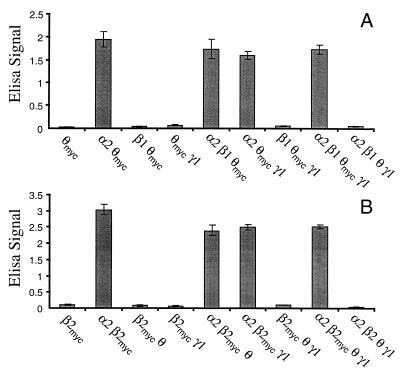
To investigate whether αβγθ subunits can coassemble in vitro, we have used a cell surface ELISA assay with intact transfected cells and myc-epitope-tagged θ subunit. The rationale behind this experiment is as follows. Because θ does not get transported to the cell surface when expressed alone (Fig. 4), then if it appears at the cell surface when coexpressed with other subunits, there must be subunit coassembly. This assay does not determine if the subunits are “correctly” assembled or if they form functional receptors. Data in Fig. 4A are from experiments using α2, β1, γ1, and θ subunits because as discussed above these appear to be the preferred subunits for coassembly in vivo. Experiments have also been performed with α1, β1, γ2 and θ, with the same findings (data not shown). From Fig. 4B, it can be seen that when expressed alone, θ-myc is not expressed to a significant level at the cell surface. Most GABA-A receptor subunits behave this way, the main exceptions being β1 and β3 (11). When θ-myc is coexpressed with α2, it appears at the cell surface. Coexpression of an α subunit with a β subunit would also result in assembly and cell surface expression (e.g., see ref. 11). When coexpressed with β1 and γ1, individually or together, there is no surface expression of θ-myc. Thus, in terms of assembly and transportation to the cell surface (but not formation of functional receptors, see below), θ can substitute for a β subunit but cannot substitute for an α subunit. When coexpressed with α2β1, α2γ1, or α2β1γ1, θ-myc is expressed on the cell surface. However, from these experiments, it is not possible to determine whether these are simply α2θ-myc combinations or coassemblies containing all the expressed subunits. In addition, we do not know whether the α2γ1 combination is able to get to the cell surface. Parallel experiments were also performed with c-myc-tagged β2 (Fig. 4B). Like θ, β2 does not get to the cell surface unless expressed with other subunits (ref. 11 and Fig. 4B). The same conclusions as above are obtained when β2-myc is expressed with other GABA-A receptor subunits. Additionally data in Fig. 4B indicate that the levels of cell surface expression of the various θ subunit-containing combinations are similar to that of α2β2. Because the latter gives robust whole cell currents (data not shown), this suggests that the absence of detectable GABA gated channels for the various dimeric and trimeric θ-containing receptors (see below) is not due to low levels of expression at the cell surface.
Figure 4.
Coassembly and cell surface expression of θ with other GABA-A receptor subunits. c-myc epitope tagged θ (A) or c-myc-epitope-tagged β2 (B) was transiently transfected into HEK 293 cells, either alone or with other GABA-A receptor subunits. Cell surface expression of θ-myc or β2-myc was measured by a whole cell surface ELISA assay. The ELISA signal (A620) is proportional to the amount of θ-myc or β2-myc at the cell surface. Background signal from mock-transfected control wells has been subtracted, and values are the mean ± SEM of triplicate determinations.
To determine the requirements for formation of functional θ-containing receptors, various subunit combinations (θ, β1θ, β1γ2θ, α1θ, α1γ2θ, α1β1θ, and/or α1β1γ2θ) were expressed in Xenopus oocytes, and their electrophysiological response to 3 mM GABA was determined. The only subunit combination that lead to the expression of functional receptors was α1β1γ2θ (data not shown). In addition to testing for responses to GABA, all cells were exposed to 1 mM pentobarbital and 100 μM picrotoxin to preclude the formation of channels gated by pentobarbital but not GABA or the formation of constitutively open channels. Thus although as discussed above αθ, αβθ, (and perhaps αθγ) coassemble and are expressed on the cell surface, they do not form functional receptors. It is interesting that expression of α1β1θ does not lead to the formation of functional receptors, but α1β1 forms robust ion channels. This suggests that although θ is coassembling with α1β1, an ion channel is not formed unless the full compliment of αβ and γ are coexpressed. Furthermore, θ is preventing α1 and β1 from forming a functional GABA gated channel.
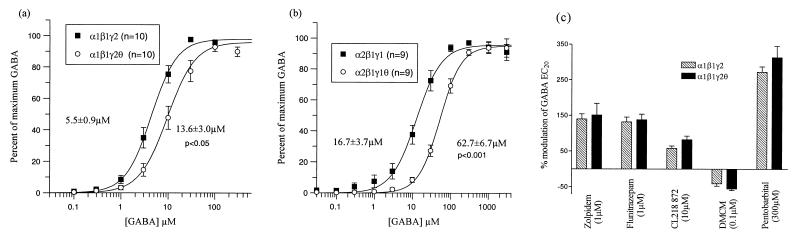
The studies described above investigating the structure of native θ-containing receptors suggested an αβγθ subunit composition, and thus electrophysiological studies that focused on this subunit arrangement, using both α1β1γ2θ and α2β1γ1θ combinations were done. GABA concentration response curves were compared for α1β1γ2 and α1β1γ2θ receptor combinations expressed in Xenopus oocytes. In this study, addition of the θ subunit produced a small but statistically significant increase in GABA EC50 [24.9 ± 4.1 μM for α1β1γ2 (n = 8) and 41.0 ± 4.1 μM for α1β1γ2θ (n = 5); P < 0.05]. These experiments were repeated with whole-cell patch-clamp methods on HEK 293 cells expressing the same subunit combinations, and a similar increase in GABA EC50 [5.5 ± 0.9 μM for α1β1γ2 (n = 10) to 13.6 ± 3.0 μM for α1β1γ2θ (n = 10), P < 0.05] was observed (Fig. 5a), suggesting that θ was incorporated into the α1β1γ2 combination as a fourth subunit. There were no marked differences in the desensitization kinetics of the θ-containing receptors expressed in HEK 293 cells, with peak to steady-state ratios of 2.2 ± 0.2 for α1β1γ2 and 2.1 ± 0.2 for α1β1γ2θ receptors. A number of different allosteric modulators were applied to α1β1γ2 and α1β1γ2θ receptors to determine other possible differences in pharmacology. The benzodiazepine ligands flunitrazepam, DMCM, CL218,872, and zolpidem, as well as the barbiturate pentobarbital, showed identical degrees of modulation to that observed at α1β1γ2 (Fig. 5c), suggesting that θ incorporation does not affect benzodiazepine or barbiturate modulation. Because studies on the composition of native θ-containing receptors had suggested that the most likely subunits that assemble in vivo (at least in the rat striatum) with θ are α2, β1, and γ1, further experiments focused on α2β1γ1 and α2β1γ1θ subunit combinations expressed in HEK 293 cells. GABA concentration–response curves revealed a similar 4-fold increase in GABA EC50 on addition of the θ subunit (Fig. 5b). These data demonstrate the coassembly of θ with α, β, and γ subunits to form a quaternary subunit combination. The pharmacology of α2β1γ1 and α2β1γ1θ receptors was also investigated. No difference in EC50 or maximum modulation by flunitrazepam or the steroid 5α-pregnan-3α-ol-20-one was observed between α2β1γ1 and α2β1γ1θ, and no differences in the IC50 for the antagonists picrotoxin or zinc were seen (Table 2).
Figure 5.
Functional effects of θ expressed with other GABA-A receptor subunits. α1β1γ2 (a) and α2β1γ1 (b) GABA-A subunit cDNAs were transiently transfected both with (○) and without (■) the θ subunit cDNA into HEK 293 cells. Whole-cell patch-clamp techniques were used to measure currents in response to GABA after receptor expression. Concentration–response curves to GABA are shown normalized to a maximum response. Data are the mean ± SEM. of the number of cells indicated. Values are the mean EC50 ± SEM calculated from fits to individual cells. (c) Modulation of the GABA EC20 response of α1β1γ2 and α1β1γ2θ expressed in oocytes by a maximum concentration of benzodiazepine ligands, flunitrazepam, zolpidem, CL218 872, and DMCM, and the barbiturate pentobarbital. Data are the mean ± SEM from at least four oocytes.
Table 2.
Activity of modulatory agents at a2b1g1 and a2b1g1q GABA-A receptors
| Drug | α2β1γ1 receptor
|
α2β1θγ1 receptor
|
||
|---|---|---|---|---|
| EC50/IC50 | Maximum modulation, % | EC50/IC50 | Maximum modulation, % | |
| Flunitrazepam | 414 ± 167 nM | 35.1 ± 4.6 | 245 ± 59 nM | 35.4 ± 4.0 |
| 5α-Pregnan-3α-ol-20-one | 9.9 ± 1.9 nM | 158 ± 34 | 14.0 ± 2.3 nM | 189 ± 16 |
| Picrotoxin | 3.5 ± 1.7 μM | 2.2 ± 0.7 μM | ||
| Zinc | 11.4 ± 2.8 μM | 23.9 ± 12.1 μM | ||
Values are determinations from six to nine oocytes and are not significantly different between subunit combinations.
DISCUSSION
The GABA-A receptor gene family is now increased by the identification of a new member that we have termed θ. Immunoprecipitation data indicates that in the rat striatum θ-containing GABA-A receptors may represent around 20% of total receptors. For comparison, this is approximately the proportion of α5-containing GABA-A receptors in the rat hippocampus (15). Quantitative immunoprecipitation experiments using subunit-specific antiserum suggest that in the rat striatum θ can coassemble with α2, β1, and γ1. Immunocytochemical studies have demonstrated the relatively abundant striatal expression of α2 immunoreactivity (9), in good agreement with this study. The coassembly of four types of subunit is also supported by the in vitro expression experiments that demonstrated that the only combination of θ-containing receptors that coassembled to form a functional receptor with distinct properties (i.e., a change in GABA EC50) is αβγθ. It is generally thought that the prototypic structure of GABA-A receptors is αβ plus a γ, δ, or ɛ subunit (2). It has been reported that native receptors containing both γ and δ subunits do exist (21), although others find that γ and δ subunits are present in different receptor subtypes (22, 23). Thus the αβγθ subunit arrangement is unexpected. It is interesting to speculate on the stoichiometry of such a receptor. αβγ receptors are thought to have the stoichiometry α2β2γ1 (24, 25), although α2β1γ2 is also possible (26). Because two α subunits per receptor is a common theme, one could propose α2β1γ1θ1.
The coassembly in the rat striatum of θ with α2 and γ1 is in accord with previous data indicating that α2 and γ1 coassemble (27–29), although it has been reported that, in membranes from whole rat brain, γ1 can also coassemble with α1 (28, 29). Native γ1-containing receptors have been reported to have a “low-affinity” [3H]flunitrazepam binding site (Kd = 40–70 nM; refs. 28 and 29), with somewhat lower affinity still for other benzodiazepine site ligands. We have been unable to confirm the binding of [3H]flunitrazepam at receptors precipitated with either γ1 or θ subunit antiserum.
It is interesting that θ does not form functional receptors when coexpressed with an α subunit or an α and β subunit, although there is no a priori reason to expect that it would. Perhaps more intriguing is that coexpression of θ with α and β subunit inhibits the formation of functional channels, further suggesting that this is not a physiological combination. The subunit combination proposed here is αβγθ. When expressed in a recombinant system (transfected mammalian cells or oocytes), this subunit combination has up to a 4-fold decrease in affinity for GABA compared with the αβγ combination. As discussed above, so far no other functional differences can be ascribed to the presence of the θ subunit.
θ-containing receptors have a striking colocalization with TH, although θ does not always colocalize with TH. This was seen in regions such as the substantia nigra pars compacta and caudate putamen, the periventricular hypothalamus and the locus coeruleus. Immunoreactivity for α2 and α3 has also been reported to be present in the substantia nigra and the striatum of the rat (9, 30). Indeed, Fritschy et al. (30) have reported that α3, but not α1, immunoreactivity is localized to TH positive cells in the substantia nigra pars compacta and the locus coeruleus. Because we have found no clear evidence for a significant population of receptors containing both α3 and θ, it could be concluded that within these TH-positive neurons, these two subunits are segregated into different receptor subtypes. That a given neuron can express multiple receptor subtypes sequestered to different subcellular domains has been documented in hippocampal pyrammidal cells (31) and cerebellar granule cells (32). Localization of θ mRNA and immunoreactivity in the locus coeruleus, a region rich in TH-positive noradrenergic neurons, is in good agreement with the study by Luque et al. (33) describing the expression of α2, α3, β1, β3, and γ1 mRNAs in these cells.
θ coassembles with γ1, and these receptors have a benzodiazepine modulatory site. Although the affinity of γ1-containing receptors for existing benzodiazepine site ligands is generally low (28, 29, 34), in the future it may be possible to develop a new generation of compounds with selectivity and efficacy at this receptor subtype.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- GABA-A receptor
GABA type A receptor
- TH
tyrosine hydroxylase
- ECn
effective concentration, n%
- TM
transmembrane
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF14468).
References
- 1.Whiting P J, McKernan R M, Wafford K A. Int Rev Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- 2.McKernan R M, Whiting P J. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 3.Seighart W. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 4.Whiting P J, McAllister G, Vasilatis D, Bonnert T, Heavens R P, Smith D W, Hewson L, O’Donnell R, Rigby M, Sirinathsinghji D, et al. J Neurosci. 1997;17:5027–5037. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies P A, Hanna M, Hales T G, Kirkness E F. Nature (London) 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 6.Hedblom E, Kirkness E F. J Biol Chem. 1997;272:15346–15350. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- 7.Wisden W, Laurie D J, Monyer H, Seeburg P H. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurie D J, Wisden W, Seeburg P H. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritschy J M, Mohler H. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 10.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 11.Connolly C N, Wooltorton J R A, Smart T G, Moss S J. Proc Natl Acad Sci USA. 1996;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak M. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- 13.Chen C A, Okayama H. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 14.Shi S-R, Key M E, Kalra K L. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 15.McKernan R M, Quirk K, Prince R, Cox P A, Gillard N P, Ragan C I, Whiting P J. Neuron. 1991;7:667–676. doi: 10.1016/0896-6273(91)90379-e. [DOI] [PubMed] [Google Scholar]
- 16.Levin M L, Chatterjee A, Pragliola A, Worley K C, Wehnert M, Zhuchenko O, Smith R F, Lee C C, Herman G E. Genome Res. 1996;6:465–477. doi: 10.1101/gr.6.6.465. [DOI] [PubMed] [Google Scholar]
- 17.Wilke K, Gaul R, Klauck S M, Poustka A. Genomics. 1997;45:1–10. doi: 10.1006/geno.1997.4885. [DOI] [PubMed] [Google Scholar]
- 18.Schofield P R, Pritchett D B, Sontheimer H, Kettenmann H, Seeburg P H. FEBS Lett. 1989;244:361–364. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- 19.Ymer S, Draguhn A, Wisden W, Werner P, Keinanen K, Schofield P R, Sprengel R, Pritchett D B, Seeburg P H. EMBO J. 1990;9:3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Heijne G. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertens S, Benke D, Mohler H. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- 22.Quirk K, Gillard N P, Ragan C I, Whiting P J, McKernan R M. J Biol Chem. 1994;269:16020–16028. [PubMed] [Google Scholar]
- 23.Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Y, Wang R, Barot S, Weiss D S. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tretter V, Ehya N, Fuchs K, Sieghart W. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backus K H, Arigoni M, Drescher U, Scheurer L, Matherbe P, Mohler H, Benson J A. NeuroReport. 1993;5:285–288. doi: 10.1097/00001756-199312000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Quirk K, Gillard N P, Ragan C I, Whiting P J, McKernan R M. Mol Pharmacol. 1994;45:1061–1070. [PubMed] [Google Scholar]
- 28.Mossier B, Togel M, Fuchs K, Sieghart W. J Biol Chem. 1994;269:25777–25782. [PubMed] [Google Scholar]
- 29.Benke D, Honer M, Michel C, Mohler H. Neuropharmacology. 1996;35:1413–1423. doi: 10.1016/s0028-3908(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 30.Fritschy J M, Benke D, Mertens S, Oertel W H, Bachi T, Mohler H. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusser Z, Sieghart W, Benke D, Fritschy J M, Somogyi P. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nusser Z, Sieghart W, Somogyi P. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luque J M, Malherbe P, Richards J G. Brain Res Mol Brain Res. 1994;24:219–226. doi: 10.1016/0169-328x(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 34.Wafford K A, Bain C J, Whiting P J, Kemp J A. Mol Pharmacol. 1993;44:437–442. [PubMed] [Google Scholar]