Abstract
Sister chromatid cohesion is established during S phase near the replication fork. However, how DNA replication is coordinated with chromosomal cohesion pathway is largely unknown. Here, we report studies of fission yeast Ctf18, a subunit of the RFCCtf18 replication factor C complex, and Chl1, a putative DNA helicase. We show that RFCCtf18 is essential in the absence of the Swi1–Swi3 replication fork protection complex required for the S phase stress response. Loss of Ctf18 leads to an increased sensitivity to S phase stressing agents, a decreased level of Cds1 kinase activity, and accumulation of DNA damage during S phase. Ctf18 associates with chromatin during S phase, and it is required for the proper resumption of replication after fork arrest. We also show that chl1Δ is synthetically lethal with ctf18Δ and that a dosage increase of chl1+ rescues sensitivities of swi1Δ to S phase stressing agents, indicating that Chl1 is involved in the S phase stress response. Finally, we demonstrate that inactivation of Ctf18, Chl1, or Swi1-Swi3 leads to defective centromere cohesion, suggesting the role of these proteins in chromosome segregation. We propose that RFCCtf18 and the Swi1–Swi3 complex function in separate and redundant pathways essential for replication fork stabilization to facilitate sister chromatid cohesion in fission yeast.
INTRODUCTION
During the course of each cell cycle, the genome must be duplicated with a high degree of accuracy. However, environmental toxins or drugs cause DNA damage and impede the proper replication of chromosome DNA. To thwart this problem, eukaryotic cells are equipped with a DNA replication stress response pathway, termed the DNA replication checkpoint or S phase checkpoint (Boddy and Russell, 2001; Nyberg et al., 2002; Osborn et al., 2002). One of its major functions is to prevent the onset of mitosis. However, emerging evidence indicates that its most important activity is to stabilize replication forks by maintaining proper assembly of replisome components and DNA structures in replication competent states when forks stall (Lopes et al., 2001; Paciotti et al., 2001; Tercero and Diffley, 2001; Sogo et al., 2002; Tercero et al., 2003). In budding yeast treated with a DNA synthesis inhibitor, hydroxyurea (HU), failure to activate the replication checkpoint kinase Rad53 is associated with collapse and regression of replication forks and gross chromosomal rearrangements (Lopes et al., 2001; Tercero and Diffley, 2001; Kolodner et al., 2002; Sogo et al., 2002). In fission yeast, we have shown that Cds1, a Rad53 homologue, prevents fork collapse in response to HU (Noguchi et al., 2003), indicating that Cds1 is required for stabilization of replication forks in replication competent states. However, how Cds1 preserves stalled forks is largely unknown. Furthermore, the precise molecular mechanisms by which stalled forks activate the replication checkpoint are incompletely understood.
We have previously shown in fission yeast that the Swi1–Swi3 complex plays an important role in efficient activation of Cds1 (Noguchi et al., 2004). swi1Δ cells display replication fork collapse and a defect in recovery from replication fork arrest provoked by HU (Noguchi et al., 2003). Moreover, we have shown that Swi1-Swi3 travels with replication forks and is required to prevent accumulation of single-stranded DNA structures near the replication forks (Noguchi et al., 2004). Taken together, we have proposed that Swi1 and Swi3 form a “replication fork protection complex” (FPC) that is required for stabilization of stalled replication forks in a configuration that is recognized by replication checkpoint sensors (Noguchi et al., 2004).
The Swi1–Swi3 complex is evolutionarily conserved and is homologous to the Tof1–Csm3 complex in budding yeast and the Timeless–Tipin complex in humans (Gotter, 2003; Lee et al., 2004; Mayer et al., 2004; Noguchi et al., 2004). Tof1- Csm3 has been shown to be part of the replisome or the replisome progression complex (RPC) and involved in Rad53 activation (Katou et al., 2003; Calzada et al., 2005; Nedelcheva et al., 2005; Gambus et al., 2006). In humans, Timeless-Tipin interacts with Chk1 and ATR to control activation of checkpoint kinase Chk1 (Chou and Elledge, 2006; Gotter et al., 2007; Unsal-Kacmaz et al., 2007; Yoshizawa-Sugata and Masai, 2007). Interestingly, in Caenorhabditis elegans, Tim-1, a Swi1 homologue, has been suggested to be involved in chromosome cohesion (Chan et al., 2003), which is essential for accurate chromosome segregation and holds replicated sister chromatids together until they are ready to be separated at anaphase. Consistently, Saccharomyces cerevisiae csm3Δ mutants seem to have a mild defect in meiotic chromosome segregation (Rabitsch et al., 2001), and recent studies have reported a partial sister chromatid cohesion defect in tof1Δ and csm3Δ cells (Mayer et al., 2004; Warren et al., 2004). These findings suggest that protection of stalled replication forks may be essential for proper establishment of chromosome cohesion. Moreover, in S. cerevisiae, it has also been reported that some proteins involved in the S phase checkpoint or DNA replication are essential in mutants that have defects in the chromosomal cohesion pathway (Mayer et al., 2004; Warren et al., 2004; Skibbens, 2005). One of them is Ctf18/Chl12, a protein related to the Rfc1 subunit of replication factor C. In budding yeast, Ctf18 associates with Rfc2, Rfc3, Rfc4, and Rfc5 to form an alternative RFCCtf18 complex and functions redundantly with Rad24 in the DNA replication checkpoint in budding yeast (Hanna et al., 2001; Mayer et al., 2001; Naiki et al., 2001). RFCCtf18 associates with two additional subunits, Dcc1 and Ctf8, to form a heptameric complex in budding yeast and humans, and it has been shown to have proliferating cell nuclear antigen (PCNA) loading and unloading activity and to play a role in sister chromatid cohesion (Hanna et al., 2001; Mayer et al., 2001; Naiki et al., 2001; Ohta et al., 2002; Bermudez et al., 2003; Merkle et al., 2003; Petronczki et al., 2004; Shiomi et al., 2004; Bylund and Burgers, 2005), although how RFCCtf18 controls the proper establishment of cohesion is unclear.
There is emerging evidence showing a strong connection between DNA replication and sister chromatid cohesion (Skibbens, 2005). However, how DNA replication proteins actually facilitate the cohesion process is largely unknown. In this report, we describe the genetic interaction between Swi1-Swi3, RFCCtf18, and Chl1 in fission yeast. We show that these proteins are involved in the protection of stalled replication forks and proper sister chromatid cohesion. Our studies suggest that the fork stabilization mechanism plays a crucial role in regulating establishment of sister chromatid cohesion in fission yeast.
MATERIALS AND METHODS
General Techniques
The methods used for genetic and biochemical analyses of fission yeast have been described previously (Moreno et al., 1991; Alfa et al., 1993). Immunoblotting and UV sensitivity assay were performed as described in our previous study (Noguchi et al., 2004). Microscopic analyses of yellow fluorescent protein (YFP) and green fluorescent protein (GFP) were performed using an Olympus PROVIS AX70 microscope equipped with a SPOT RT camera model 2.3.1 (Diagnostic Instruments, Sterling Heights, MI). Images were acquired with OpenLab software (Improvision, Lexington, MA).
Gene Cloning, Plasmids, Primers, and Schizosaccharomyces pombe Strain Construction
The S. pombe strains used in this study were constructed using standard techniques (Alfa et al., 1993), and their genotypes are listed in Supplemental Table S1. The 3.6-kb swi1+ genomic fragment was amplified by EXtaq polymerase (TaKaRa, Ohtsu, Japan) and introduced into the SmaI site of pUC28, resulting in pUC28-Swi1. The swi1+ SacI–XbaI fragment was excised and transferred into the SacI/XbaI site of pDblet (Brun et al., 1995), resulting in pDblet-Swi1. Finally, the 2.86-kb ade6+ fragment was introduced into pDblet-swi1, resulting in pDblet-Swi1-Ade6. ctf18-5FLAG (ctf18-5FLAG-Kanr) and ctf18-TAP (ctf18-TAP-Kanr) were generated by a one-step polymerase chain reaction (PCR) method (Bähler et al., 1998) by using primers P532 and P533 to construct a 5xFLAG and a TAP tag at the C terminus of ctf18, respectively. chk1Δ (chk1::Kanr) was generated by a two-step PCR method (Krawchuk and Wahls, 1999) by using primers P534, P535, P538, and P539 to replace the chk1+ open reading frame with the Kanr gene. ctf18Δ (ctf18::Kanr) was generated by a two-step PCR method (Krawchuk and Wahls, 1999) by using primers P545, P547, P548, and P574 to replace the ctf18+ open reading frame with the Kanr gene. chl1Δ (chl1::Kanr) was generated by a two-step PCR method (Krawchuk and Wahls, 1999) by using primers P525, P526, P529, and P530 to replace the chl1+ open reading frame with the Kanr gene. chl1Δ (chl1::hph) was generated from the chl1::Kanr strain by a one-step marker switch method as described previously (Sato et al., 2005). ctf18Δ (ctf18::his3+) was generated by replacing the ctf18+ open reading frame with the his3+ fragment that was amplified using primers Ctf18-KO1 and Ctf18-KO2. rad3Δ (rad3::Kanr) was generated by transforming a rad3::ura4+ strain (Bentley et al., 1996) with the Kanr fragment amplified using primers UraKan-T1 and UraKan-B1. pFA6a-KanMX6 (Bähler et al., 1998), pFA6a-5FLAG-KanMX6 (Noguchi et al., 2004), and pFA6a-TAP-KanMX6 (Saitoh et al., 2002) were used as the templates for the PCR-base gene deletion and tagging. The primer sequences used in these procedures are listed in Supplemental Table S2. Mutations and epitope-tagged genes have been described previously for cdc25-22 (Fantes, 1979), swi1Δ (swi1::Kanr) (Noguchi et al., 2003), swi3Δ (swi3::Kanr) (Noguchi et al., 2004), cds1Δ (cds1::ura4+) (Boddy et al., 1998), chk1Δ (chk1::ura4+) (al-Khodairy et al., 1994), rad3Δ (rad3::ura4+) (Bentley et al., 1996), rad22-YFP (rad22-YFP-Kanr) (Noguchi et al., 2003), rad21-K1 (rad21-K1-ura4+) (Tatebayashi et al., 1998) rqh1Δ (rqh1::ura4+) (Stewart et al., 1997), nda3-KM311 (Hiraoka et al., 1984), and lys1+-lacO repeat his7+-dis1promoter-GFP-LacI-NLS (Ding et al., 2004).
Isolation of the ctf18+ and chl1+ Genes
The S. pombe ade6 mutants form red colonies due to accumulation of an adenine-intermediate-derived pigment. swi1Δ ade6-210 cells were transformed with a plasmid pDblet-Swi1-Ade6 that contains swi1+, ura4+, and ade6+ genes. Transformants formed white colonies in the absence of uracil, whereas they formed red or red-white sectored colonies on YES agar medium because of plasmid loss. These cells (2 × 108 cells) were washed in water, resuspended in sodium phosphate buffer, and treated with 3% ethyl methanesulfonate for 95 min at 30°C. The mutagenized cells were washed once in water and twice with 5% sodium thiosulfate and plated on YEA agar medium to allow cells to lose the plasmid pDblet-Swi1-Ade6. Strains that formed white colonies were further tested for sensitivity to 5-fluoroorotic acid (5-FOA). Only one strain, which we designated Y660, seemed to be dependent on the swi1+ (pDblet-Swi1-Ade6) plasmid for viability. Y660 was backcrossed twice with a swi1Δ strain, and we confirmed that Y660 contained a single mutation that is synthetically lethal with swi1Δ. To identify the mutated gene in Y660, we transformed this strain with S. pombe genomic library cloned into a pARS2004LEU2 vector. Transformed cells were plated on agar medium lacking leucine, incubated for 2 d at 30°C and replica-plated on agar medium containing 5-FOA followed by another 5-d incubation. Library derived genomic DNAs cloned into pARS2004LEU2 were then isolated from 5-FOA–resistant clones. Among four 5-FOA–resistant clones, two contained the swi1+ gene, other two contained the ctf18+ and SPAC3G6.11+ genes, respectively. As described in Results, SPAC3G6.11 seemed to be a homologue of CHL1 of budding yeast and Chl1 of humans.
Precipitation of Tandem Affinity Purification (TAP)-tagged Protein
Cells expressing the TAP and Myc fusion proteins at their own genomic loci were cultured in YES medium until an optical density of 1.2 at 600 nm was reached, and cells were collected. Cells were lysed with glass beads in lysis buffer A (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% NP-40, 10% glycerol, 50 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 5 mM N-methylmaleimide, 1 μM microcyctin, 0.1 μM okadaic acid, 0.2 mM p-4-amidoinophenyl-methane sulfonyl fluoride hydrochloride monohydrate [p-APMSF] and Roche Complete EDTA-free protease inhibitor cocktail [Roche Diagnostics, Basel, Switzerland]) by using a FastPrep cell disrupter (Qbiogene, Irvine, CA) for two cycles of 20 s at speed 6, with a 1-min interval on ice between the two cycles. Protein extracts were clarified by centrifugation at 13,000 rpm in an Eppendorf microcentrifuge 5415D for 10 min at 4°C, mixed with immunoglobulin G-Sepharose beads (GE Healthcare, Piscataway, NJ) and incubated for 2 h at 4°C. The Sepharose beads were collected and washed three times in lysis buffer A. Proteins associated with the beads were analyzed by immunoblotting. TAP and Myc fusion proteins were probed with the anti-c-Myc 9E10 monoclonal antibody (Covance, Berkeley, CA) and peroxidase anti-peroxidase (Sigma-Aldrich, St. Louis, MO), respectively.
Cds1 Kinase Assay
Cds1 kinase assay was performed essentially as described previously (Lindsay et al., 1998). Exponentially growing cells were washed in STOP buffer (150 mM NaCl, 50 mM NaF, 10 mM EDTA, and 1 mM NaN3) and lysis buffer B (50 mM Tris, pH 7.5, 80 mM β-glycerophosphate, 250 mM NaCl, 15 mM nitrophenylphosphate, 50 mM NaF, 5 mM EDTA, 1 mM dithiothreitol [DTT], and 0.1% NP-40 supplemented with protease inhibitor cocktail [Complete EDTA-free protease inhibitor cocktail; Roche Diagnostics], and p-APMSF). Protein extract was prepared as described in the previous section and incubated at 4°C for 90 min with 20 μl of protein A agarose (Sigma-Aldrich) preincubated with the Cds1 antibody. The protein A-agarose beads were washed three times each with lysis buffer B and kinase buffer (10 mM HEPES, pH 7.5, 75 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, and 1 mM DTT). The immunocomplex containing Cds1 bound on protein A-agarose beads was incubated with 10 μl of 2X kinase buffer, 5 μCi of [γ-32P]ATP, 0.2 μl 10 mM ATP, and 0.5 μl of 10 mg/ml myelin basic protein (MBP; Cds1 substrate) at 30°C for 15 min. The reaction was stopped by the addition of 25 μl of 2X SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer and subsequent boiling for 5 min. MBP was separated on 15% polyacrylamide gels and detected by Coomassie Brilliant Blue staining. The gel was dried, and radioactivity incorporated in MBP was detected with a Storm 840 machine (GE Healthcare). After imaging, the radioactivity levels (cpm) of MBP were determined in an LS6500 liquid scintillation counter (Beckman Coulter, Fullerton, CA).
Detection of Rad22-YFP DNA Repair Foci by Fluorescent Microscopy
Cells expressing Rad22-YFP from its own promoter were grown at 25°C in YES liquid medium until mid-log phase. We have used 25°C to obtain stronger YFP signal. Cells were concentrated by centrifugation and kept on ice before microscopic analysis. Rad22-YFP localization was analyzed and imaged as described in General Techniques. Quantification of Rad22-YFP foci has been performed at least four times, and at least 200 cells were counted for each strain in each experiment. The cell cycle position of cells containing Rad22-YFP foci was estimated by analyzing cell length, nuclei number and position, and the presence of a division plate.
Pulsed Field Gel Electrophoresis (PFGE)
Exponentially growing cells were treated with 12 mM HU for 4 h at 30°C, and then they were washed and released into fresh media. Cells were collected at the indicated times at a concentration of 2.5 × 108 and washed in 20 ml of CSE (20 mM citric acid, 20 mM sodium phosphate [Na2HPO4·7H2O], adjusted to pH 5.6, 1.2 M sorbitol, and 40 mM EDTA, pH 8.0). Cell pellets were suspended in 1 ml of CSE + 1 mg/ml Zymolase 100T and incubated at 37°C for 2 h. Cells were resuspended to a concentration of 8 × 108 cells/ml in 300 μl of TSE (10 mM Tris-HCl, pH 7.5, 0.9 M sorbitol, and 45 mM EDTA, pH 8.0). Cell suspension was warmed to 42°C and mixed with 300 μl of 1.1% low melting temperature agarose in TSE. Aliquots were dispensed into plug molds and allowed to solidify at 4°C, and then they were suspended in 3 ml of Tris-EDTA-SDS (0.25 M EDTA, pH 8.0, 50 mM Tris-HCl, pH 7.5, and 1% SDS) and incubated at 55°C for 90 min. Plugs were then incubated at 55°C for 48 h in 3 ml of NDS (10 mM Tris-HCl, 0.5 M EDTA, pH 8.0, pH adjusted to 9.5, and 1% lauryl sarcosine) supplemented with 1 mg/ml proteinase K (Invitrogen, Carlsbad, CA). Plugs were equilibrated in 1 ml of TE and stored in 5 ml of 0.5 M EDTA at 4°C. To analyze chromosome DNA embedded in plugs, the plugs were equilibrated in TE and run on 0.8% Megabase agarose gel (Bio-Rad, Hercules, CA) in 1X TAE by using a CHEF-DR II system (Bio-Rad) at the following settings: block 1, 2 V/cm, initial and final switch time of 1800 s; 14°C; and pump speed, 70, for 72 h. Gels were stained with 0.5 μg/ml ethidium bromide in H2O for 30 min and destained with water for 1–2 h.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed essentially as described previously (Noguchi and Noguchi, 2007). Briefly, S. pombe cells (5 × 108) were fixed in 1% formaldehyde for 20 min at room temperature, and then they were quenched in 125 mM glycine for 5 min. Cells were washed in Tris-buffered saline and disrupted in lysis buffer (50 mM HEPES-KOH, pH 7.5, 140 mM NaCl, 1 mM EDTA, and 1% Triton X-100) supplemented with protease inhibitors ~0.2 mM p-APMSF and Roche protease inhibitor cocktail). The broken cells were sonicated six times for 20 s each with a Misonix Sonicator 3000 until chromatin DNA was sheared into 500- to 700-base pair fragments. Cell lysate was clarified by two rounds of maximum speed centrifugation in an Eppendorf 5415C microcentrifuge at 4°C. Immunoprecipitations were performed in these cell extracts using anti-FLAG M2 agarose (Sigma-Aldrich). PCR amplification conditions and the specific primers used in these studies have also been described previously (Ogawa et al., 1999).
Chromosome Cohesion Assay
We used a strain harboring bacterial LacO tandem repeat sequences inserted in the vicinity of the centromere on chromosome I (Ding et al., 2004). This strain is engineered to express the LacI repressor fused to GFP-nuclear localization signal (NLS), which is recruited to LacO repeat sequences, allowing us to visualize the centromere I (Ding et al., 2004). Cohesion assays were performed using the following three conditions. 1) Cells were grown to exponential phase in liquid YES medium at 25°C, synchronized in early S phase in the presence of 10 mM HU for 2 h, and released into medium containing 100 μg/ml thiabendazole (TBZ). At the indicated time, GFP foci were monitored and imaged as described in General Techniques. 2) cdc25-22 temperature-sensitive cells were grown to mid-log phase at 25°C and synchronized at the G2-M transition at 33°C for 3 h. Cells were then released at 25°C into medium containing 100 μg/ml TBZ. At the indicated time, GFP foci were monitored and imaged. 3) nda3-KM311 cold-sensitive cells were grown to mid-log phase at 30°C and shifted to a restrictive temperature, 20°C. At the indicated time, GFP foci were monitored and imaged. Quantification of GFP foci has been performed at least three times, and at least 200 cells were counted for each strain in each experiment.
RESULTS
Synthetic Lethal Genetic Interactions Involving RFCCtf18, Chl1, and Swi1-Swi3
To further understand the role of the Swi1–Swi3 replication fork protection complex in genomic integrity, we carried out a genetic screen to isolate mutations that are synthetically lethal with deletion of swi1+ (see Materials and Methods). This screen identified one mutant strain that was inviable in a swi1Δ background. Therefore, we designated the mutation carried by the isolated strain lws1-1 (lethal with swi1 deletion). To isolate dosage suppressors of lws1-1 swi1Δ lethality, lws1-1 swi1Δ double mutant cells harboring a swi1+-ura4+ plasmid was transformed with an S. pombe genomic library. We sought clones that could grow in the presence of 5-fluoroorotic acid, a compound that forces the loss of a swi1+-ura4+ plasmid via counterselection of ura4+ cells. This screen then identified plasmids containing swi1+, SPAC3G6.11+, or ctf18+ as dosage suppressors of the lethality of lws1-1 swi1Δ. Our genetic analyses found that the lws1-1 was not allelic to either of SPAC3G6.11+ or ctf18+. Identify of lws1-1 is currently under investigation. Because SPAC3G6.11 had not been characterized in S. pombe, we performed a BLAST search by using SPAC3G6.11 open reading frame as the query protein sequence and found that SPAC3G6.11 is highly homologous to the DEAD box DNA helicase Chl1 in humans (E-value of 1 × 10−143) (Amann et al., 1996) and budding yeast (E-value of 2 × 10−126) (Gerring et al., 1990). Therefore, we named the SPAC3G6.11 gene as S. pombe chl1+.
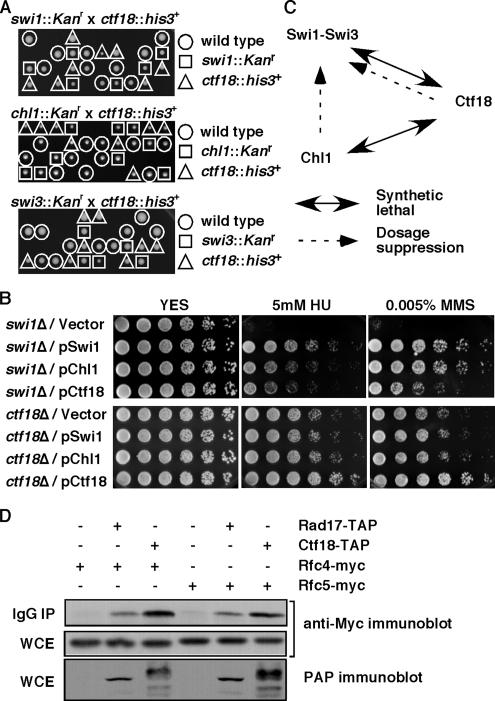
To understand the relationship among the genes identified in our screen, we performed tetrad analyses to examine genetic interactions of these genes. As shown in Figure 1A, none of the swi1Δ ctf18Δ and chl1Δ ctf18Δ double mutant strains grew after tetrad analyses, whereas swi1Δ chl1Δ double mutants showed a normal growth comparable to swi1Δ mutants (Figure 6B and Table 1). These data established that ctf18Δ is synthetically lethal with swi1Δ or chl1Δ. We have noticed that spore viability is somewhat low in swi1Δ or swi3Δ crosses (Figure 1A). This might be due to improper chromosome segregation during meiosis, because C. elegans Tim-1, a homologue of Swi1, has been suggested to play an important role in proper chromosome segregation by regulating both mitotic and meiotic sister chromatid cohesion (Chan et al., 2003). We also found that swi3Δ showed similar genetic interaction with chl1Δ and ctf18Δ (Figure 1A and Table 1), indicating that RFCCtf18 plays an essential role for cell survival in the absence of the Swi1–Swi3 complex. Because Swi1-Swi3 is required for the stabilization of replication forks and activation of the replication checkpoint (Noguchi et al., 2004), we have hypothesized that RFCCtf18 is also involved in these cellular mechanisms in fission yeast. Therefore, in this report, we investigated this possibility.
Figure 1.
Genetic interaction involving Swi1-Swi3, RFCCtf18, and Chl1. (A) None of the viable spores from swi1::Kanr x ctf18::his3+, chl1::Kanr × ctf18::his3+, and swi3::Kanr × ctf18::his3+ crosses were able to grow on YES medium containing G-418 and EMM2, Edinburgh minimal media lacking histidine, indicating that swi1Δ ctf18Δ, chl1Δ ctf18Δ, and swi3Δ ctf18Δ cells are inviable. Genotypes of viable spores are shown. Representative images of >40 tetrad dissections from each cross are shown. (B) Damage sensitivities of swi1Δ were suppressed by an increased gene dosage of chl1+ or ctf18+. swi1Δ or ctf18Δ cells were transformed with the indicated plasmid and plated on YES medium containing no drug (YES), 5 mM HU, or 0.005% MMS. Fivefold serial dilution of cells were plated and incubated for 2–3 d at 32°C. Representative images of repeat experiments are shown. (C) Summary of genetic interaction involving Swi1-Swi3, RFCCtf18, and Chl1. (D) Ctf18 associates with Rfc4 and Rfc5. Protein extracts were prepared from cells expressing the indicated fusion proteins. Ctf18-TAP was precipitated and probed with anti-myc antibodies. Rfc4-Myc and Rfc5-Myc are shown to be equally expressed in each cell line (whole-cell extract, WCE). As a positive control, Rad17-TAP is shown to associate with Rfc4 and Rfc5. IgG IP, precipitated fraction.
Figure 6.
Chl1 is involved in an S phase response pathway. (A and B) For drug sensitivity assays, fivefold serial dilution of cells were incubated on YES agar medium supplemented with the indicated amounts of MMS, CPT, HU, or TBZ for 2–4 d at 32°C. For UV survival assays, fivefold serial dilution of cells of the indicated genotypes were spotted onto YES agar medium and exposed to the indicated doses of UV irradiation. The plates were then incubated for 2–3 d at 32°C. Representative images of repeat experiments are shown.
Table 1.
Synthetic genetic interaction described in this study
| Genotype | Phenotype | Source |
|---|---|---|
| swi1Δ ctf18Δ | Lethal | This study |
| swi3Δ ctf18Δ | Lethal | This study |
| swi1Δ chl1Δ | No apparent phenotypic enhancement | This study |
| swi3Δ chl1Δ | No apparent phenotypic enhancement | This study |
| chl1Δ ctf18Δ | Lethal | This study |
| swi1Δ rad21-K1 | Lethal | This study |
| ctf18Δ rad21-K1 | Phenotypic enhancement | This study |
| chl1Δ rad21-K1 | Phenotypic enhancement | This study |
| swi1Δ rqh1Δ | Severe growth defect | Noguchi et al. (2003) |
| swi3Δ rqh1Δ | Lethal | This study |
| ctf18Δ rqh1Δ | Phenotypic enhancement | This study |
| ctf18Δ cds1Δ | No apparent increase in UV and HU sensitivity | This study |
| Phenotypic enhancement in MMS and CPT sensitivity | This study | |
| ctf18Δ chk1Δ | Phenotypic enhancement in UV and HU sensitivity | This study |
| Phenotypic enhancement in MMS and CPT sensitivity | This study | |
| ctf18Δ rad3Δ | No apparent increase in UV and HU sensitivity | This study |
| Phenotypic enhancement in MMS and CPT sensitivity | This study | |
| chl1Δ cds1Δ | No apparent increase in MMS, CPT, and TBZ sensitivity | This study |
| chl1Δ chk1Δ | Phenotypic enhancement in MMS and CPT sensitivity | This study |
| chl1Δ rad3Δ | Phenotypic enhancement in MMS, CPT, and TBZ sensitivity | This study |
Inactivation of Swi1 is known to render cells sensitive to HU and methylmethane sulfonate (MMS) (Noguchi et al., 2003; Sommariva et al., 2005). HU depletes the dNTP pool and inhibits DNA synthesis, whereas MMS introduces alkylation to template DNA and also causes an arrest of replication fork progression. To further characterize genetic interactions between the three genes, we examine whether HU and MMS sensitivity of swi1Δ is suppressed by a multicopy vector carrying swi1+, ctf18+, or chl1+ (Figure 1B). MMS sensitivity of swi1Δ cells was greatly suppressed by a chl1+ plasmid and significantly by a ctf18+ plasmid. In fact, the suppression conferred by the chl1+ plasmid was comparable to the suppression by the swi1+ plasmid. In contrast, HU sensitivity of swi1Δ cells was significantly suppressed both by chl1+ and ctf18+, but it was not as dramatic as the suppression by swi1+ plasmid. These results suggest that RFCCtf18 and Chl1 are both involved in mechanisms that are important for proper cellular responses to S phase stress. We have also examined whether ctf18Δ phenotypes can be suppressed by an increased dosage of swi1+ or chl1+ (Figure 1B). As described later, ctf18Δ cells showed weak sensitivity to HU and MMS. Unlike the case for swi1Δ cells, HU and MMS sensitivity of ctf18Δ cells were not significantly suppressed by swi1+ or chl1+ dosage increases (Figure 1B). Results of our genetic interaction analyses are summarized in Figure 1C, and Tables 1 and Table 2. Because chl1Δ is synthetically lethal with ctf18Δ but not with swi1Δ, and Chl1 overexpression can suppress MMS and HU sensitivity of swi1Δ but not ctf18Δ, our data are consistent with the notion that Chl1 is closely involved in the S phase stress response pathway regulated by Swi1. In contrast, the role of Ctf18 in the S phase stress response may represent a mechanism that is independent of but partially redundant with the mechanisms regulated by the Swi1–Swi3 complex.
Table 2.
Dosage suppression described in this study
| Mutant | Multicopy plasmid | Degree of suppressiona |
||
|---|---|---|---|---|
| HU | MMS | TBZ | ||
| swi1Δ | Vector | − | − | − |
| swi1Δ | pSwi1 | ++ | ++ | ++ |
| swi1Δ | pChl1 | + | ++ | + |
| swi1Δ | pCtf18 | + | + | + |
| ctf18Δ | Vector | − | − | − |
| ctf18Δ | pSwi1 | − | − | − |
| ctf18Δ | pChl1 | − | − | ++ |
| ctf18Δ | pCtf18 | ++ | ++ | ++ |
a ++, strong suppression; +, partial suppression; and −, no suppression.
Ctf18 Associates with Rfc4 and Rfc5 in S. pombe Cell Extract
Ctf18 is related to the replication factor C subunit Rfc1 and is known to form the RFCCtf18 complex together with Rfc2, Rfc3, Rfc4, Rfc5, Ctf8, and Dcc1 in budding yeast. RFCCtf18 has been shown to have a role in PCNA loading or unloading in budding yeast and humans and play an important role during sister chromatid cohesion and the replication checkpoint (Hanna et al., 2001; Mayer et al., 2001; Naiki et al., 2001; Ohta et al., 2002; Bermudez et al., 2003; Merkle et al., 2003; Petronczki et al., 2004; Shiomi et al., 2004; Bylund and Burgers, 2005). In S. pombe, ctf18+, ctf8+, or dcc1+ has been shown to be essential in the absence of functional RFCRfc1 (Kim et al., 2005), also suggesting the role of RFCCtf18 in PCNA loading. However, how RFCCtf18 controls these mechanisms is poorly understood. To address whether Ctf18 forms a similar complex in S. pombe, we generated an S. pombe strain that expresses the Ctf18–TAP fusion protein at endogenous levels from the ctf18+ promoter. This strain is engineered to express Rfc4-13myc or Rfc5-13myc from their genomic loci to determine the interaction between Ctf18 and Rfc subunits. These tagged alleles had no apparent effect on cell viability or growth. Ctf18-TAP was precipitated from S. pombe cell extracts by using immunoglobulin (Ig)G-Sepharose, and precipitates were then probed with anti-myc antibody. As shown in Figure 1D, both Rfc4-13myc and Rfc5-13myc copurified with Ctf18-TAP, indicating that RFCCtf18 complex exists in S. pombe. Another alternative RFC complex involving Rad17 served as a positive control for coprecipitation with Rfc4 and Rfc5 (Figure 1D). Although Rfc2 and Rfc3 were not tested in this current report, it is likely that these proteins also bind Ctf18 to form the RFCCtf18 complex.
Ctf18 Is Required for Survival after UV- or HU-induced Fork Arrest and for Proper Activation of Replication Checkpoint Kinase Cds1
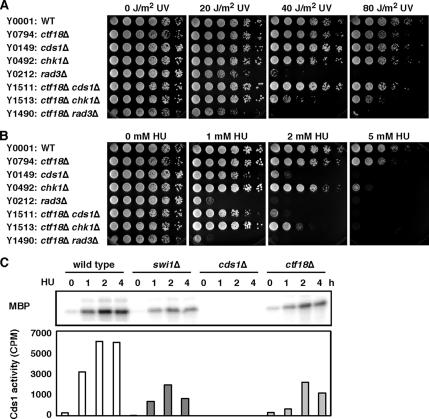
Ctf18 is shown to have some redundant roles with Rad24 in the DNA replication checkpoint in budding yeast (Naiki et al., 2001). Therefore, the genetic interactions involving RFCCtf18 and Swi1-Swi3 suggest that RFCCtf18 plays an important role, not only in the activation of the replication checkpoint, but also in the stabilization of replication forks in fission yeast. To address this possibility, we first examined whether Ctf18 is involved in tolerance of fork arrest. To induce replication fork arrest, we used UV irradiation, which creates DNA lesions that arrest forks (Friedberg et al., 1995), and hydroxyurea to inhibit ribonucleotide reductase and deplete dNTP pools (Boddy and Russell, 2001; Nyberg et al., 2002; Osborn et al., 2002). ctf18Δ cells were weakly sensitive to both UV and HU (Figure 2, A and B), suggesting that Ctf18 plays a role in cellular tolerance of fork arrest.
Figure 2.
Ctf18 is involved in the Cds1-dependent replication checkpoint. (A and B) Synergistic interaction of ctf18Δ and chk1Δ in UV and HU survival assays indicates that Ctf18 is required for cellular tolerance to fork arrest. For UV survival assays, fivefold serial dilution of cells were plated on YES agar medium and exposed to the indicated doses of UV. Agar plates were then incubated for 2–3 d at 32°C. For HU sensitivity assays, fivefold serial dilution of cells were incubated on YES agar medium supplemented with the indicated amounts of HU for 2–4 d at 32°C. Representative images of repeat experiments are shown. (C) Cds1 activation is strongly reduced in ctf18Δ cells. Cells of the indicated genotypes were incubated in YES liquid medium supplemented with 12 mM HU for 0, 1, 2, and 4 h at 30°C. Kinase activity of immunoprecipitated Cds1 was measured using MBP as a substrate. The radiolabeled MBP was detected after gel electrophoresis (top). The radioactivity levels (cpm) of MBP were then determined in a liquid scintillation counter (bottom). Representative results from repeat experiments are shown.
Next, we performed epistasis analysis between ctf18Δ and deletion mutations of checkpoint kinases Chk1 and Cds1 in a UV-survival assay. The ctf18Δ chk1Δ cells were substantially more sensitive than either single mutant (Figure 2A and Table 1). In contrast, there was no significant genetic interaction between ctf18Δ and cds1Δ in UV-survival assays (Figure 2A and Table 1). We also examined the effect of inactivating Ctf18 in chk1Δ and cds1Δ backgrounds in an HU-survival assay. Again, HU sensitivity of ctf18Δ cells was further enhanced by chk1 deletion (Figure 2B and Table 1), the effector kinase of the G2-M DNA damage checkpoint. However, there was no significant genetic interaction between ctf18Δ and cds1Δ in HU survival assay (Figure 2B and Table 1). Therefore, these results suggest that UV- or HU-induced stalled fork structures accumulated in ctf18Δ mutants are converted to different fork forms that activate the Chk1-dependent G2-M DNA damage checkpoint pathway. Similar results have been reported for inactivation of Swi1 and Swi3, components of the replication fork protection complex (Noguchi et al., 2003; Noguchi et al., 2004).
Cds1 and Chk1 define redundant pathways of checkpoint activation in response to fork arrest (O'Connell et al., 2000; Rhind and Russell, 2000a, b; Boddy and Russell, 2001; Nyberg et al., 2002). Because both Cds1 and Chk1 pathways are controlled by the Rad3 kinase, we examined genetic interaction between ctf18Δ and rad3Δ in UV- and HU-survival assays. As shown in Figure 2, A and B, rad3Δ and ctf18Δ rad3Δ cells were similarly sensitive to UV and HU. Thus, the strong genetic interaction in HU- or UV-survival assays involving ctf18Δ and chk1Δ (Figure 2, A and B, and Table 1) suggests that Ctf18 is involved in activation of the replication checkpoint enforced by Cds1. To test this possibility, we measured Cds1 kinase activity in wild-type and ctf18Δ cells treated with HU. As shown in Figure 2C, HU treatment induced a robust activation of the Cds1 kinase. However, Cds1 activation was strongly decreased in ctf18Δ cells (Figure 2C). A similar defect in Cds1 activation was observed in swi1Δ cells (Figure 2C) (Noguchi et al., 2003), supporting the idea that Ctf18 is involved in the activation of the Cds1 replication checkpoint kinase. The residual Cds1 activation observed in swi1Δ or ctf18Δ cells might be due to a redundant requirement of Swi1 and Ctf18 in the activation of Cds1. However, we were unable to test this possibility directly because swi1Δ ctf18Δ cells are not viable (Figure 1A).
Ctf18 Is a Component of Checkpoint-independent S Phase Stress Response Pathways to Alkylation Damage and Replication Fork Breakage
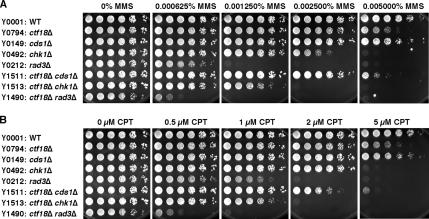
To understand the role of Ctf18 in the S phase DNA damage response, we examined the sensitivity of ctf18Δ cells to MMS, which lead to alkylation damage that interferes with DNA replication. Cells repair these damages by cell cycle checkpoint, postreplication repair, recombination repair, and base excision repair pathways (Xiao et al., 1996; Chang et al., 2002). ctf18Δ cells showed significant sensitivity to 0.005% MMS (Figure 3A). As is the case for HU- and UV-sensitivity assays, this sensitivity was further enhanced by Chk1 inactivation (Figure 3A and Table 1), suggesting that ctf18Δ cells accumulate abnormal DNA structures that must be repaired by the Chk1-dependent G2-M checkpoint. Unlike the situation upon UV exposure, there was a reproducible synergistic interaction between ctf18Δ and cds1Δ at 0.005% MMS (Figure 3A). This genetic interaction suggests that Ctf18 has a Cds1-independent role in tolerance of alkylation damage. In addition, our analyses showed that ctf18Δ rad3Δ cells were much more sensitive to MMS than either single mutant (Figure 3A and Table 1). rad3Δ mutants are defective for the activation of both Cds1 and Chk1, thereby lacking checkpoint-dependent cell cycle arrest in response to replication block or DNA damage (O'Connell et al., 2000; Rhind and Russell, 2000b; Boddy and Russell, 2001; Nyberg et al., 2002). Therefore, the synergistic interaction between ctf18Δ and rad3Δ in MMS sensitivity assays suggests that Ctf18 has an important function that is independent of cell cycle checkpoints, and this function may contribute to the recovery from alkylation damage that causes stalled replication forks and other lesions.
Figure 3.
Ctf18 constitutes a checkpoint-independent S phase DNA damage response pathway. (A and B) Fivefold serial dilution of cells were incubated on YES agar medium supplemented with the indicated amounts of MMS or CPT for 2–4 d at 32°C. Representative images of repeat experiments are shown.
To further examine the role of Ctf18 in the S phase DNA damage response, we introduced replication fork breakage during S phase by exposure to camptothecin (CPT), a drug that traps topoisomerase I on DNA (Pommier, 2006). ctf18Δ cells showed significant sensitivity to CPT (Figure 3B), suggesting that Ctf18 has an important role in the tolerance of DNA damage during S phase. As with MMS, ctf18Δ cds1Δ, ctf18Δ chk1Δ, and ctf18Δ rad3Δ double mutant cells showed stronger CPT sensitivity than either single mutant (Figure 3B and Table 1), indicating that Ctf18's role in recovery from fork breakage during S phase cannot solely be accounted for by the defect in cell cycle checkpoint controls. Together, our results indicate that Ctf18 constitutes an S phase DNA damage response pathway that is independent of checkpoints.
Replication Abnormalities in ctf18Δ Cells
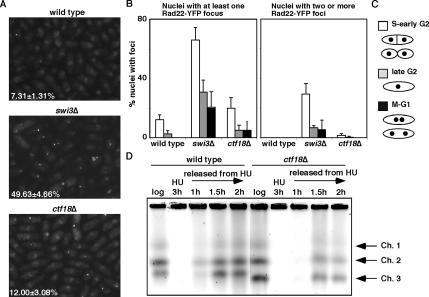
Our data thus far demonstrate that Ctf18 plays an important role in cell survival after fork arrest or damage. Importantly, the genetic interaction involving ctf18Δ and rad3Δ (Figure 3) has suggested that Ctf18 has a checkpoint-independent function during the S phase stress response. Because Ctf18 is essential in cells defective for the replication fork protection complex that consists of Swi1 and Swi3 (Figure 1), we speculated that Ctf18 has a critical role in stabilization of replication forks in a manner independent of Swi1-Swi3. In previous studies, we have shown that swi1Δ or swi3Δ cells accumulate spontaneous DNA damage near replication forks (Noguchi et al., 2004). Therefore, we investigated whether ctf18Δ cells also accumulate DNA damage in the absence of genotoxic agents. ctf18Δ cells were engineered to express Rad22-YFP from its genomic locus. Rad22 is a homologue of budding yeast Rad52 and shown to bind single-stranded DNA (ssDNA) during homologous recombination and at double-strand breaks and other sites that have an exposed ssDNA segment, leading the formation of Rad22-YFP DNA repair foci at the site of DNA damage (Ostermann et al., 1993; Kim et al., 2000; Noguchi et al., 2003). As reported previously (Noguchi et al., 2004), a dramatic increase in spontaneous Rad22-YFP foci was detected in swi3Δ cells (49.63 ± 4.66% of total swi3Δ nuclei compared with 7.31 ± 1.31% of wild-type nuclei) (Figure 4A). A significant increase in Rad22-YFP foci was also observed in ctf18Δ cells (12.00 ± 3.08% of ctf18Δ nuclei) (Figure 4A). Particularly, we reproducibly found that ctf18Δ cells but not wild-type cells contained nuclei with multiple Rad22 foci (Figure 4B). These data indicate that both swi3Δ and ctf18Δ cells accumulate spontaneous DNA damage although a smaller number of ctf18Δ cells displayed Rad22-YFP foci. To address whether Rad22 foci arose from replication abnormalities, the cell cycle position of cells containing Rad22-YFP foci was evaluated (Figure 4B). Cell cycle position in fission yeast can be estimated by noting cell length, nuclear morphology, and the appearance of septum as shown in Figure 4C. This analysis demonstrated that Rad22-YFP foci formed predominantly in S or early G2 phase (Figure 4B). Importantly, we found that accumulation of Rad22-YFP foci in ctf18Δ cells was specifically noticeable in S phase, suggesting that replication fork abnormality causes spontaneous DNA damage in ctf18Δ cells. After S phase, the number of foci then decreased throughout the cell cycle. Similar results have been obtained with swi1Δ and swi3Δ cells (Figure 4B) (Noguchi et al., 2003, 2004).
Figure 4.
Ctf18 is involved in stabilization of replication forks. (A) Rad22-YFP foci formation was significantly elevated in ctf18Δ cells. Cells of the indicated genotype expressing genomic Rad22-YFP were grown in YES medium at 25°C until mid-log phase. The percentages of nuclei with at least one Rad22-YFP focus are shown. The standard deviations were obtained from four independent experiments (B) Quantification of Rad22-YFP foci according to cell cycle stages. S and early G2 cells had the most Rad22-YFP foci. The percentages of nuclei that have at lest one focus or harbor two or more foci are shown. At least 200 cells were counted for each strain. Error bar corresponds to the standard deviation obtained from four independent experiments. (C) Schematic drawing for nuclear and morphological changes during the S. pombe cell cycle. (D) Ctf18 is required for the efficient resumption of replication after fork arrest. Chromosome samples from either wild-type or ctf18Δ cells were examined by PFGE. Cells were grown until mid-log phase and then incubated in the presence of 12 mM HU for 3 h at 30°C. Cells were then washed and released into fresh medium. Chromosomal DNA samples were prepared at the indicated times. Representative results from repeat experiments are shown.
To further address the role of Ctf18 in the stabilization of replication forks, we examined the recovery of DNA replication after fork arrest provoked by HU exposure. Chromosome samples of wild-type and ctf18Δ cells were prepared before (log) and at 3 h after HU treatment, and also at different time points during recovery after the removal of HU (Figure 4D). These chromosomes were then resolved by PFGE, which allows only a fully replicated chromosome to enter the gel. Chromosomes from exponentially growing cells (log) in both wild-type and ctf18Δ migrated into the gel, indicating that ctf18Δ cells have no significant defect in replicating DNA (Figure 4D, log). HU treatment caused an arrest of DNA synthesis, leading to the reduction in the amount of chromosomes migrated into the gel in both wild-type and ctf18Δ cells (Figure 4D, HU 3 h). When cells are released into fresh medium without HU, chromosomes from wild-type cells entered into the gel at 1 h after the HU removal due to the completion of DNA synthesis. However, chromosomes from ctf18Δ cells did not migrate at this time point and displayed a reduced capacity to enter the gel at either 1.5 or 2 h during recovery (Figure 4D). We also performed PFGE of chromosomes prepared from swi1Δ cells and found that swi1Δ cells show even more severe delay in recovery of DNA replication after fork arrest (Supplemental Figure S1). These data are consistent with our aforementioned results that swi1Δ cells had a greater number of Rad22-YFP foci and stronger HU sensitivity compared with ctf18Δ cells (Figures 1B and 4, A and B). We have also observed that ctf18Δ cells harbor a shorter chromosome III that contains ribosomal DNA repeats in S. pombe. We and other groups have reported similar findings in a number of mutants defective for replication and replication fork stabilization including swi1Δ, swi3Δ, and sap1-48 (Supplemental Figure S1) (Sommariva et al., 2005; Noguchi and Noguchi, 2007). Taken together, the fact that ctf18Δ cells accumulate DNA damage during S phase, are delayed in recovering from replication arrest, and are sensitive to fork damaging agents, we conclude that Ctf18 is involved in the stabilization of replication forks.
We have previously shown that Swi1-Swi3 is required for the stabilization of replication forks and that swi1Δ or swi3Δ shows strong synthetic genetic interaction with rqh1Δ (Table 1) (Noguchi et al., 2003, 2004). Rqh1, RecQ-like DNA helicase, and its S. cerevisiae orthologue Sgs1, are thought to be required for the stabilization of replication forks (Doe et al., 2002; Cobb et al., 2003; Bjergbaek et al., 2005). Therefore, to understand pathways involved in fork stabilization or S phase stress response, we examined genetic interaction between rqh1Δ and ctf18Δ. rqh1Δ cells showed significant sensitivity to MMS and CPT (Supplemental Figure S2A). rqh1Δ ctf18Δ double mutant cells were much more sensitive to MMS and CPT than either single mutant cells (Table 1 and Supplemental Figure S2A). Together with the fact that swi1Δ or swi3Δ has strong genetic interaction with rqh1Δ (Table 1), we conclude that Swi1-Swi3, Ctf18, and Rqh1 play separate but redundant roles in the stabilization of replication forks.
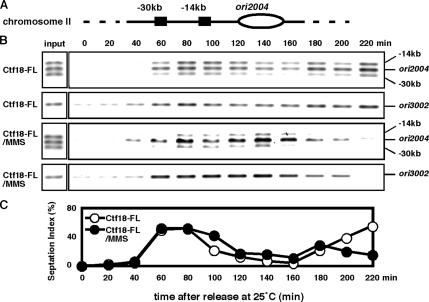
Ctf18 Is Recruited to Chromatin during S Phase
In budding yeast, Ctf18 has been shown to be recruited to replication forks in response to HU, although whether Ctf18 is localized at replication forks in the absence of genotoxic agents is unknown (Lengronne et al., 2006; Ogiwara et al., 2007). Therefore, we examined whether Ctf18 is associated with chromatin in unperturbed fission yeast cells. The cdc25-22 strain was engineered to express Ctf18-5FLAG via its endogenous promoter. ctf18-5FLAG strains showed normal growth rate and no detectable sensitivity to UV, HU, MMS, or CPT, indicating that Ctf18-5FLAG is functional (data not shown). The cdc25-22 allele was used to synchronize cells at the G2-M boundary. The localization of Ctf18-5FLAG was monitored by ChIP analysis at the well-characterized replication origin 2004 (ori2004) and at two positions 14 and 30 kb away on S. pombe chromosome II (Figure 5A). On release from the cdc25-22 arrest, Ctf18-5FLAG was observed to strongly associate with the ori2004 region at 60 min, which subsequently declined between 120 and 160 min and increased again at 180 min (Figure 5B). We also examined septation, which in fission yeast occurs in S phase, to monitor cell cycle progression (Figure 5C). The level of Ctf18 association with the ori2004 region was found to correlate with an increase in the septation index, which also coincided with the onset of S phase, and this association was found to decline as the septation index decreased (Figure 5, B and C), indicating that Ctf18 tightly associates with the ori2004 region during unperturbed S phase. Similar association of Ctf18 with chromatin was observed at the ori3002 region (Figure 5B), another active replication origin (Dubey et al., 1996), indicating that Ctf18 interacts with chromatin during DNA replication. A weaker association of Ctf18 with chromatin was also observed at 14- and 30-kb positions near ori2004 (Figure 5B). It should be noted that similar chromatin association has been reported with Mcm6, a component of putative replicative DNA helicase in S. pombe. Therefore, our results suggest that Ctf18 is involved in DNA replication in unperturbed cells. To further understand the role of Ctf18 in S phase response pathways, we performed ChIP assays of Ctf18-5FLAG in the presence of MMS. As shown in Figure 5B, Ctf18-5FLAG was observed to associate with ori2004 and ori3002 as the septation index increase. This association seemed to be slightly stronger than that in unperturbed cells and persisted throughout extended S phase due to the MMS treatment, further strengthening our conclusion that Ctf18 tightly associate with chromatin during S phase. This result also suggests that the association of Ctf18 with chromatin plays an important role in the S phase stress response.
Figure 5.
Ctf18 associates with chromatin during S phase. (A) Diagram of the ori2004 region on S. pombe chromosome II used in ChIP is shown. (B) ChIP assay of Ctf18-5FLAG were performed at ori2004, at sites located 14 or 30 kb away from this origin and at ori3002 as indicated. cdc25-22 cells were synchronized at the G2-M boundary by incubation at 36°C for 4 h and then released into fresh YES medium containing 0 or 0.03% MMS at 25°C as indicated. ChIP assays were performed at the indicated times. ChIP of input (whole-cell extract) samples shows that 3 PCR products at ori2004 amplify equally with the primers. Representative results from repeat experiments are shown. (C) An increase in the septation index indicates the onset of S phase.
Chl1 Is Involved in an S Phase Stress Response Pathway
The dosage suppression of swi1Δ cells by chl1+ in HU and MMS sensitivity assays (Figure 1B) suggested that Chl1 is also involved in the tolerance of S phase stresses. To confirm this idea, we have performed a series of drug sensitivity assays of chl1Δ cells. chl1Δ cells seemed to have no significant sensitivity to MMS or CPT (Figure 6A). However, when combined with chk1Δ, chl1Δ chk1Δ double deletion cells showed a significant increase in MMS and CPT sensitivity, suggesting that, in response to S phase DNA-damaging agents, chl1Δ cells accumulate unusual DNA structures that must be repaired by the Chk1-dependent G2-M checkpoint. Moreover, chl1Δ rad3Δ cells are much more sensitive to MMS and CPT than either single mutant (Figure 6A and Table 1), suggesting that the involvement of Chl1 in an S phase DNA damage response is independent of cell cycle checkpoints. To further investigate the role of Chl1 in S phase stress response pathway, we examined whether swi1Δ chl1Δ double mutant cells showed increased levels of sensitivity to S phase-stressing agents and found that there was no significant genetic interaction between swi1Δ and chl1Δ in drug sensitivity assays including UV, HU, MMS, CPT, and TBZ (Figure 6B and Table 1). Considering the fact that an increased dosage of chl1+ suppresses the HU and MMS sensitivity of swi1Δ cells (Figure 1B), our results are consistent with the notion that Chl1 and Swi1 are in the same pathway required for tolerance of S phase stresses. It should also be noted that swi1Δ, swi3Δ, and chl1Δ all showed synthetic lethal interaction with ctf18Δ (Figure 1A), again strengthening the idea that the Chl1-dependent S phase stress response pathway is independent of, but partially redundant with, the Ctf18-regulated mechanism.
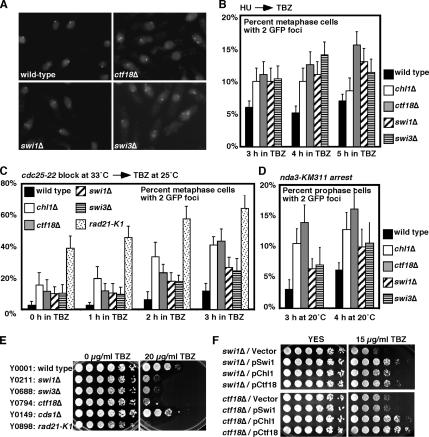
Swi1-Swi3, Ctf18, and Chl1 Are Involved in Sister Chromatid Cohesion
In budding yeast, Ctf18 has been shown to be required for proper chromosome cohesion (Hanna et al., 2001; Mayer et al., 2001). It has also been reported that some S phase checkpoint proteins are essential in mutants defective in the sister chromatid cohesion pathway (Mayer et al., 2004; Warren et al., 2004), suggesting a tight link between DNA replication and sister chromatid cohesion. Consistent with this notion, we found that swi1Δ or swi3Δ are synthetically lethal with a mutation in Rad21/Scc1 (rad21-K1) (Table 1), a subunit of the cohesin complex. In addition, we have also found that ctf18Δ or chl1Δ shows synergistic genetic interaction with rad21-K1 (Table 1 and Supplemental Figure S2B), suggesting the importance of Swi1-Swi3, Ctf18, and Chl1 in sister chromatid cohesion in S. pombe. To monitor cohesion defects in swi1, swi3, ctf18, and chl1 mutants, we used a strain that has the bacterial LacO tandem repeat sequences inserted at the lys1 locus located in the vicinity of the centromere on chromosome I. This strain is engineered to express the LacI repressor fused to GFP-NLS, which is recruited to LacO repeat sequences, allowing us to visualize the centromere I (Ding et al., 2004). If sister chromatids are properly adhered to one another, the GFP signal should occur as a single focus in the nuclei of metaphase cells. However, if sister chromatids are prematurely separated, two distinct GFP foci will occur in the nuclei of metaphase cells (Figure 7A). Using this system, we determined the effect of swi1, swi3, ctf18, or chl1 mutations on cohesion at the centromere region. For synchronization, cells were first arrested at the beginning of S phase by a 2-h incubation in the presence of HU. To obtain metaphase-arrested cells, cells were then released into medium containing TBZ, a compound that depolymerizes tubulin. Because sister chromatids are still attached to one another at metaphase, most of wild-type cells showed a single centromere focus in nuclei (Figure 7, A and B). In contrast, the experiments revealed a significant increase in the number of swi1Δ nuclei with two foci (Figure 7, A and B). We also obtained similar results with swi3Δ, ctf18Δ, and chl1Δ cells (Figure 7, A and B), indicating a higher frequency of precocious sister chromatid separation in these mutants. To further confirm the importance of these factors in cohesion pathways, we repeated cohesion assays using two additional methods. In the first method (Figure 7C), cdc25-22 cells were synchronized at G2-M and released into medium containing TBZ. As reported previously (Takeda et al., 2001), rad21-K1, which contains a temperature-sensitive mutation in a cohesin subunit, showed a strong defect in sister chromatid cohesion. There is an increase in the percentage of cells with two centromere I foci in swi1Δ, swi3Δ, ctf18Δ, and chl1Δ cells (Figure 7C), further confirming our conclusion that these factors play important roles in sister chromatid cohesion. In the second method (Figure 7D), nda3-KM311 cold-sensitive cells (Hiraoka et al., 1984) were arrested at prophase by culturing them at 20°C. Again, we observed reproducible precocious sister chromatid separation in all the mutants we tested.
Figure 7.
Ctf18, Chl1, and FPC play an important role for proper sister chromatid cohesion. (A) Cells of the indicated genotypes that had LacO repeats near centromere 1 and expressed LacI-GFP-NLS were grown to mid-log phase and supplemented with 10 mM HU for 2 h to synchronize cells in early S phase. Cells were then released into fresh YES medium containing 100 μg/ml TBZ for 3, 4, and 5 h to obtain metaphase cells. Representative images at 4 h in TBZ are shown for cells of the indicated genotypes. (B) Quantification of metaphase cells that had two GFP foci shown in A. At least 200 cells were counted for each strain. Error bar corresponds to the standard deviations obtained from three independent experiments. (C) cdc25-22 cells with the indicated genotypes were arrested at G2-M and released into the cell cycle in the presence of 100 μg/ml TBZ. Quantification of metaphase cells that had two GFP foci was performed at the indicated times as described above. (D) nda3-KM311 cells with the indicated genotypes were arrested at prophase, and cells with two GFP foci was quantified at the indicated times as described above. (E) Swi1-Swi3 and RFCCtf18 are required for cellular tolerance to a microtubule drug, TBZ. Fivefold serial dilution of cells of the indicated genotypes were incubated on YES agar medium supplemented with 0 or 20 μg/ml TBZ for 3 d at 30°C. (F) Genetic interaction between swi1, chl1 and ctf18. swi1Δ or ctf18Δ cells were transformed with the indicated plasmid and plated on YES medium containing 0 or 15 μg/ml TBZ. Fivefold serial dilution of cells were incubated for 2–3 d at 32°C. Representative images of repeat experiments are shown.
Interestingly, swi1Δ, swi3Δ, and ctf18Δ cells, and rad21-K1 cells, were found to be sensitive to TBZ (Figure 7E). Although chl1Δ cells were not sensitive to TBZ, chl1Δ showed synergistic genetic interaction with rad3Δ in TBZ sensitivity assays (Figure 6A and Table 1). TBZ sensitivity is found among mutants that affect general sister chromatid cohesion and segregation (Tatebayashi et al., 1998; Wang et al., 2002; Williams and McIntosh, 2002; Silverstein et al., 2003). Therefore, we used TBZ sensitivity to further understand the pathways involving Swi1-Swi3, Ctf18, and Chl1 (Figure 7F and Table 2). TBZ sensitivity of swi1Δ was partially rescued by a dosage increase in chl1+ or ctf18+, whereas TBZ sensitivity of ctf18Δ is strongly rescued by chl1+, but not by swi1+. This is a striking contrast with our earlier observation that a dosage increase in chl1+ failed to rescue HU or MMS sensitivity of ctf18Δ (Figure 1B and Table 2). Therefore, our results suggest that Ctf18 and Chl1 have partially redundant roles in cellular tolerance to tubulin poison TBZ.
DISCUSSION
Roles of RFCCtf18 and Swi1-Swi3 in Activation of Replication Checkpoint and Fork Stabilization
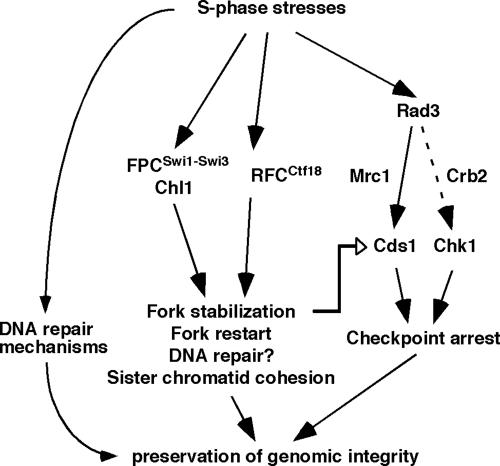
Ctf18 is an RFC1-like subunit of the alternative replication factor C complex and thought to be involved in loading or unloading of PCNA, a trimeric sliding clamp required for DNA synthesis in the budding yeast S. cerevisiae and humans (Bermudez et al., 2003; Shiomi et al., 2004; Bylund and Burgers, 2005). Ctf18 is also implicated in the replication checkpoint and functions redundantly with Rad24 to activate Rad53, a budding yeast homolog of Cds1 (Naiki et al., 2001), although it is not clear whether Ctf18 is involved in replication fork stabilization in this organism. The results of the present study suggest that fission yeast Ctf18 also forms an alternative RFC complex, RFCCtf18 (Figure 1D) and that it is involved in the Cds1-dependent replication checkpoint pathway and stabilization of stalled replication forks. As with swi1Δ, ctf18Δ shows a significant decrease in HU-induced activation of Cds1 (Figure 2C) and a significant delay in resumption of DNA replication after fork arrest (Figure 4D). The decrease of Cds1 activity in ctf18Δ cells may be explained by one of the following mechanisms, or a combination of the three: 1) RFCCtf18 directly interacts with Cds1 to modulate its activity. 2) RFCCtf18 interacts with Mrc1, which is the mediator of Cds1 activation, to promote the replication checkpoint. 3) Inactivation of RFCCtf18 causes fork structures to become unstable, which in turn attenuates replication checkpoint activation. In this third model, we envision that RFCCtf18 preserves replication fork structures in a configuration that is recognized by the replication checkpoint sensors (Figure 8). ctf18Δ cells showed a significant increase in S phase-dependent accumulation of Rad22 DNA repair foci (Figure 4B), indicating that these cells generate ssDNA stretches during DNA synthesis in the absence of genotoxic agents. This suggests that Ctf18 has a checkpoint independent function, which is required for stabilization of replication forks during normal S phase. Therefore, we prefer the third model in which RFCCtf18 acts at replication forks during normal DNA synthesis to modulate replication fork or replisome structures in a replication competent state to facilitate proper Cds1 activation (Figure 8). Consistently, our result suggests that Ctf18 may associate with replication origins during both unperturbed and arrested S phase (Figure 5). Although we are not able to detect relocation of Ctf18 along the chromosomes, it is still possible that Ctf18 moves with replication forks to stabilize them as in the case of Swi1-Swi3. Similarly, Mcm6 has been observed to display no significant association with sites at 14 or 30 kb away from ori2004 in S. pombe (Ogawa et al., 1999), although budding yeast Mcm proteins have been shown to travel with replication forks (Aparicio et al., 1997). It is possible that not all cells initiate DNA synthesis at ori2004 in fission yeast but all ori2004 sites recruits Ctf18, resulting in dilution of fork-bound Ctf18 proteins in our cell extract preparation. Therefore, we speculate that Ctf18 recognizes replication origins and relocates along the chromosome. In contrast, we have previously shown that Swi1, Swi3, and RPA relocate along the chromosome (Noguchi et al., 2004), suggesting that these proteins may recognize replication fork structures after origin unwinding.
Figure 8.
Models for S phase stress response mechanisms in S. pombe. RFCCtf18 is involved in Cds1-dependent replication checkpoint. FPCSwi1-Swi3, RFCCtf18, and Chl1 also have checkpoint independent functions that are important for fork protection and DNA repair. In this model, FPCSwi1-Swi3, RFCCtf18, and Chl1 stabilize replication forks in a configuration that is recognized by replication checkpoint sensors. Chl1 may work together with FPCSwi1-Swi3to facilitate fork protection. In addition, FPCSwi1-Swi3and RFCCtf18 act in parallel to facilitate proper chromosome cohesion.
Role of RFCCtf18 and Swi1-Swi3 in Sister Chromatid Cohesion
Emerging evidence suggests that DNA replication is coupled with sister chromatid cohesion (Skibbens, 2005). Cohesin proteins are loaded onto chromatin during G1 phase, and chromosomal cohesion is established during DNA replication when replication forks pass through cohesin rings (Skibbens, 2005; Lengronne et al., 2006). This is probably when cohesin and fork components interact together to establish chromosomal cohesion.
In this study, we showed that both RFCCtf18 and Swi1-Swi3 play an important role in the activation of the replication checkpoint, fork stabilization, and sister chromatid cohesion. However, it is still unclear how Ctf18 is acting to facilitate chromosomal cohesion. Our data suggest that Ctf18 may recognize replication origins. This is interesting in light of the fact that the origin recognition complex functions in sister chromatid cohesion in budding yeast (Shimada and Gasser, 2007). Therefore, Ctf18-origin interaction might be important for proper establishment of cohesion. Another possibility is that Ctf18 is required to either recruit cohesin or related factors onto DNA, or to help maintain their association with chromosomes. Several different mechanisms can be proposed for the importance of replication fork maintenance in sister chromatid cohesion: 1) The cohesin ring may be an obstacle for replication fork progression. In this model, cohesin may cause a pausing of replication forks. Because paused replication forks are prone to collapse, there may be an increased requirement for fork stabilizing proteins, such as Swi1-Swi3 and RFCCtf18, at cohesin sites. 2) Components of the replication fork might be required to aid in stabilizing cohesin complexes during DNA synthesis. Lengronne et al. (2006) have proposed a model in which cohesin rings may transiently dissociate when forks pass through them. This is possible if the replisome complex is too large to fit through the cohesin ring. This suggests that fork components might preserve cohesin structures or tether cohesin-related proteins to DNA when forks pass through the ring. We speculate Ctf18 and/or the Swi1–Swi3 complex may be required for these functions, thereby facilitating proper establishment of sister chromatid cohesion. In support of this idea, a human Ctf18 homologue has been shown to interact with various cohesin proteins (Bermudez et al., 2003). 3) Ctf18 might be needed to act as a clamp loader or unloader through cohesin-rich regions. Bylund et al. has suggested that Ctf18-dependent unloading of PCNA might loosen the replication fork structure so that replication forks are able to pass through the cohesin ring without its temporal dissociation (Bylund and Burgers, 2005). Alternatively, Ctf18-dependent unloading and reloading of PCNA may facilitate a polymerase switch at cohesin sites. Consistent with this notion, it has been reported that human RFCCtf18 physically interact with DNA polymerase η and stimulate its activity (Shiomi et al., 2007). In the future, it would therefore be interesting to examine whether RFCCtf18 and Swi1-Swi3 are required for this mechanism.
Role of Chl1 in Preservation of Genomic Integrity
We have also identified a putative DNA helicase, Chl1, as a dosage suppressor of lws1-1 swi1Δ synthetic lethality. In budding yeast, Chl1 has been thought to be involved in DNA damage response, preservation of genomic integrity during S phase, and efficient sister chromatid cohesion (Mayer et al., 2004; Petronczki et al., 2004; Skibbens, 2004; Warren et al., 2004). In humans, Chl1 has been shown to exhibit DNA helicase activity and to be involved in sister chromatid cohesion (Hirota and Lahti, 2000; Parish et al., 2006). These Chl1 functions seemed to be evolutionarily conserved, because, in our present study, we have shown that fission yeast Chl1 is involved in the S phase stress response and efficient sister chromatid cohesion. Our data also suggest that chl1Δ cells accumulate abnormal DNA structures that activate the checkpoint response and that Chl1 is involved in the maintenance of replication forks. Interestingly, our genetic studies involving chl1+, ctf18+, and swi1+ suggested the possibility that Chl1 and Swi1 are in the same pathway to preserve genomic integrity and that this pathway is working in parallel with the pathway involving Ctf18 (Figure 8). In support of this idea, budding yeast Chl1 and Tof1-Csm3 have been shown to be in the same genetic pathway required for sister chromatid cohesion (Xu et al., 2007). It has also been reported that Tof1-Csm3 and Ctf18 function in different pathways (Xu et al., 2007), indicating evolutional conservation in pathways involving Swi1-Swi3, Ctf18, and Chl1. Therefore, we speculate that Chl1 and Swi1 cooperate to stabilize replication forks as ancillary components of the replisome and promote proper establishment of sister chromatid cohesion, thereby preserving genomic integrity (Figure 8).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Teresa Wang (Stanford University, CA) for generously providing the anti-Cds1 antibody; Dr. Hisao Masukata (Osaka University, Japan) for the S. pombe genomic library; and Drs. Hideo Ikeda (University of Tokyo), Hisao Masukata, Paul Russell (The Scripps Research Institute), Shigeaki Saitoh (Kurume University, Japan), Katsunori Tanaka (Kwansei Gakuin University, Japan), and Mitsuhiro Yanagida (Kyoto University, Japan) for donating the S. pombe strains. We also thank Drs. Joseph Nickels and Mark Lechner and Adam Leman and anonymous reviewers for helpful comments. This work was supported by a Leukemia Research Foundation grant (to E.N.) and Drexel University College of Medicine start-up funds (to E.N.). T.M.N. is a Sidney Kimmel Scholar (SKF-05-070).
Abbreviations used:
- CPT
camptothecin
- FPC
fork protection complex
- HU
hydroxyurea
- MMS
methylmethane sulfonate
- PCNA
proliferating cell nuclear antigen
- PFGE
Pulsed field gel electrophoresis
- RFC
replication factor C
- TBZ
thiabendazole.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-06-0618) on November 28, 2007.
REFERENCES
- al-Khodairy F., Fotou E., Sheldrick K. S., Griffiths D. J., Lehmann A. R., Carr A. M. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol. Biol. Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa C., Fantes P., Hyams J., McLeod M., Warbrick E. Experiments with Fission Yeast. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1993. [Google Scholar]
- Amann J., Valentine M., Kidd V. J., Lahti J. M. Localization of chi1-related helicase genes to human chromosome regions 12p11 and 12p 13, similarity between parts of these genes and conserved human telomeric-associated DNA. Genomics. 1996;32:260–265. doi: 10.1006/geno.1996.0113. [DOI] [PubMed] [Google Scholar]
- Aparicio O. M., Weinstein D. M., Bell S. P. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bentley N. J., Holtzman D. A., Flaggs G., Keegan K. S., DeMaggio A., Ford J. C., Hoekstra M., Carr A. M. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- Bermudez V. P., Maniwa Y., Tappin I., Ozato K., Yokomori K., Hurwitz J. The alternative Ctf18-Dcc1-Ctf8-replication factor C complex required for sister chromatid cohesion loads proliferating cell nuclear antigen onto DNA. Proc. Natl. Acad. Sci. USA. 2003;100:10237–10242. doi: 10.1073/pnas.1434308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjergbaek L., Cobb J. A., Tsai-Pflugfelder M., Gasser S. M. Mechanistically distinct roles for Sgs1p in checkpoint activation and replication fork maintenance. EMBO J. 2005;24:405–417. doi: 10.1038/sj.emboj.7600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy M. N., Furnari B., Mondesert O., Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Boddy M. N., Russell P. DNA replication checkpoint. Curr. Biol. 2001;11:R953–R956. doi: 10.1016/s0960-9822(01)00572-3. [DOI] [PubMed] [Google Scholar]
- Brun C., Dubey D. D., Huberman J. A. pDblet, a stable autonomously replicating shuttle vector for Schizosaccharomyces pombe. Gene. 1995;164:173–177. doi: 10.1016/0378-1119(95)00497-t. [DOI] [PubMed] [Google Scholar]
- Bylund G. O., Burgers P. M. Replication protein A-directed unloading of PCNA by the Ctf18 cohesion establishment complex. Mol. Cell. Biol. 2005;25:5445–5455. doi: 10.1128/MCB.25.13.5445-5455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada A., Hodgson B., Kanemaki M., Bueno A., Labib K. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 2005;19:1905–1919. doi: 10.1101/gad.337205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. C., Chan A., Jeon M., Wu T. F., Pasqualone D., Rougvie A. E., Meyer B. J. Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature. 2003;424:1002–1009. doi: 10.1038/nature01697. [DOI] [PubMed] [Google Scholar]
- Chang M., Bellaoui M., Boone C., Brown G. W. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proc. Natl. Acad. Sci. USA. 2002;99:16934–16939. doi: 10.1073/pnas.262669299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou D. M., Elledge S. J. Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function. Proc. Natl. Acad. Sci. USA. 2006;103:18143–18147. doi: 10.1073/pnas.0609251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb J. A., Bjergbaek L., Shimada K., Frei C., Gasser S. M. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003;22:4325–4336. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D. Q., Yamamoto A., Haraguchi T., Hiraoka Y. Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev. Cell. 2004;6:329–341. doi: 10.1016/s1534-5807(04)00059-0. [DOI] [PubMed] [Google Scholar]
- Doe C. L., Ahn J. S., Dixon J., Whitby M. C. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J. Biol. Chem. 2002;277:32753–32759. doi: 10.1074/jbc.M202120200. [DOI] [PubMed] [Google Scholar]
- Dubey D. D., Kim S. M., Todorov I. T., Huberman J. A. Large, complex modular structure of a fission yeast DNA replication origin. Curr. Biol. 1996;6:467–473. doi: 10.1016/s0960-9822(02)00514-6. [DOI] [PubMed] [Google Scholar]
- Fantes P. Epistatic gene interactions in the control of division in fission yeast. Nature. 1979;279:428–430. doi: 10.1038/279428a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., Walker G. C., Siede W. Washington, DC: ASM Press; 1995. DNA Repair and Mutagenesis. [Google Scholar]
- Gambus A., Jones R. C., Sanchez-Diaz A., Kanemaki M., van Deursen F., Edmondson R. D., Labib K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 2006;8:358–366. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- Gerring S. L., Spencer F., Hieter P. The CHL 1 (CTF 1) gene product of Saccharomyces cerevisiae is important for chromosome transmission and normal cell cycle progression in G2/M. EMBO J. 1990;9:4347–4358. doi: 10.1002/j.1460-2075.1990.tb07884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter A. L. Tipin, a novel timeless-interacting protein, is developmentally co-expressed with timeless and disrupts its self-association. J. Mol. Biol. 2003;331:167–176. doi: 10.1016/s0022-2836(03)00633-8. [DOI] [PubMed] [Google Scholar]
- Gotter A. L., Suppa C., Emanuel B. S. Mammalian TIMELESS and Tipin are evolutionarily conserved replication fork-associated factors. J. Mol. Biol. 2007;366:36–52. doi: 10.1016/j.jmb.2006.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J. S., Kroll E. S., Lundblad V., Spencer F. A. Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol. Cell. Biol. 2001;21:3144–3158. doi: 10.1128/MCB.21.9.3144-3158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y., Toda T., Yanagida M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Lahti J. M. Characterization of the enzymatic activity of hChlR1, a novel human DNA helicase. Nucleic Acids Res. 2000;28:917–924. doi: 10.1093/nar/28.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katou Y., Kanoh Y., Bando M., Noguchi H., Tanaka H., Ashikari T., Sugimoto K., Shirahige K. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424:1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- Kim J., Robertson K., Mylonas K. J., Gray F. C., Charapitsa I., MacNeill S. A. Contrasting effects of Elg1-RFC and Ctf18-RFC inactivation in the absence of fully functional RFC in fission yeast. Nucleic Acids Res. 2005;33:4078–4089. doi: 10.1093/nar/gki728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. J., Lee S., Park M. S., Jang Y. K., Kim J. B., Park S. D. Rad22 protein, a rad52 homologue in Schizosaccharomyces pombe, binds to DNA double-strand breaks. J. Biol. Chem. 2000;275:35607–35611. doi: 10.1074/jbc.M007060200. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D., Putnam C. D., Myung K. Maintenance of genome stability in Saccharomyces cerevisiae. Science. 2002;297:552–557. doi: 10.1126/science.1075277. [DOI] [PubMed] [Google Scholar]
- Krawchuk M. D., Wahls W. P. High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast. 1999;15:1419–1427. doi: 10.1002/(SICI)1097-0061(19990930)15:13<1419::AID-YEA466>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. S., Grewal S. I., Klar A. J. Biochemical interactions between proteins and mat1 cis-acting sequences required for imprinting in fission yeast. Mol. Cell. Biol. 2004;24:9813–9822. doi: 10.1128/MCB.24.22.9813-9822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengronne A., McIntyre J., Katou Y., Kanoh Y., Hopfner K. P., Shirahige K., Uhlmann F. Establishment of sister chromatid cohesion at the S. cerevisiae replication fork. Mol Cell. 2006;23:787–799. doi: 10.1016/j.molcel.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Lindsay H. D., Griffiths D. J., Edwards R. J., Christensen P. U., Murray J. M., Osman F., Walworth N., Carr A. M. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M., Cotta-Ramusino C., Pellicioli A., Liberi G., Plevani P., Muzi-Falconi M., Newlon C. S., Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412:557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Gygi S. P., Aebersold R., Hieter P. Identification of RFC(Ctf18p, Ctf8p, Dcc1p): an alternative RFC complex required for sister chromatid cohesion in S. cerevisiae. Mol Cell. 2001;7:959–970. doi: 10.1016/s1097-2765(01)00254-4. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Pot I., Chang M., Xu H., Aneliunas V., Kwok T., Newitt R., Aebersold R., Boone C., Brown G. W., Hieter P. Identification of protein complexes required for efficient sister chromatid cohesion. Mol. Biol. Cell. 2004;15:1736–1745. doi: 10.1091/mbc.E03-08-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle C. J., Karnitz L. M., Henry-Sanchez J. T., Chen J. Cloning and characterization of hCTF18, hCTF8, and hDCC1. Human homologs of a Saccharomyces cerevisiae complex involved in sister chromatid cohesion establishment. J. Biol. Chem. 2003;278:30051–30056. doi: 10.1074/jbc.M211591200. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Naiki T., Kondo T., Nakada D., Matsumoto K., Sugimoto K. Chl12 (Ctf18) forms a novel replication factor C-related complex and functions redundantly with Rad24 in the DNA replication checkpoint pathway. Mol. Cell. Biol. 2001;21:5838–5845. doi: 10.1128/MCB.21.17.5838-5845.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcheva M. N., Roguev A., Dolapchiev L. B., Shevchenko A., Taskov H. B., Shevchenko A., Stewart A. F., Stoynov S. S. Uncoupling of unwinding from DNA synthesis implies regulation of MCM helicase by Tof1/Mrc1/Csm3 checkpoint complex. J. Mol. Biol. 2005;347:509–521. doi: 10.1016/j.jmb.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Noguchi C., Noguchi E. Sap1 promotes the association of the replication fork protection complex with chromatin and is involved in the replication checkpoint in Schizosaccharomyces pombe. Genetics. 2007;175:553–566. doi: 10.1534/genetics.106.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi E., Noguchi C., Du L. L., Russell P. Swi1 prevents replication fork collapse and controls checkpoint kinase Cds1. Mol. Cell. Biol. 2003;23:7861–7874. doi: 10.1128/MCB.23.21.7861-7874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi E., Noguchi C., McDonald W. H., Yates J. R., 3rd, Russell P. Swi1 and Swi3 are components of a replication fork protection complex in fission yeast. Mol. Cell. Biol. 2004;24:8342–8355. doi: 10.1128/MCB.24.19.8342-8355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg K. A., Michelson R. J., Putnam C. W., Weinert T. A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Walworth N. C., Carr A. M. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Takahashi T., Masukata H. Association of fission yeast Orp1 and Mcm6 proteins with chromosomal replication origins. Mol. Cell. Biol. 1999;19:7228–7236. doi: 10.1128/mcb.19.10.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara H., Enomoto T., Seki M. The INO80 chromatin remodeling complex functions in sister chromatid cohesion. Cell Cycle. 2007;6:1090–1095. doi: 10.4161/cc.6.9.4130. [DOI] [PubMed] [Google Scholar]
- Ohta S., Shiomi Y., Sugimoto K., Obuse C., Tsurimoto T. A proteomics approach to identify proliferating cell nuclear antigen (PCNA)-binding proteins in human cell lysates. Identification of the human CHL12/RFCs2–5 complex as a novel PCNA-binding protein. J. Biol. Chem. 2002;277:40362–40367. doi: 10.1074/jbc.M206194200. [DOI] [PubMed] [Google Scholar]
- Osborn A. J., Elledge S. J., Zou L. Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 2002;12:509–516. doi: 10.1016/s0962-8924(02)02380-2. [DOI] [PubMed] [Google Scholar]
- Ostermann K., Lorentz A., Schmidt H. The fission yeast rad22 gene, having a function in mating-type switching and repair of DNA damages, encodes a protein homolog to Rad52 of Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:5940–5944. doi: 10.1093/nar/21.25.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciotti V., Clerici M., Scotti M., Lucchini G., Longhese M. P. Characterization of mec1 kinase-deficient mutants and of new hypomorphic mec1 alleles impairing subsets of the DNA damage response pathway. Mol. Cell. Biol. 2001;21:3913–3925. doi: 10.1128/MCB.21.12.3913-3925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish J. L., Rosa J., Wang X., Lahti J. M., Doxsey S. J., Androphy E. J. The DNA helicase ChlR1 is required for sister chromatid cohesion in mammalian cells. J. Cell Sci. 2006;119:4857–4865. doi: 10.1242/jcs.03262. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Chwalla B., Siomos M. F., Yokobayashi S., Helmhart W., Deutschbauer A. M., Davis R. W., Watanabe Y., Nasmyth K. Sister-chromatid cohesion mediated by the alternative RF-CCtf18/Dcc1/Ctf8, the helicase Chl1 and the polymerase-α-associated protein Ctf4 is essential for chromatid disjunction during meiosis II. J. Cell Sci. 2004;117:3547–3559. doi: 10.1242/jcs.01231. [DOI] [PubMed] [Google Scholar]
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- Rabitsch K. P., et al. A screen for genes required for meiosis and spore formation based on whole-genome expression. Curr. Biol. 2001;11:1001–1009. doi: 10.1016/s0960-9822(01)00274-3. [DOI] [PubMed] [Google Scholar]
- Rhind N., Russell P. Checkpoints: it takes more than time to heal some wounds. Curr. Biol. 2000a;10:R908–911. doi: 10.1016/s0960-9822(00)00849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N., Russell P. Chk1 and Cds 1, linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 2000b;113:3889–3896. doi: 10.1242/jcs.113.22.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S., Chabes A., McDonald W. H., Thelander L., Yates J. R., Russell P. Cid13 is a cytoplasmic poly(A) polymerase that regulates ribonucleotide reductase mRNA. Cell. 2002;109:563–573. doi: 10.1016/s0092-8674(02)00753-5. [DOI] [PubMed] [Google Scholar]
- Sato M., Dhut S., Toda T. New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast. 2005;22:583–591. doi: 10.1002/yea.1233. [DOI] [PubMed] [Google Scholar]
- Shimada K., Gasser S. M. The origin recognition complex functions in sister-chromatid cohesion in Saccharomyces cerevisiae. Cell. 2007;128:85–99. doi: 10.1016/j.cell.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Shiomi Y., Masutani C., Hanaoka F., Kimura H., Tsurimoto T. A second PCNA loader complex, Ctf18-RFC, stimulates DNA polymerase eta activity. J. Biol. Chem. 2007;282:20906–21014. doi: 10.1074/jbc.M610102200. [DOI] [PubMed] [Google Scholar]
- Shiomi Y., Shinozaki A., Sugimoto K., Usukura J., Obuse C., Tsurimoto T. The reconstituted human Chl12-RFC complex functions as a second PCNA loader. Genes Cells. 2004;9:279–290. doi: 10.1111/j.1356-9597.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Silverstein R. A., Richardson W., Levin H., Allshire R., Ekwall K. A new role for the transcriptional corepressor SIN3; regulation of centromeres. Curr. Biol. 2003;13:68–72. doi: 10.1016/s0960-9822(02)01401-x. [DOI] [PubMed] [Google Scholar]
- Skibbens R. V. Chl1p, a DNA helicase-like protein in budding yeast, functions in sister-chromatid cohesion. Genetics. 2004;166:33–42. doi: 10.1534/genetics.166.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbens R. V. Unzipped and loaded: the role of DNA helicases and RFC clamp-loading complexes in sister chromatid cohesion. J. Cell Biol. 2005;169:841–846. doi: 10.1083/jcb.200503129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Lopes M., Foiani M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science. 2002;297:599–602. doi: 10.1126/science.1074023. [DOI] [PubMed] [Google Scholar]
- Sommariva E., Pellny T. K., Karahan N., Kumar S., Huberman J. A., Dalgaard J. Z. Schizosaccharomyces pombe Swi1, Swi3, and Hsk1 are components of a novel S-phase response pathway to alkylation damage. Mol. Cell. Biol. 2005;25:2770–2784. doi: 10.1128/MCB.25.7.2770-2784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E., Chapman C. R., Al-Khodairy F., Carr A. M., Enoch T. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 1997;16:2682–2692. doi: 10.1093/emboj/16.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Ogino K., Tatebayashi K., Ikeda H., Arai K., Masai H. Regulation of initiation of S phase, replication checkpoint signaling, and maintenance of mitotic chromosome structures during S phase by Hsk1 kinase in the fission yeast. Mol. Biol. Cell. 2001;12:1257–1274. doi: 10.1091/mbc.12.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi K., Kato J., Ikeda H. Isolation of a Schizosaccharomyces pombe rad21ts mutant that is aberrant in chromosome segregation, microtubule function, DNA repair and sensitive to hydroxyurea: possible involvement of Rad21 in ubiquitin-mediated proteolysis. Genetics. 1998;148:49–57. doi: 10.1093/genetics/148.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercero J. A., Diffley J. F. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- Tercero J. A., Longhese M. P., Diffley J. F. A central role for DNA replication forks in checkpoint activation and response. Mol. Cell. 2003;11:1323–1336. doi: 10.1016/s1097-2765(03)00169-2. [DOI] [PubMed] [Google Scholar]
- Unsal-Kacmaz K., Chastain P. D., Qu P. P., Minoo P., Cordeiro-Stone M., Sancar A., Kaufmann W. K. The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol. Cell. Biol. 2007;27:3131–3142. doi: 10.1128/MCB.02190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. W., Read R. L., Norbury C. J. Fission yeast Pds5 is required for accurate chromosome segregation and for survival after DNA damage or metaphase arrest. J. Cell Sci. 2002;115:587–598. doi: 10.1242/jcs.115.3.587. [DOI] [PubMed] [Google Scholar]
- Warren C. D., Eckley D. M., Lee M. S., Hanna J. S., Hughes A., Peyser B., Jie C., Irizarry R., Spencer F. A. S-phase checkpoint genes safeguard high-fidelity sister chromatid cohesion. Mol. Biol. Cell. 2004;15:1724–1735. doi: 10.1091/mbc.E03-09-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., McIntosh J. R. mcl1+, the Schizosaccharomyces pombe homologue of CTF4, is important for chromosome replication, cohesion, and segregation. Eukaryot. Cell. 2002;1:758–773. doi: 10.1128/EC.1.5.758-773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Chow B. L., Rathgeber L. The repair of DNA methylation damage in Saccharomyces cerevisiae. Curr. Genet. 1996;30:461–468. doi: 10.1007/s002940050157. [DOI] [PubMed] [Google Scholar]
- Xu H., Boone C., Brown G. W. Genetic dissection of parallel sister-chromatid cohesion pathways. Genetics. 2007;176:1417–1429. doi: 10.1534/genetics.107.072876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa-Sugata N., Masai H. Human Tim/Timeless-interacting protein, Tipin, is required for efficient progression of S phase and DNA replication checkpoint. J. Biol. Chem. 2007;282:2729–2740. doi: 10.1074/jbc.M605596200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.