Abstract
Drotecogin alfa (activated; DrotAA) was approved in 2001 by the US Food and Drug Administration for the treatment of patients with severe sepsis who are at high risk for death. The European Agency for the Evaluation of Medical Products also recommended that DrotAA could be administered to patients with severe sepsis and multiple organ dysfunction when added to the best standard care. Following the initial publication of the PROWESS (Protein C Worldwide Evaluation in Severe Sepsis) findings, multiple subgroup analyses supported the need for additional studies to explore the various hypotheses raised by this study. This review discusses all large clinical trials exploring the efficacy and safety of DrotAA and proposes recommendations for the use of DrotAA in severe sepsis.
Introduction
Drotecogin alfa (activated [DrotAA]; Xigris®; Eli Lilly and Company, Indianapolis, IN, USA) is a recombinant form of the natural anticoagulant activated protein C, and it was approved by the US Food and Drug Administration (FDA) in November 2001 for the treatment of adult patients with severe sepsis at high risk of death (for instance, as indicated by Acute Physiology and Chronic Health Evaluation [APACHE] II score). In 2002 the European Agency for the Evaluation of Medical Products recommended that its use be restricted to patients with two or more sepsis-induced organ dysfunctions.
Together with these recommendations, other studies were mandated by the FDA to explore the hypotheses generated by subgroup analyses from PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis), which suggested that patients at low risk for death might be harmed by DrotAA and that heparin prophylaxis could reduce the survival benefit conferred by DrotAA. Finally, at the time of FDA approval, no randomized controlled trial had been conducted in children with severe sepsis. An FDA-mandated paediatric trial, RESOLVE (REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective), was therefore conducted. This review discusses the potential indications for DrotAA based on data from all large clinical trials reported to date and provides recommendations for its use.
Clinical trials
Table 1 provides a summary of the clinical trials in severe sepsis evaluating DrotAA in adults and children. In the following sections the individual trials are discussed in greater detail. As in Table 1, the adult trials are discussed first followed by the paediatric trials.
Table 1.
Summary of clinical trials with drotrecogin alfa (activated) in severe sepsis
| Study | Patients (n) | Study type | Main findings | Comments |
| Adults | ||||
| Phase II [1] | 131 | RCT | Reduction in D-dimer and interleukin-6 plasma levels with DrotAA; reduction in 28-day all-cause mortality (not significant); no difference in bleeding events | Dose-finding study; optimal dose defined as 24 μg/kg per hour; benefit more pronounced in high-risk patients |
| PROWESS [2] | 1,690 | RCT | Significant reduction in 28-day, all-cause mortality; faster resolution of organ dysfunction; consistent survival benefit in more than 70 subgroups; reduced ospital and 3 month mortality | Increased survival benefit in patients at high risk for death; no benefit in single organ dysfunction and low APACHE II score; increased incidence of serious bleeding events |
| ENHANCE [11] | 2,378 | Open label | Similar 28-day, all-cause mortality compared with PROWESS; earlier intervention associated with improved outcome (<24 hours) | Increased incidence of bleeding events compared with PROWESS |
| ADDRESS [12] | 2,640 | RCT | No difference in 28-day and hospital all-cause mortality in patients at low risk for death | Increased incidence of bleeding events; no increased incidence in ICH |
| XPRESS [13] | 1,994 | RCT | Concomitant heparin does not increase 28-day mortality; heparin prophylaxis should not be discontinued before DrotAA | Small increase in nonserious bleeding; prophylactic heparin reduces incidence of ischaemic stroke |
| Children | ||||
| Phase Ib [14] | 83 | Open label | Safety and pharmacokinetic/pharmacodynamic study; pharmacokinetics/pharmacodynamics similar to adults | Safety similar to adults |
| RESOLVE [15] | 477 | RCT | No difference in time to organ failure resolution; no difference in 28-day mortality; no difference in the incidence of serious bleeding events | More ICH in children younger than 60 days in DrotAA arm |
ADDRESS, Administration of Drotrecogin alfa (activated) in early stage Severe Sepsis; APACHE, Acute Physiology and Chronic Health Evaluation; DrotAA, drotecogin alfa (activated); ENHANCE, Extended Evaluation of Recombinant Human Activated Protein C; ICH, intracerebral haemorrhage; PROWESS, Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis; RCT, randomized controlled trial; RESOLVE, REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective; XPRESS, Xigris and Prophylactic hepaRin Evaluation in Severe Sepsis.
Adults
Phase II trial
In a phase II, placebo-controlled, dose-finding study [1], 131 patients with severe sepsis were analyzed. This study demonstrated that an infusion of 24 μg/kg per hour for 96 continuous hours was associated with the greatest decrease in D-dimer and interleukin-6 plasma levels, without significant increase in bleeding risk compared with placebo. Also, although not powered to evaluate this end-point, the 28-day, all-cause mortality in the 24 to 32 μg/kg per hour arm was reduced compared with placebo (34.1% versus 28.9%).
Based on these promising results, the 24 μg/kg per hour dose was selected for use in the subsequent phase III PROWESS study [2].
Phase III trial: PROWESS
This large, multicentre, randomized, double-blind, placebo-controlled trial included 1,690 patients with sepsis and one or more organ dysfunction that had been present for no longer than 24 hours [2]. Patients were randomly assigned to receive either a continuous infusion of DrotAA at a dose of 24 μg/kg per hour or placebo for 96 hours. The primary endpoint was 28-day, all-cause mortality.
The study was stopped early after the second interim analysis by an independent scientific board, because it met the stopping rule for efficacy, established a priori. More than 70% of the patients had shock, defined as cardiovascular dysfunction, and required mechanical ventilation.
On the basis of the prospectively defined primary analysis, treatment with DrotAA was associated with absolute and relative reductions in the risk for death of 6.1% and 19.4%, respectively (P = 0.005). The mortality rate was 30.8% in the placebo group and only 24.7% in the DrotAA group (P = 0.005). Also, further analysis showed that patients treated with DrotAA exhibited a faster resolution in cardiovascular and respiratory dysfunction [3]. However, the incidence of serious bleeding was higher in the DrotAA group than in the placebo group (3.5% versus 2.0%; P = 0.06). Two patients treated with the drug developed intracerebral haemorrhage (ICH) during the infusion period (0.2%).
After the initial report of the overall results, multiple subgroup analyses were performed to explore whether the observed efficacy of DrotAA could be attributed to one particular subgroup of patients [4], and it confirmed that no specific subpopulations were more likely to be harmed by the drug. These subgroup analyses have important limitations, including decreased statistical power and increased variance; they should therefore be interpreted with caution. In PROWESS more than 70 subgroup analyses have demonstrated a consistent reduction in 28-day mortality when treated with DrotAA. A survival benefit was observed independent of sex, age, type of infection, type of pathogen, surgical status and biochemical measures of disease severity, including baseline protein C levels [5-7]. However, in patients with a single organ dysfunction or APACHE II score of less than 20, no apparent survival benefit was found.
This observation supported the decision of the FDA to indicate the use of DrotAA for the treatment of patients with severe sepsis at high risk for death. Also, based on the fact that patients receiving concomitant heparin prophylaxis with DrotAA had a lower survival benefit than did patients without heparin prophylaxis, it was suggested that heparin may interact with DrotAA. This finding prompted the FDA to mandate a subsequent study to explore this hypothesis (see XPRESS, below).
In a subsequent morbidity analysis, Angus and coworkers [8] demonstrated there was no increase in resource use in patients treated with DrotAA. Apart from the drug acquisition cost, both placebo and DrotAA-treated patients had similar intensive care unit and hospital lengths of stay [8]. In a long-term follow-up study of the initially enrolled population, Laterre and colleagues [9] showed that the hospital and 3-month survival benefit from DrotAA was maintained. The 1-year and 2.5-year follow up by Angus and coworkers [10], which was not adequately powered to evaluate a survival endpoint, identified a nonsignificant benefit in patients treated with DrotAA in the overall population but a highly significant benefit in patients with baseline APACHE II score greater than 24.
Phase IIIB trial: ENHANCE
After PROWESS had been prematurely interrupted because of efficacy and before FDA approval of DrotAA for the treatment of severe sepsis, an open-label, single-arm trial (ENHANCE [Extended Evaluation of Recombinant Human Activated Protein C]) [11] was begun in March 2001. The main objectives of ENHANCE were to provide more safety and efficacy data on the use of DrotAA in patients with severe sepsis. Despite the limitations of an unblinded study without a placebo arm, selecting a population with similar inclusion and exclusion criteria could allow some comparisons with PROWESS with respect to the efficacy and safety of DrotAA.
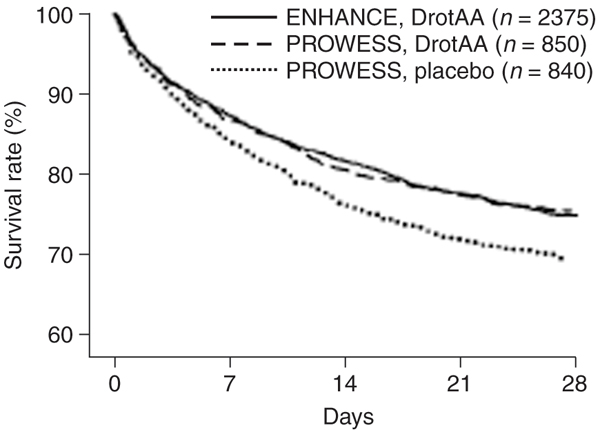
The only major difference with PROWESS regarding the inclusion criteria was the time window for intervention. In ENHANCE, DrotAA infusion could be initiated up to 48 hours after the first sepsis-induced organ dysfunction. A total of 2,378 patients received DrotAA in this study. Baseline characteristics revealed that the enrolled population was more severely ill than in the PROWESS population. More patients required vasopressors and mechanical ventilation, they had a higher mean Sequential Organ Failure Assessment score, and they had undergone surgery before enrolment. Therefore, the observed 28-day, all-cause mortality of 25.3%, similar to that in PROWESS, was the main reassuring result in terms of efficacy. The Kaplan-Meier survival curve of ENHANCE was almost superimposable on that of the DrotAA-treated arm of PROWESS (Figure 1).
Figure 1.
Kaplan-Meier survival curves. Comparison of ENHANCE (Extended Evaluation of Recombinant Human Activated Protein C) 28-day survival with that of PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis). DrotAA, drotecogin alfa (activated). Reproduced with permission from Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, Artigas A, Fumagalli R, Macias W, Wright T, Wong K, Sundin DP, Turlo MA, Janes J: Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med 2005, 33:2266–2277.
In terms of safety, however, the incidence of serious bleeding events was 3.6% in ENHANCE as compared with 2.4% in PROWESS during the infusion period. Also, 15 patients (0.6%) experienced one ICH event during the same period, as opposed to two (0.2%) in PROWESS. This greater proportion of bleeding complications might reflect the higher proportion of surgical patients and the higher hepatic and haematological Sequential Organ Failure Assessment scores than in PROWESS.
The second important finding of this study was the potential time dependency of the intervention's effect. Patients treated within the first 24 hours of organ dysfunction had a higher survival rate than did patients treated later (22.9% versus 27.4%). Moreover, earlier treatment (within 24 hours) was associated with improved survival, particularly in patients at high risk for death. This difference persisted even after adjusting for baseline differences in severity.
Phase IIIB trial: ADDRESS
After reviewing the subgroups analyses from PROWESS, the FDA mandated that a study be conducted to evaluate the efficacy and safety of DrotAA in adults with severe sepsis and at low risk of death: ADDRESS (Administration of Drotrecogin alfa [activated] in early stage Severe Sepsis). Patients eligible for this double-blind, placebo-controlled, randomized trial were required to have been diagnosed with severe sepsis, as defined by the presence of a suspected or known infection but (in accordance with the country label for DrotAA) associated with a single sepsis-induced organ failure, or if they had an APACHE II score below 25 [12]. Therefore, patients with two sepsis-related organ dysfunctions (ODs) but an APACHE II score below 25, and patients with a single OD and an APACHE II score above 25 could be enrolled in the trial in the US and Europe, respectively. The primary end-point was death from any cause assessed 28 days after the start of the study drug infusion. The study sample was initially calculated to detect a statistically significant difference between placebo and DrotAA at a two-sided P-value of 0.05.
The number of patients to be enrolled would have been 11,444. However, after a second interim analysis, the data monitoring committee recommended early termination of enrolment in accordance with the futility guidelines. The chances of successfully meeting the defined objective was less than 5% for a significant reduction in risk for death from any cause at 28 days after starting the infusion. A total of 2,640 patients were finally enrolled and evaluated.
The 28-day, all-cause mortality was 17% in the placebo versus 18.5% in the DrotAA arm (P = 0.34). Hospital mortality rates were 20.5% and 20.6% in placebo and DrotAA groups, respectively (P = 0.98). The expected increase in the risk for bleeding associated with the use of DrotAA was confirmed. Serious bleeding events occurred in 2.4% of DrotAA-treated patients versus 1.2% in the placebo group (P = 0.02) during the drug-infusion period. However, no difference was observed in the number of bleeding events involving the central nervous system (CNS) with the use of DrotAA compared with placebo (0.3% versus 0.2%).
Pre-specifed subgroup analyses were performed. Patients with an APACHE II score of below 25 represented 87.7% of the evaluated population. The 28-day and hospital mortality rates were 16% and 18.8% in the placebo group, respectively; the corresponding figures in DrotAA-treated patients were 16.9% and 18.9% (not significant). In patients with an APACHE II score above 24 and those who had two or more sepsis-induced organ failures, the use of DrotAA was not associated with a reduction in mortality, and the observed results were not consistent. Indeed, although the 28-day mortality rate was 24.7% in the placebo group as compared with 29.5% in the DrotAA group for patients with an APACHE II score above 25, in patients with multiple ODs the placebo mortality was 21.9% as compared with 20.7% in DrotAA-treated patients (not significant). Not only was the sample size too small to detect a statistical difference, but these data are difficult to compare with those from PROWESS. Indeed, the mean APACHE II score in this subgroup was lower in ADDRESS than in PROWESS. More importantly, the 28-day mortality in placebo patients with an APACHE II score above 24 in this study was 24.7% as opposed to 43.7% in PROWESS. Similar observations were made for patients with two or more organ failures, who had a 28-day mortality of 21.9% as compared with 33.9% in PROWESS, which confirms that the enrolled patients in ADDRESS were at lower risk for death.
Interestingly, heparin use at baseline apparently had no influence on outcome in the two groups and in particular within the placebo group. Finally, in a post hoc exploratory analysis, the subgroup of patients who had undergone recent surgery, had a single organ failure, and were receiving DrotAA had a higher 28-day and hospital mortality compared with the placebo group (20.7% versus 14.1% [P = 0.03] and 23.4% versus 19.8% [P = 0.26], respectively). In a small subgroup of 98 patients in PROWESS with a single organ failure and recent surgery, a similar effect was detected. Increased postoperative bleeding and delayed drug administration might have contributed to worsening organ failure and higher mortality. Unfortunately, the limited data on surgery and the absence of a statistically significant increase in the bleeding event rate in the surgical population with one organ failure did not allow confirmation of these hypotheses, but they have led to the recommendation that DrotAA is not used in this setting.
Phase IV trial: XPRESS
Subgroup analyses from PROWESS suggested that co-administration of prophylactic heparin with DrotAA was associated with a higher 28-day mortality, prompting the FDA to request that the sponsor design a subsequent trial to explore this hypothesis. XPRESS (Xigris and Prophylactic hepaRin Evaluation in Severe Sepsis) was a randomized, double-blind, phase IV, equivalence-design trial comparing DrotAA plus heparin versus DrotAA plus placebo, which enrolled 1,994 patients [13]. Unfractionated/low-molecular-weight heparin or placebo were administered every 12 hours during DrotAA infusion. If commercial heparin was being administered at baseline, then it was a protocol requirement to stop the heparin because patients were being randomly assigned to heparin or placebo.
The 28-day mortality was 28.3% in the heparin arm as compared with 31.9% in the placebo group (P = 0.08). The incidence of all thrombotic events, including deep venous thrombosis and pulmonary embolism, was low and no different between the two groups (5.7% for heparin versus 7% for placebo; not significant). However, ischaemic stroke incidence was greater in the placebo group for the entire study period (0.5% in heparin versus 1.8% in placebo; P = 0.01).
In subgroup analyses, heparin and placebo patients not exposed to heparin at baseline had similar mortality rates. Patients randomly assigned to placebo after heparin exposure at baseline had a higher mortality than did patients remaining on heparin. The difference in mortality was mostly driven by non-sepsis-related deaths in the placebo group. A greater number of these patients died from cardiac, cerebral, bleeding and respiratory events as compared with the patients receiving heparin. The incidence of ischaemic stroke was 1.8% for placebo and 0.5% in the heparin group (P = 0.01) for the 28-day follow up. Finally, the incidence of 'any bleeding event' was greater in the heparin group; however, for serious bleeding events or ICH, no difference was observed between the two groups. Results of the XPRESS study indicate that prophylactic heparin administration with DrotAA appears to be safe and that prophylactic heparin should not be abruptly discontinued in patients with severe sepsis or septic shock.
Children
Phase Ib trial
An open-label, nonrandomized, sequential study in children was initiated in March 2000 [14]. A total of 83 paediatric patients with severe sepsis (age range: ≥38 weeks of gestation to <18 years) were enrolled. The primary objectives were to evaluate the safety and pharmacokinetic properties of DrotAA in children, as well as the pharmacodynamic responses in adults with severe sepsis. One-third of the patients had positive blood cultures, and in approximately 20% of these the CNS was the site of infection.
The pharmacokinetic and pharmacodynamic effects of DrotAA in children were similar to those observed in adults. Two children (2.4%) had a serious bleeding event during the infusion period, and in a third child with meningitis an ICH occurred during the study period. Overall, the safety profile in this paediatric population was comparable to that in adults and supported a large phase III trial in children.
Phase III trial: RESOLVE
A phase III, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of DrotAA (24 μg/kg per hour for 96 hours) in severe sepsis and included 477 paediatric patients (age range: ≥38 weeks of gestation to <18 years) [15]. The primary end-point of RESOLVE was a prospectively defined Composite Time to Complete Organ Failure Resolution score. Mortality at 28-days, major amputations and safety were secondary end-points. The median age was close to 2.5 years, with more than 6% of children being younger than 12 months. At baseline, one-third of the population had purpura and more than 50% were diagnosed with disseminated intravascular coagulation.
At the end of the defined follow-up period, there was no significant difference between the two groups in Composite Time to Complete Organ Failure Resolution score. Mortality at 28 days was 17.5% in the placebo group and 17.2% in the DrotAA group (P = 0.93). Hospital mortality also exhibited no significant differences between treatment arms. Although the bleeding event rates were similar in the two groups, there were numerically more episodes of ICH in the DrotAA group (11 [4.6%]) than in the placebo group (5 [2.1%]; P = 0.13). This difference was predominantly observed in children younger than 60 days at the time of enrolment.
Despite the limitations of this study (for example, small population size with imbalances in baseline characteristics, lower protein C levels than in PROWESS and potentially insufficient dosing), DrotAA cannot be recommended for use in children with severe sepsis.
Safety
The main expected adverse event in patients treated with DrotAA is bleeding. A serious bleeding event was prospectively defined in the PROWESS trial as a life-threatening bleed, CNS haemorrhage, any bleeding event considered serious by the investigator, or a requirement of three or more red blood cell units for more than 2 consecutive days. The same definition was used for all subsequent trials after PROWESS. Table 2 summarizes bleeding events observed in clinical trials, including all bleeding events, serious bleeding events and CNS haemorrhage, when described. In all randomized controlled trials, the incidence of bleeding was increased in DrotAA-treated patients as compared with placebo. Most bleeding occurred during the infusion period, and more than half were procedure-related events. For the 28-day study period, serious bleeding events were observed in 3.5% to 6.5% in DrotAA-treated patients as compared with 2.0% to 5.0% in the placebo group. These serious bleeding events were often correlated with more pronounced baseline severity, coagulopathy and platelet count below 30,000/mm3 during the infusion period. With the exception of ENHANCE, the incidence of CNS bleeding during DrotAA infusion was lower than 0.5% in all other trials.
Table 2.
Bleeding events for the 28-day study (period) in clinical trials evaluating DrotAA in severe sepsis
| All bleeding events (%) | Serious bleeding events (%) | CNS bleeding (%) | |||||||
| Study | DrotAA | Placebo | P | DrotAA | Placebo | P | DrotAA | Placebo | P |
| Adults | |||||||||
| Phase II [1] | ND | ND | 4.0 | 5.0 | 0.99 | 0 | 0 | NS | |
| PROWESS [2] | ND | ND | 3.5 | 2.0 | 0.06 | 0.2 | 0 | NS | |
| ENHANCE [11] | ND | NA | 6.5 | NA | 1.5 | NA | NA | ||
| ADDRESS [12] | ND | ND | 3.9 | 2.2 | 0.01 | 0.5 | 0.4 | NS | |
| XPRESS [13]a | 12.4 | 10.9 | 0.32 | 3.9 | 5.2 | 0.16 | 1.0 | 0.7 | 0.49 |
| Children | |||||||||
| Phase Ib [14] | 20.5 | NA | 4.8 | NA | 2.4 | NA | NA | ||
| RESOLVE [15] | ND | ND | 6.7 | 6.8 | 0.97 | 4.6 | 2.1 | 0.13 | |
aFor the XPRESS trial all patients received drotecogin alfa (activated; DrotAA). Placebo refers to absence of concomitant heparin prophylaxis during DrotAA infusion. ADDRESS, Administration of Drotrecogin alfa (activated) in early stage Severe Sepsis; ENHANCE, Extended Evaluation of Recombinant Human Activated Protein C; NA, not applicable; ND, not described; PROWESS, Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis; RESOLVE, REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective; XPRESS, Xigris and Prophylactic hepaRin Evaluation in Severe Sepsis.
In children included in randomized controlled trials, the overall incidence of serious bleeding was no different in DrotAA-treated than in placebo patients [15]. However, there were numerically more CNS bleeding episodes in DrotAA-treated children, and these occurred mainly in children younger than 2 months.
Post-marketing surveys
After DrotAA was launched, post-marketing surveys were conducted in numerous countries throughout the world. The main common findings of these surveys were a higher overall mortality and incidence of serious bleeding events compared with PROWESS [16,17]. However, the incidence of CNS haemorrhage was found to be lower than 1% and similar to incidence reported in randomized controlled trials. A survival benefit for patients treated with DrotAA was suggested, but this was assessed by using the expected mortality based on severity scores or using the untreated population as controls.
These findings indicate that, in everyday clinical practice, the indications and use of the drug may largely differ from the population initially enrolled in controlled studies. Indeed, elevated severity scores and presence of relative or absolute contraindications at baseline were much more common based on post-marketing analyses than in PROWESS. Also, in a large proportion of patients drug administration was initiated well after 48 hours of organ dysfunction suggesting that DrotAA was sometimes used as a rescue therapy. This suggests that a better earlier severity assessment and defined guidelines for use of DrotAA in patients with severe sepsis who are at high risk for death should be established.
Clinical trial concerns
The initial publication of the PROWESS study report, secondary subgroups analyses and additional clinical trials were followed by multiple comments and criticisms [18-20]. Indeed, it was suggested that the positive results of PROWESS had to be questioned for the following reasons: an amendment took place during the study; recombinant activated protein C was produced by a new cell lot midway during the trial; blinding could ultimately not be assured because there was a change in the placebo; some subgroup analyses exhibited inconsistencies; and some sites had been closed during the study and others opened. Additional criticism was made after ADDRESS was stopped because of futility and subgroups analyses compared with PROWESS. Also, the bleeding risks of DrotAA were considered to be a limitation.
These criticisms need to be addressed. First, the second batch of recombinant activated protein C was demonstrated to have the same in vitro activity as the first lot and appears not to have played a role in the overall results. Unblinding (because of the placebo change to saline in a single country because human albumin was not allowed) is rather unlikely to account for the difference in mortality.
The amendment that took place during PROWESS consisted mainly of clarification on the definitions of inclusion and exclusion criteria, and reminding investigators that patients with advanced cancer or severe liver failure were not the optimal population, especially because of the bleeding risks. A small reduction in the number of patients with cancer was observed after the amendment, but this occurred in both treated and placebo arms. Interestingly, patients with significant underlying conditions at baseline had an apparent large benefit with DrotAA and therefore are not expected to have positively influenced the second part of the trial. Also, many investigators enrolled patients under both versions of the protocol, and at these sites the survival benefit was constant over the course of the study [21].
When the ADDRESS data were reviewed it was noted that, because of the study design and different DrotAA labels in the USA and Europe, a proportion of patients had multiple organ dysfunction (MOD) or a baseline APACHE II score above 25. For these two subgroups, the mortality results were not consistent and differed from those in PROWESS. Survival at day 28 was superior in placebo compared with DrotAA for patients with an APACHE II score above 25, but it was inferior in the presence of MOD. Not only are these subgroups not adequately powered to allow conclusions to be drawn, but also the placebo mortality in ADDRESS was much lower than in the corresponding subgroups of PROWESS (24.7% versus 43.7%), which supports the evidence indicating that the two study populations should not be compared.
Conclusion
In light of the various clinical trials evaluating the efficacy and safety of DrotAA in severe sepsis, we can conclude that DrotAA can reduce mortality in patients with severe sepsis at high risk for death. We can define this risk using severity scores, such as high APACHE II score or multiple organ failure, but probably also by sustained organ dysfunction that is not improving or worsening despite optimal care and adequate source control. However, this survival benefit is achieved at the expense of a slight increase in the risk for bleeding events, which can be minimized by adequate patient selection. If indicated, the drug should be used within the first 24 hours of sepsis-induced organ dysfunction.
Heparin prophylaxis given before DrotAA administration should not be interrupted because it is not associated with an increased incidence of serious bleeding events and may reduce the risk for ischaemic stroke in severe sepsis.
DrotAA is not indicated for use in patients at low risk for mortality, as defined by a single organ failure or rapidly improving MOD. Also, surgical patients at low risk for death (single organ dysfunction) or with organ dysfunction not induced by sepsis should not be considered appropriate candidates for this therapy. Currently, DrotAA is not indicated in children presenting with severe sepsis.
Future clinical trials should be designed to define better the target population for DrotAA, based on clinical signs of severity combined with a bedside biomarker. Also, the optimal timing of intervention requires confirmation.
Abbreviations
ADDRESS = Administration of Drotrecogin alfa (activated) in early stage Severe Sepsis; APACHE = Acute Physiology and Chronic Health Evaluation; CNS = central nervous system; DrotAA = drotecogin alfa (activated); ENHANCE = Extended Evaluation of Recombinant Human Activated Protein C; FDA = Food and Drug Administration; ICH = intracerebral haemorrhage; MOD, multiple organ dysfunction; OD = organ dysfunction; PROWESS = Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis; RESOLVE = REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective; XPRESS = Xigris and Prophylactic hepaRin Evaluation in Severe Sepsis.
Competing interests
P-FL has served as a consultant for Eli Lilly and Company and participated in Eli Lilly and Company sponsored trials.
Acknowledgments
Acknowledgements
This article is part of Critical Care Volume 11 Supplement 5: Severe sepsis and drotrecogin alfa (activated). The full contents of the supplement are available online at http://ccforum.com/supplements/11/S5. Publication of the supplement has been sponsored by Eli Lilly and Company.
References
- Bernard GR, Ely EW, Wright TJ, Fraiz J, Stasek JE, Jr, Russell JA, Mayers I, Rosenfeld BA, Morris PE, Yan SB, et al. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001;29:2051–2059. doi: 10.1097/00003246-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely W, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Angus DC, Artigas A, Kalil A, Basson BR, Jamal HH, Johnson G, III, Bernard GR. Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med. 2003;31:834–840. doi: 10.1097/01.CCM.0000051515.56179.E1. [DOI] [PubMed] [Google Scholar]
- Ely EW, Laterre PF, Angus DC, Helterbrand JD, Levy H, Dhainaut JF, Vincent JL, Macias WL, Bernard GR. Drotrecogin alfa (activated) administration across clinically important subgroups of patients with severe sepsis. Crit Care Med. 2003;31:12–19. doi: 10.1097/00003246-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Ely EW, Angus DC, Williams MD, Bates B, Qualy R, Bernard GR. Drotrecogin alfa (activated) treatment of older patients with severe sepsis. Clin Infect Dis. 2003;37:187–195. doi: 10.1086/375775. [DOI] [PubMed] [Google Scholar]
- Opal SM, Garber GE, LaRosa SP, Maki DG, Freebairn RC, Kinasewitz GT, Dhainaut JF, Yan SB, Williams MD, Graham DE, et al. Systemic host responses in severe sepsis analyzed by causative microorganism and treatment effects of drotrecogin alfa (activated) Clin Infect Dis. 2003;37:50–58. doi: 10.1086/375593. [DOI] [PubMed] [Google Scholar]
- Laterre PF, Garber G, Levy H, Wunderink R, Kinasewitz GT, Sollet JP, Maki DG, Bates B, Yan SB, Dhainaut JF. Severe community-acquired pneumonia as a cause of severe sepsis: Data from the PROWESS study. Crit Care Med. 2005;33:952–961. doi: 10.1097/01.CCM.0000162381.24074.D7. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Clermont G, Ball DE, Basson BR, Ely EW, Laterre PF, Vincent JL, Bernard G, van Hout B. Cost effectiveness of drotrecogin alfa (activated) in the treatment of severe sepsis. Crit Care Med. 2003;31:1–11. doi: 10.1097/00003246-200301000-00001. [DOI] [PubMed] [Google Scholar]
- Laterre PF, Levy H, Clermont G, Ball DE, Garg R, Nelson DR, Dhainaut JF, Angus DC. Hospital mortality and resource use in subgroups of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial. Crit Care Med. 2004;32:2207–2218. doi: 10.1097/01.ccm.0000145231.71605.d8. [DOI] [PubMed] [Google Scholar]
- Angus DC, Laterre PF, Helterbrand J, Ely EW, Ball DE, Garg R, Weissfeld LA, Bernard GR, PROWESS Investigators The effect of drotrecogin alfa (activated) on long-term survival after severe sepsis. Crit Care Med. 2004;32:2199–2206. doi: 10.1097/01.CCM.0000114816.62331.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, Artigas A, Fumagalli R, Macias W, Wright T, et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005;33:2266–2277. doi: 10.1097/01.CCM.0000181729.46010.83. [DOI] [PubMed] [Google Scholar]
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Francois B, Guy JS, Brückmann M, Rea-Neto A, et al. Drotecogin alfa (activated) for adult patients with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- Levi M, Levy M, Williams MD, Douglas I, Artigas A, Antonelli M, Duncan W, Janes J, Booth FV, Wang D, et al. Prophylactic heparin in patients with severe sepsis treated with drotecogin alfa (activated) Am J Respir Crit Care Med. 2007;176:483–490. doi: 10.1164/rccm.200612-1803OC. [DOI] [PubMed] [Google Scholar]
- Barton P, Kalil AC, Nadel S, Goldstein B, Okhuysen-Cawley R, Brilli RJ, Takano JS, Martin LD, Quint P, Yeh TS, et al. Safety, pharmacokinetics, and pharmacodynamics of drotrecogin alfa (activated) in children with severe sepsis. Pediatrics. 2004;113:7–17. doi: 10.1542/peds.113.1.7. [DOI] [PubMed] [Google Scholar]
- Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- Kanji S, Perreault MM, Chant C, Williamson D, Burry L. Evaluating the use of Drotrecogin alfa (activated) in adult severe sepsis: a Canadian multicenter observational study. Intensive Care Med. 2007;33:517–523. doi: 10.1007/s00134-007-0555-9. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Rossi C, Anghileri A, Livigni S, Addis A, Poole D. Use of drotrecogin alfa (activated) in Italian intensive care units: the results of a nationwide survey. Intensive Care Med. 2007;33:426–434. doi: 10.1007/s00134-007-0554-x. [DOI] [PubMed] [Google Scholar]
- Warren HS, Suffredini AF, Eichacker PQ, Munford RS. Risks and benefits of activated protein C treatment for severe sepsis. N Engl J Med. 2002;347:1027–1030. doi: 10.1056/NEJMsb020574. [DOI] [PubMed] [Google Scholar]
- Mackenzie AF. Activated protein C: do more survive? Intensive Care Med. 2005;31:1624–1626. doi: 10.1007/s00134-005-2829-4. [DOI] [PubMed] [Google Scholar]
- Carlet J. Prescribing indications based on successful clinical trials in sepsis: a difficult exercise. Crit Care Med. 2006;34:525–529. doi: 10.1097/01.CCM.0000198329.85851.8E. [DOI] [PubMed] [Google Scholar]
- Macias WL, Vallet B, Bernard GR, Vincent JL, Laterre PF, Nelson DR, Derchak PA, Dhainaut JF. Sources of variability on the estimate of treatment effect in the PROWESS trial: implications for the design and conduct of future studies in severe sepsis. Crit Care Med. 2004;32:2385–2391. doi: 10.1097/01.CCM.0000147440.71142.AC. [DOI] [PubMed] [Google Scholar]