Abstract
Glycerol 3-phosphate acyltransferase-1 (GPAT1), catalyzes the committed step in phospholipid and triacylglycerol synthesis. Because both GPAT1 and carnitine-palmitoyltransferase 1 are located on the outer mitochondrial membrane (OMM) it has been suggested that their reciprocal regulation controls acyl-CoA metabolism at the OMM. To determine whether GPAT1, like carnitine-palmitoyltransferase 1, is enriched in both mitochondrial contact sites and OMM, and to correlate protein location and enzymatic function, we used Percoll and sucrose gradient fractionation of rat liver to obtain submitochondrial fractions. Most GPAT1 protein was present in a vesicular membrane fraction associated with mitochondria (MAV) but GPAT specific activity in this fraction was low. In contrast, highest GPAT1 specific activity was present in purified mitochondria. Contact sites from crude mitochondria, which contained markers for both endoplasmic reticulum (ER) and mitochondria, also showed high expression of GPAT1 protein but low specific activity, whereas contact sites isolated from purified mitochondria lacked ER markers and expressed highly active GPAT1. To determine how GPAT1 is targeted to mitochondria, recombinant protein was synthesized in vitro and its incorporation into crude and purified mitochondria was assayed. GPAT1 was rapidly incorporated into mitochondria, but not into microsomes. Incorporation was ATP-driven, and lack of GPAT1 removal by alkali and a chaotropic agent showed that GPAT1 had become an integral membrane protein after incorporation. These results demonstrate that two pools of GPAT1 are present in rat liver mitochondria: an active one, located in OMM and a less active one, located in membranes (ER-contact sites and mitochondrial associated vesicles) associated with both mitochondria and ER.
Keywords: Triacylglycerol synthesis, Protein targeting, Mitochondria-endoplasmic reticulum interaction
1. Introduction
The first and committed step in de novo synthesis of glycerolipids is catalyzed by glycerol 3-phosphate acyltransferase (GPAT). Three GPAT isoforms have been characterized in mammalian tissues, two N-ethylmaleimide (NEM)-sensitive microsomal and mitochondrial isoforms [1,2] and an NEM-resistant-mitochondrial isoform (GPAT1) [1,3] which was cloned from mouse and rat liver [4,5].
The specific role of each GPAT isoform in glycerolipid metabolism is not well understood, but numerous studies strongly suggest that GPAT1 initiates the synthesis of TAG under conditions of dietary fat or carbohydrate excess. We have reported that over-expression of GPAT1 in CHO cells [6] and in rat hepatocytes [7] increases TAG content and [14C]oleate incorporation into TAG, but does not increase VLDL secretion. In contrast, in livers from GPAT1-null mice, TAG mass is 40% lower than in wild-type mice [8].GPAT1 mRNA and protein are up-regulated when fasted mice are refed [9] and after insulin is provided to streptozotocin diabetic mice [10]. In addition, GPAT1 specific activity is inhibited by AMP-activated protein kinase, which is active when cellular energy stores are low [11,12]. Unlike GPAT1, the specific activity of the microsomal GPAT isoform does not appear to change with nutritional alterations and a second mitochondrial GPAT, GPAT2, is not normally expressed in rat liver [2].
GPAT1, an intrinsic protein of the outer mitochondrial membrane, contains two transmembrane domains and its catalytic site faces the cytosol [13]. The presence of GPAT1 in the outer mitochondrial membrane is somewhat surprising because the enzymes that catalyze the final steps of TAG synthesis are located in the endoplasmic reticulum [1]. However, when GPAT1 is overexpressed in rat hepatocytes fatty acid oxidation decreases [7], whereas in GPAT1 null mice fatty acid oxidation increases[14], supporting our hypothesis that GPAT1 competes with carnitine palmitoyltransferase-1 (CPT1), the rate limiting step for fatty acid β-oxidation.
Because GPAT1 and CPT1 are reciprocally regulated in their apparent competition for long-chain-acyl-CoAs at the outer mitochondrial membrane (OMM) and CPT1 is either present or enriched in mitochondrial contact sites (CS) [15,16], we hypothesized that GPAT1 might also be present in these CS. Thus, we investigated the submitochondrial location of GPAT1, in order to correlate protein location and enzymatic function.
2. Materials and methods
2.1. Subcellular fractionation of rat liver
Male Wistar rats weighing 200 g were maintained on Cargill chow (4% fat, 60% carbohydrate) and kept on 12 h light/dark cycles. The animals were euthanized according to protocols approved by the Committee for Care and Use of Laboratory Animals, School of Medicine, University of La Plata, Argentina. Liver was removed, rinsed with ice cold PBS and submerged in precooled buffer H (10 mM HEPES-KOH, pH 7.4, 0.25 M sucrose, 1 mM EDTA and 1 mM DTT) with 0.002% V/V protease inhibitor cocktail (Cat. #P2714 Sigma). Total homogenate was prepared by 10 up-and-down strokes in a Teflon-glass motorized homogenizing vessel. Debris and nuclei were removed by centrifuging twice at 600×g at 4 °C for 10 min, and the supernatant (postnuclear homogenate) was centrifuged for 10 min at 10,000×g at 4 °C in a Sorvall refrigerated centrifuge. The resulting pellet was resuspended in 1 ml of buffer H/g of liver and homogenized in a Dounce tissue grinder with glass pestle to obtain the crude mitochondrial fraction. The supernatant was centrifuged for 1 h at 100,000×g (Beckman LE-80K ultracentrifuge, rotor 70.1 Ti) to obtain microsomes. All steps were performed at 4 °C.
2.2. Purification of crude mitochondria
Mitochondria were purified by a self-forming Percoll gradient centrifugation [17,18] (Fig. 1). Briefly, 1 ml of the crude mitochondria suspension was loaded in a tube containing 9 ml 30% (v/v) Percoll in buffer H and centrifuged for 30 min at 95,000×g in a 70.1 Ti rotor, in a Beckman LE-80K ultracentrifuge. After centrifugation, the tubes contained two distinct bands separated by a clear zone. The lower brownish band was collected and diluted 4-fold in cooled buffer H. To remove the Percoll, the samples were centrifuged for 10 min at 6300×g and the pellet was washed twice with cold buffer H to obtain pure mitochondria. The whitish-upper layers of the self-forming Percoll gradient were collected and diluted 4-fold in cold buffer H. They were centrifuged at 10,000×g for 10 min, and the resulting pellet was washed twice with cold buffer H to obtain the mitochondrial associated vesicle (MAV) fraction. The supernatant of this centrifugation was centrifuged at 100,000×g for 1 h to obtain the MAM fraction.
Fig. 1.
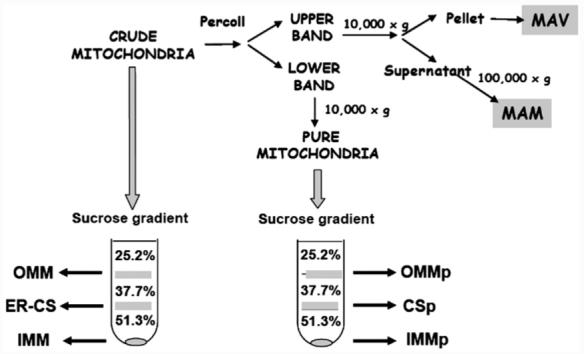
Submitochondrial fractionation and purification of rat-liver mitochondria. Rat liver crude mitochondrial fraction (pellet from a 10,000×g centrifugation, 10 min) was further purified by a 30% self-forming Percoll gradient. Two bands were separated, an upper band, which was separated into mitochondrial associated membranes (MAM) and mitochondrial associated vesicles (MAV), and a lower band corresponding to pure mitochondria. Crude and pure mitochondria were further fractionated in a discontinuous sucrose gradient. Outer mitochondrial membrane (OMM), inner mitochondrial membrane (IMM) and contact sites (ER-CS) were obtained from crude mitochondria. Outer membrane (OMMp), inner membrane (IMMp) and contact sites (CSp) were obtained from pure mitochondria.
2.3. Subfractionation of rat liver mitochondria
To obtain submitochondrial fractions from either crude or pure mitochondria, we modified a method described previously [15,19]. The mitochondrial pellet was resuspended in a hypotonic 10 mM potassium phosphate buffer pH 7.4 (50 mg of protein/ml) and incubated on ice for 20 min before adding 60% (w/v) sucrose. After a 20 min incubation on ice, the suspension was sonicated for three periods of 30 s, during a period of 3 min with constant cooling on ice in a Branson S-450A sonicator. The resulting suspension was centrifuged at 6300×g to remove debris, and the supernatant was layered on top of a discontinuous sucrose gradient [19] (1.2 ml each of 51.3%, 37.7%, and 25.2% sucrose in 10 mM potassium phosphate pH 7.4) and centrifuged for 60 min at 4 °C at 121,000×g (Beckman LE-80K ultracentrifuge, SW 60 rotor). The outer membrane fraction (OMM) was recovered at the 25.2%/37.7% interface, and the contact-site fraction (CS) was recovered at the 37.7%/51.2% interface (Fig. 1). These fractions were washed by diluting them 10-fold with 10 mM potassium phosphate buffer pH 7.4 and centrifuging at 180,000×g (Beckman LE-80K ultracentrifuge, 70.1 Ti rotor). The inner mitochondrial fraction (IMM) was collected from the pellet of the tube.
2.4. Enzyme assays
Total GPAT activity was assayed in each fraction (200 μg of total protein) as described previously [20] with modifications. Assays contained 0.8 mM glycerol-3-phosphate (0.5 μCi [14C]glycerol-3-phosphate, Amersham Biosciences), 60 μM palmitoyl-CoA, 75 mM Tris-HCl, pH 7.4, 4 mM MgCl2, 2 mg/ml BSA, 8 mM NaF and 1 mM DTT and were incubated at 37 °C for 10 min. To measure mitochondrial GPAT, samples were preincubated for 30 min on ice with 1mM N-ethylmaleimide to inhibit the microsomal GPAT. Arylesterase activity (ER marker) was measured as described previously [21], cytochrome c oxidase (IMM marker) was determined using Cytochrome c oxidase assay kit (Sigma) and monoamine oxidase (MAO, an OMM marker) was measured as described [22]. One MAO unit catalyzes the formation of 1 μmol of benzaldeyde per min at 30 °C; one cytochrome c oxidase unit oxidizes 1 μmol of ferrocytochrome c per min at 37 °C.
2.5. In vitro synthesis of GPAT1 and import into mitochondria
Radiolabeled GPAT1 was synthesized by an in vitro transcription-translation system (TNT® T7-coupled reticulocyte lysate system, Promega) in the presence of [35S]methionine (GE Healthcare SJ1515, 0.06 pmol, 60 μCi in a 50-μl reaction) according to the manufacturer’s instructions. When co-translational incorporation into microsomes was tested, 100 μg protein from washed rat-liver micosomes was added. Recombinant GPAT1 subcloned in pGEM12zf (+) (Promega) was used as template. In vitro import of radiolabeled protein into mitochondria or microsomes (100 μg/tube) was carried out at 30 °C in a buffer containing 10 mM HEPES-KOH, 0.25 M sucrose, 1 mM EDTA, 2 mg/ml BSA, 1 mM DTT, 100 mM potassium acetate, 2 mM ATP, 5 mM phosphocreatine, 100 μg/ml phosphocreatine kinase, 1 mM NADH, and 0.6 mM spermidine, pH 7.4. After incubation the import reaction was stopped by dilution into 5 volumes of ice cold buffer H and centrifuged at 16,000×g in a refrigerated microcentrifuge for 10 min for mitochondria and at 100,000×g for 1 h for microsomes, in order to completely recover all mitochondrial and microsomal protein. To determine whether the GPAT1 associated with the mitochondrial fraction was an intrinsic membrane protein, the mitochondria were washed with either a 0.5 M KCl or a 0.1 M Na2CO3, pH 11.5, solution, or with both solutes simultaneously. After centrifugation at 100,000×g for 1 h, the pellet was then resuspended in sample buffer, heated for 5 min at 70 °C and loaded on an 8% SDS-PAGE. After running, the gel was fixed for 30 min (50% methanol, 10% acetic acid, 40% H2O), incubated for 5 min in 7% acetic acid, 7% methanol, 1% glycerol, soaked briefly in DMSO and then incubated for 3 h in 20% 2,5-diphenyloxazole (PPO) DMSO. The 2,5-diphenyloxazole was then precipitated by adding water, and the gel was dried and exposed to X-ray film for 72 h.
2.6. Electron microscopy
Mitochondrial and submitochondrial fractions were checked for purity and structural integrity by electron microscopy. They were fixed in 2.5% glutaralde-hyde in 0.1 M phosphate buffer (pH 7.2) for 2 h, post-fixed in 1% osmium tetroxide and embedded in epoxi-resin. Ultrathin sections were stained with uranyl acetate and lead citrate and observed with a JEM-1200 EXII electron microscope.
2.7. Immunoblotting
50 μg of total protein from each fraction was separated on an 8% or 12% SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Bio-Rad), and probed with 1/10,000 anti-GPAT1 polyclonal antibody, 1/1,000 anti-voltage-dependent anion channel (VDAC) and anti-GRP78 antibodies (Affinity Bioreagents) or 100 μg/ml anti-phosphatidylethanolamine methyltransferase 2 (PEMT2) antibody (kindly provided by Dr. Dennis Vance, University of Alberta, Canada). The membranes were then washed extensively and probed with horseradish peroxidase-conjugated goat antimouse IgG (Pierce). For chemiluminescent detection, the membranes were incubated with Super Signal detection kit (Pierce). Immunoreactive bands were quantified using Kodak Digital Science 1D software.
3. Results
3.1. Purified mitochondria contain highest GPAT1 specific activity, but GPAT1 protein expression is maximal in the MAV fraction
To determine the precise location of GPAT1, both NEM-resistant GPAT activity and GPAT1 protein expression were assayed in rat liver subcellular fractions. To assess the purity of each fraction, marker proteins were analyzed by Western Blot (Fig. 2 panel I) and by enzyme activity (Table 1). VDAC (Fig. 2-I, A), an OMM marker protein, and cytochrome c oxidase (Table 1), an inner-mitochondrial-membrane (IMM) marker enzyme, were highly enriched in the pure mitochondrial fraction. The presence of immunoreactive PEMT2, a specific marker of MAM [23] identified the MAM fraction (Fig. 2-I, B) and arylesterase activity (Table 1), an ER marker, was abundant in MAM and maximal in microsomes. ER marker protein GRP78 (Fig. 2-I, D) was maximal in microsomes, and most of the marker present in crude mitochondria was recovered in the MAV fraction, whereas in pure mitochondria GRP78 could not be visualized. The subcellular distribution of NEM-sensitive GPAT activity matched the arylesterase activity, and no NEM-sensitive GPAT activity was present in purified rat liver mitochondria.
Fig. 2.
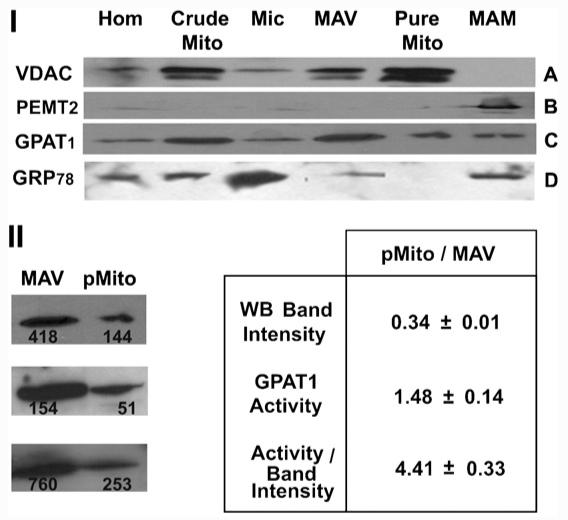
Subcellular localization of GPAT1, a mitochondrial outer membrane, an ER and a MAM-marker protein. I—Rat liver subcellular fractions were analyzed by Western Blot. Fifty μg of total protein from each fraction was loaded onto 12% SDS-PAGE (A and B) or 8% SDS-PAGE (C and D). Panel A: probed with antiVDAC antibody. Panel B: probed with antiPEMT2 antibody. Panel C: probed with antiGPAT1 antibody. Panel D: probed with antiGRP78 antibody Hom, homogenate; Crude Mito, crude mitochondria; Mic, microsomes; MAV, 10,000 g pellet from band 1 of Percoll tube; Pure Mito, pure mitochondria; MAM, 100,000 g pellet from the supernatant of MAV. Results are representative of three independent experiments. II—GPAT1 activity and expression in MAV and pure mitochondria. The GPAT1 specific activity (nmol/min/mg) and protein expression (western blot band intensity, arbitrary units) were quantified in MAV and pure mitochondria (pMito). Ratios between pMito and MAV were calculated and the values in Table 1 represent the average±SD of three independent experiments. Western blots of the three experiments, as well as the quantified areas are shown.
Table 1.
Fold enrichment of marker enzymes and GPAT activity compared to rat-liver homogenate
| Arylesterase | Cytochrome c oxidase | NEM-resistant GPAT | NEM-sensitive GPAT | |
|---|---|---|---|---|
| Crude mitochondria | 0.36±0.06 | 1 | 2.0±0.4 | 0.80±0.10 |
| Microsomes | 1.70±0.30 | 0.19±0.02 | 0.29±0.06 | 1.50±0.20 |
| MAV | 0.29±0.07 | 1.60±0.02 | 2.60±0.10 | 0.74±0.02 |
| Pure mitochondria | ND | 2.70±0.50 | 4.40±0.20 | ND |
| MAM | 1.20±0.40 | ND | 1.30±0.30 | 1.60±0.30 |
Cytochrome oxidase enrichment was normalized to crude mitochondria (0.045±0.015 U/mg). Results are shown as the means±SD from three independent experiments. Specific activities for the homogenate were: arylesterase 0.95±0.24 mmol/min mg, NEM-resistant GPAT 0.28±0.08 nmol/min mg and NEM-sensitive GPAT 0.40±0.06 nmol/min mg. ND: non-detectable values.
Our fractionation procedure recovered a mitochondrial subfraction that we have termed mitochondrial-associated vesicles (MAV). The MAV fraction was obtained by centrifuging the upper band obtained from the Percoll gradient at 10,000×g 10 min (Fig. 1). Electron microscopy showed that MAV consisted of large vesicular membranes (0.5 μm) that sedimented at 10,000×g even though no mitochondria were present (Fig. 3). MAV contained markers from OMM (VDAC protein), ER (arylesterase activity and GRP78 protein) and IMM (cytochrome c oxidase activity). The presence of these marker proteins is consistent with previous studies showing the presence of a vesicular fraction different from the traditionally-isolated ER [24,25], and suggested that MAV might be enriched in mitochondrial contact sites that had co-partitioned with MAM in the Percoll purification step, as well as interacting fragments of the ER membranes. In pure mitochondria GPAT1 expression was only 34% of MAV fraction value (Fig. 2-I, C and Fig. 2-II), but NEM-resistant GPAT specific activity was 1.5-fold higher (Table 1). GPAT1 specific activity relative to protein expression (quantified by western blots) was 4.4-fold higher in pure mitochondria than in MAV (Fig. 2-II). Thus, the large amount of GPAT1 protein present in MAV was relatively inactive.
Fig. 3.

Electron micrographs from pure mitochondria and the MAV fraction. The pellets obtained from centrifugation at 10,000×g after Percoll purification of mitochondria were visualized by electron microscopy at two magnifications. (A and B) pure mitochondria. (C and D) MAV fraction.
3.2. The ER-CS fraction from crude mitochondria contains a less-active GPAT1 pool and GPAT1 specific activity is highest in the OMM
Since the Percoll-gradient purification of crude mitochondria showed an inconsistency between GPAT1 expression and activity, we further fractionated crude mitochondria in order to correlate GPAT1 expression and activity with the presence of mitochondrial and ER components. A discontinuous gradient centrifugation was performed to obtain OMM, a pellet enriched in IMM, and OMM-IMM contact sites from crude mitochondria (Fig. 1). A low-density membrane band was obtained at the 25.2%-37.7% sucrose interface, highly enriched in OMM-markers MAO and VDAC compared to crude mitochondria. This fraction was called OMM, as in previous reports [26]. The pellet from this centrifugation contained a high-density mixed-membrane fraction enriched in cytochrome-c oxidase activity, an IMM marker. An intermediate-density band was collected at the 37.7%-51.3% sucrose interface, which is enriched in both OMM (VDAC and MAO) (Fig. 4-I, B) and IMM (cytochrome-c-oxidase) markers. This fraction has historically been named “contact sites” between OMM and IMM. However, when crude mitochondria that contain an ER component, are used as the starting material for the submitochondrial fraction isolation, the ER components fractionate primarily in the contact-site fraction which has the highest ER-marker enzyme activity [26] as well as NEM-sensitive GPAT activity consistent with the ER-marker; thus, we have called this fraction ER-contact sites (ER-CS) (Fig. 1). Our results corroborate the finding that the ER-component that fractionates with mitochondria, represented by MAM plus MAV, was not evenly distributed among the mitochondrial subfractions, but interacted primarily in the OMM-IMM CS regions. When we performed the same separation starting from Percoll-purified mitochondria which did not contain any ER-marker activity, we obtained pure OMM (OMMp), pure IMM (IMMp) and OMM-IMM-contact-sites (CS) (Fig. 1). The enrichment of the OMM-markers VDAC and MAO in the low-density band and the IMM-marker cytochrome-c-oxidase in the pellet was evident (Fig. 5A, B). The intermediate-density band contained both IMM and OMM markers at about 90% of their maximal activity, indicating that neither OMM nor IMM predominated in the CS fraction (Fig. 5-I). The data showed a discordance between GPAT1 protein expression and activity. The western blot (Figs. 4, 5 II-B) showed higher expression of GPAT1 protein in ER-CS obtained from crude mitochondria, but GPAT1 specific activity was highest in OMMp, obtained from pure mitochondria. The low percentage of GPAT1 activity present in the other mitochondrial subfractions from pure mitochondria, including CS, was consistent with the distribution of MAO activity and the presence of VDAC, suggesting that the GPAT1 protein in these fractions resulted from OMM contamination. Thus, GPAT1 was present, but not enriched in CS. Within crude mitochondria, the highest GPAT1 specific activity was observed in OMM and the highest protein expression in ER-CS, whereas in fractions obtained from pure mitochondria, both GPAT activity and expression were highest in OMMp.
Fig. 4.

GPAT1 protein distribution is consistent with the amount of the ER marker in crude mitochondria. I—arylesterase, monoamine oxidase (MAO), cytochrome c oxidase (Cyt ox) and NEM resistant GPAT (GPAT) activities were measured in crude mitochondria (Crude Mito) and in the subfractions obtained from them: outer mitochondrial membrane (OMM), inner mitochondrial membrane (IMM) and contact sites (ER-CS). Values represent the average±SD of three independent experiments. II—50 μg of crude mitochondria (Crude Mito) and their subfractions OMM, IMM and ER-CS , were separated on a 12% (A) or 8% (B) SDS-PAGE and probed with anti-VDAC and anti-GPAT1 antibodies, respectively. Results are representative of three independent experiments.
Fig. 5.

The distribution of GPAT1 enzymatic activity is consistent with the outer mitochondrial marker in pure mitochondria. I—monoamine oxidase (MAO), cytochrome c oxidase (Cyt ox) and NEM resistant GPAT (GPAT) activities were measured in pure mitochondria (Pure Mito) and in subfractions obtained from them: outer mitochondrial membrane (OMMp), inner mitochondrial membrane (IMMp), and contact sites (CS). Values represent the average±SD of three independent experiments. II—50 μg of pure mitochondria (Pure Mito) and their subfractions: OMMp, IMMp and CS, were separated on a 12% (A) or 8% (B) SDS-PAGE and probed with anti-VDAC and anti-GPAT1 antibodies, respectively. Results are representative of three independent experiments.
3.3. GPAT1 is rapidly imported into mitochondria in an ATP-dependent fashion
Because a large amount of GPAT1 was present in the MAV fraction which contains ER markers, we asked whether GPAT1 might be initially synthesized in the ER and subsequently imported into the OMM. We synthesized recombinant GPAT1 protein in vitro that had the predicted mass of 92 kDa. This radiolabeled GPAT1 was efficiently incorporated into pure mitochondria in a time- and temperature-dependent manner, with incorporation kinetics that follow a hyperbolic-Hill-three-parameter equation (Fig. 6-I). When the autoradiographic bands were quantified as radioactivity incorporated into mitochondria, they showed rapid incorporation kinetics and reached saturation at 10 min (Fig. 6-II). With incubations at 4 °C for as long as 60 min, however, no GPAT1 was incorporated into mitochondria, showing that incorporation was temperature-dependent. In the absence of ATP the autoradiographic band for GPAT1 was similar to the one corresponding to time 0 (Fig. 7-I), showing that internalization of GPAT1 into mitochondria at 30 °C required an external source of ATP. Further, when the in vitro synthesis was performed in the presence of microsomal membranes, GPAT1 was not targeted to microsomes (Fig. 7-II). Similar results were obtained when microsomes were added after GPAT1 synthesis had been completed. Thus, GPAT1 incorporation into OMM was not preceded by incorporation into a microsomal fraction. The radiolabeled GPAT1 incorporated into pure mitochondria (Fig. 7-II) was resistant to both alkaline and saline extractions. When mitochondrial membranes were washed sequentially with alkali, then salt, we still detected radioactive GPAT1 retained in the mitochondrial membranes (Fig. 7-II), consistent with its insertion into the OMM as an intrinsic protein. The time- and ATP-dependence import experiments of GPAT1 were also performed with crude mitochondria, and the results were similar (data not shown).
Fig. 6.

GPAT1 synthesized in vitro is targeted to mitochondria in a time and temperature dependent manner. Labeled recombinant GPAT1 was synthesized in vitro and incubated at 30 °C with pure mitochondria for different periods of time. Panel I: Mitochondrial proteins were separated on an 8% SDS-PAGE and autoradiographed. Panel II: Quantitative determination of the radioactivity incorporated into mitochondria. Results are expressed as radioactivity recovered from mitochondria and are representative of three independent experiments. The incorporation kinetics followed a hyperbolic-Hill-three-parameter equation with y = axb/(cb+xb), for the values: a = 4130, b = 0.72 and c = 5.95.
Fig. 7.

The incorporation of GPAT1 into mitochondria is ATP-dependent and does not require ER components. I—Incorporation of GPAT1 synthesized in vitro to pure mitochondria was performed either in the absence (lanes 1-3) or the presence (lanes 4-6) of ATP and an ATP recycling system. The incubations were performed for 0 (lanes 1 and 4), 15 (lanes 2 and 5) and 60 (lanes 3 and 6) min. Input corresponds to the radioactivity from the labeled GPAT1 incubated with the mitochondria. II—100 μg of pure mitochondrial (Mito) and microsomal (Micros.) fractions were used as targets for labeled GPAT1 synthesized in vitro. After a 60 min incubation peripheral proteins were extracted by saline and alkaline washes (Washed Mito). Alk: mitochondria washed with 0.1 M sodium carbonate, pH 11.5; Chaotr: mitochondria washed with 0.5 M potassium chloride; (A + C): mitochondria washed with both the alkali and the chaotrope. Microsomal incorporation was tested co-translationally. Results are representative of three independent experiments.
4. Discussion
The aim of our work was to establish without ambiguity the subcellular and sub-mitochondrial location of GPAT1. To do this, we used a polyclonal antibody raised against recombinant GPAT1 protein expressed in Escherichia coli and assayed the NEM-resistant GPAT activity. The anti-GPAT1 antibody does not recognize the microsomal isoform of GPAT, but it does recognize the two mitochondrial GPAT isoforms, GPAT1 and 2 [2]. GPAT1 (92 kDa) and 2 (87 kDa) differ in molecular mass. The anti-GPAT1 antibody identified only a single band with identical molecular mass in both MAV and pure mitochondria fractions. In the present studies, the antibody primarily probes only the GPAT1 isoform because the amount of NEM-sensitive GPAT2 is very low in mouse liver (Wang, S. and Coleman RA, unpublished results). In fact, all NEM-sensitive GPAT activity present in the crude mitochondrial fraction disappears when pure mitochondria are obtained. Moreover, the NEM-sensitive GPAT activity distribution in MAV and crude mitochondria is consistent with the distribution of the ER-marker enzyme arylesterase and the ER-marker protein GRP78 (Table 1 and Fig. 2-I D), implying that all NEM-sensitive GPAT activity corresponds exclusively to the ER isoform. These data support the point that little GPAT2 protein and activity are present in rat liver mitochondria [2]. Physical connections between components of the endoplasmic reticulum and the OMM have been well documented [27-33]. ER-contact sites from crude mitochondria were obtained by discontinuous sucrose gradient centrifugation, and electron micrographs of the “contact site” band show ER and mitochondrial membranes that contain interacting regions. The identity of ER-CS as a concrete subfraction of crude mitochondria was also confirmed by other authors by centrifugation in a continuous sucrose gradient, supporting the idea that ER-mitochondria interactions are not casual, but instead, actually occur in vivo [26]. A portion of this ER compartment co-sediments at low speed with crude mitochondria and is termed mitochondria associated membranes (MAM), a fraction that can also be obtained by Percoll gradient centrifugation [18,23]. This specialized MAM fraction is enriched in lipid biosynthetic enzyme activities such as phosphatidylserine synthase [18], acyl-CoA:cholesterol acyltransferase [23], diacylglycerol acyltransferase [23], acyl-CoA synthetase 4 [34], and phosphatidylethanolamine N-methyltransferase [18]. Although the function of MAM has not been determined, it has been variously suggested that MAM is involved in phospholipid transport [35], VLDL secretion [23] and aminophospholipid synthesis [36].
We did not find that GPAT1 was enriched in MAM. Instead, compared to the homogenate, GPAT1 was enriched in MAV, a novel vesicular compartment, in which the specific activity of GPAT was similar to that in crude mitochondria and MAM. Recovery of GPAT1 activity in MAV plus MAM (both derived from the upper band in the Percoll purification) was only about 20% of the total GPAT activity present in crude mitochondria. The remaining GPAT activity was recovered in the pure mitochondria fraction. Thus, although the highest specific activity was found in pure mitochondria, the maximum protein expression was present in MAV. This inconsistency between activity and protein expression was confirmed by the fact that, although MAM and crude mitochondria had similar specific activities, protein expression was significantly higher in crude mitochondria. We interpret this inconsistency to mean that the relatively inactive pool of GPAT1 present in crude mitochondria partitioned with the MAV fraction during the Percoll purification and left a more active pool of GPAT1 in the purified mitochondria.
What is the nature of the MAV fraction? It differs from MAM because it did not contain PEMT2, the MAM-marker protein [37]. Instead, MAV exhibited features of both ER and mitochondria. By electron microscopy, MAV contained large vesicles that sedimented at 10,000×g even in the absence of mitochondria (Fig. 3), as well as marker proteins for ER, IMM and OMM. Although some studies using the MAV fraction have been reported, in general, the MAV and MAM fractions have not been considered independently. For example, in CHO and thymoma cells, the 10,000-g pellet from the upper band of the Percoll gradient (called MAMp) and the associated ER-mitochondrial fraction were both essential for glycosylphosphatidylinositol biosynthesis [25]. The fraction containing both MAV and MAM has also been reported to traffic a viral protein between ER and mitochondria [38]. Thus, we hypothesize that MAV might be comprised of an interacting region between ER and mitochondria or a region enriched in mitochondrial membranes that interact either with MAM or ER and that contain both ER and mitochondrial markers. To determine the validity of this hypothesis, we used ER-CS isolated from crude mitochondria because, unlike pure mitochondria, the ER-CS contains ER markers. In accordance with data from others [26], most of the ER marker activity present in crude mitochondria was subsequently recovered in the ER-CS fraction. It has been hypothesized that either ER or MAM interacts with mitochondria at specific regions that can be isolated by centrifugation, and that these regions constitute a sub-mitochondrial compartment in which processes like lipid trafficking would occur [36]. Although we detected the highest expression of GPAT1 protein in ER-CS, GPAT specific activity was low in this fraction. Further, GPAT specific activity was only about 33% lower in MAV compared to pure mitochondria, suggesting that the dense GPAT1 protein band in this fraction (Fig. 2-II) was largely inactive. This inactive pool of GPAT1 could be obtained from crude mitochondria both by sucrose gradient centrifugation to produce ER-CS and by Percoll centrifugation to produce MAV (Fig. 1). Because GPAT1 is a mitochondrial enzyme whose lysophosphatidic acid product must be transported to the endoplasmic reticulum to complete the biosynthesis of TAG and glycerophospholipids, it is not surprising that GPAT1 is located in a submitochondrial compartment closely associated with the ER. This compartment may be important for shuttling lysophosphatidic acid and phosphatidic acid intermediates from the mitochondria to the ER [1].
The presence of GPAT1 protein in MAV and in ER-CS suggested the possibility that the protein might be targeted first to the ER, and then transferred to the mitochondria for activation. GPAT1 is encoded by a nuclear gene [39] and does not contain a mitochondrial targeting signal sequence. Because incorporation kinetics were identical with both pure and crude mitochondria as targets, our data showed that GPAT1 directly entered the mitochondria in the absence of an ER component. In addition, GPAT1 was not incorporated into microsomal membranes. These data are similar to studies of CPT1, which is also targeted directly to mitochondria and not to ER [40]. Moreover, the ATP requirement for GPAT1 import into the OMM and the absence of protease cleavage is consistent with the import mechanism generally accepted for an OMM protein [41,42]. Thus, we conclude that GPAT1 reaches MAV through the OMM, probably through IMM and OMM contact sites that interact with ER.
Although GPAT1 was highly expressed in ER-CS, enzyme activity was highest in pure mitochondria. How is this activity distributed in mitochondrial subfractions? To answer this question we screened GPAT1 expression and activity in subfractions obtained from pure mitochondria and found maximal specific activity in OMMp and CS. However, because the submitochondrial fraction distribution of GPAT1 activity is consistent with the OMM marker MAO, it does not appear that GPAT1 is selectively enriched in the CS. In other words, the distribution of GPAT1 is similar to the submitochondrial localization proposed for CPT1 [16].
Our major finding is that two pools of GPAT1 are present in rat liver mitochondria: an active one, located in the OMM and a less active one, located in membranes that are associated with both mitochondria and ER (MAV and ER-CS). Because the molecular mass of GPAT1 is identical in both locations, activation by post-translational protease cleavage is unlikely. Post-translational phosphorylation/dephosphorylation or protein-protein interactions may regulate GPAT1 activity in these fractions.
Acknowledgments
We are grateful to Dennis Vance, PhD (University of Alberta) for anti-PEMT2 antibodies. This work was supported by grants from the National Institutes of Health (DK52172, P30-DK350, and TW06034-01) and CONICET PIP6503. MPM and MAM are research fellows and MRGB is a member of the Carrera del Investigador Científico y Tecnológico, CONICET, Argentina.
Abbreviations
- CHO
Chinese Hamster Ovary
- CPT
carnitine-palmitoyl transferase
- ER-CS
contact sites obtained from crude mitochondria
- CS
contact sites obtained from pure mitochondria
- DTT
dithiothreitol
- GPAT
glycerol-3-phosphate acyltransferase
- IMM
inner mitochondrial membrane obtained from crude mitochondria
- IMMp
inner mitochondrial membrane obtained from pure mitochondria
- MAM
mitochondrial associated membrane
- MAO
monoamine oxidase
- MAV
mitochondrial associated vesicles
- NEM
N-ethylmaleimide
- OMM
outer mitochondrial membrane obtained from crude mitochondria
- OMMp
outer mitochondrial membrane obtained from pure mitochondria
- PEMT
phosphatidylethanolamine methyltransferase
- TAG
triacylglycerol
- VDAC
voltage-dependent anion channel
5. Note added in proof
An ER-microsomal isoform has been recently cloned (Cao J, Li JL, Li D, Tobin JF, Gimeno RE. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc Natl Acad Sci U S A. 2006 Dec 26;103(52):19695-700.)
References
- [1].Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- [2].Lewin TM, Schwerbrock NM, Lee DP, Coleman RA. Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J. Biol. Chem. 2004;279:13488–13495. doi: 10.1074/jbc.M314032200. [DOI] [PubMed] [Google Scholar]
- [3].Coleman RA, Lewin TM, Muoio DM. Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu. Rev. Nutr. 2000;20:77–103. doi: 10.1146/annurev.nutr.20.1.77. [DOI] [PubMed] [Google Scholar]
- [4].Yet SF, Lee S, Hahm YT, Sul HS. Expression and identification of p90 as the murine mitochondrial glycerol-3-phosphate acyltransferase. Biochemistry. 1993;32:9486–9491. doi: 10.1021/bi00087a029. [DOI] [PubMed] [Google Scholar]
- [5].Bhat BG, Wang P, Kim JH, Black TM, Lewin TM, Fiedorek FT, Jr., Coleman RA. Rat sn-glycerol-3-phosphate acyltransferase: molecular cloning and characterization of the cDNA and expressed protein. Biochim. Biophys. Acta. 1999;1439:415–423. doi: 10.1016/s1388-1981(99)00103-1. [DOI] [PubMed] [Google Scholar]
- [6].Igal RA, Wang S, Gonzalez-Baro M, Coleman RA. Mitochondrial glycerol phosphate acyltransferase directs the incorporation of exogenous fatty acids into triacylglycerol. J. Biol. Chem. 2001;276:42205–42212. doi: 10.1074/jbc.M103386200. [DOI] [PubMed] [Google Scholar]
- [7].Lewin TM, Wang S, Nagle CA, Van Horn CG, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 directs the meta-bolic fate of exogenous fatty acids in hepatocytes. Am. J. Physiol.: Endocrinol. Metab. 2005;288:E835–E844. doi: 10.1152/ajpendo.00300.2004. [DOI] [PubMed] [Google Scholar]
- [8].Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol. Cell. Biol. 2002;22:8204–8214. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lewin TM, Granger DA, Kim JH, Coleman RA. Regulation of mitochondrial sn-glycerol-3-phosphate acyltransferase activity: response to feeding status is unique in various rat tissues and is discordant with protein expression. Arch. Biochem. Biophys. 2001;396:119–127. doi: 10.1006/abbi.2001.2604. [DOI] [PubMed] [Google Scholar]
- [10].Shin DH, Paulauskis JD, Moustaid N, Sul HS. Transcriptional regulation of p90 with sequence homology to Escherichia coli glycerol-3-phosphate acyltransferase. J. Biol. Chem. 1991;266:23834–23839. [PubMed] [Google Scholar]
- [11].Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem. J. 1999;338(Pt 3):783–791. [PMC free article] [PubMed] [Google Scholar]
- [12].Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J. Biol. Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- [13].Gonzalez-Baro MR, Granger DA, Coleman RA. Mitochondrial glycerol phosphate acyltransferase contains two transmembrane domains with the active site in the N-terminal domain facing the cytosol. J. Biol. Chem. 2001;276:43182–43188. doi: 10.1074/jbc.M107885200. [DOI] [PubMed] [Google Scholar]
- [14].Hammond LE, Neschen S, Romanelli AJ, Cline GW, Ilkayeva OR, Shulman GI, Muoio DM, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J. Biol. Chem. 2005;280:25629–25636. doi: 10.1074/jbc.M503181200. [DOI] [PubMed] [Google Scholar]
- [15].Fraser F, Zammit VA. Enrichment of carnitine palmitoyltransferases I and II in the contact sites of rat liver mitochondria. Biochem. J. 1998;329(Pt 2):225–229. doi: 10.1042/bj3290225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoppel C, Kerner J, Turkaly P, Tandler B. Rat liver mitochondrial contact sites and carnitine palmitoyltransferase-I. Arch. Biochem. Biophys. 2001;392:321–325. doi: 10.1006/abbi.2001.2463. [DOI] [PubMed] [Google Scholar]
- [17].Hovius R, Lambrechts H, Nicolay K, de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta. 1990;1021:217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- [18].Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- [19].Hoppel C, Kerner J, Turkaly P, Minkler P, Tandler B. Isolation of hepatic mitochondrial contact sites: previously unrecognized inner membrane components. Anal. Biochem. 2002;302:60–69. doi: 10.1006/abio.2001.5531. [DOI] [PubMed] [Google Scholar]
- [20].Pellon-Maison M, Coleman RA, Gonzalez-Baro MR. The C-terminal region of mitochondrial glycerol-3-phosphate acyltransferase-1 interacts with the active site region and is required for activity. Arch. Biochem. Biophys. 2006;450:157–166. doi: 10.1016/j.abb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- [21].Shephard EH, Hubscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem. J. 1969;113:429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Salach JI. Monoamine oxidase from beef liver mitochondria: simplified isolation procedure, properties, and determination of its cysteinyl flavin content. Arch. Biochem. Biophys. 1979;192:128–137. doi: 10.1016/0003-9861(79)90078-x. [DOI] [PubMed] [Google Scholar]
- [23].Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J. Biol. Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- [24].Pottekat A, Menon AK. Subcellular localization and targeting of N-acetylglucosaminyl phosphatidylinositol de-N-acetylase, the second enzyme in the glycosylphosphatidylinositol biosynthetic pathway. J. Biol. Chem. 2004;279:15743–15751. doi: 10.1074/jbc.M313537200. [DOI] [PubMed] [Google Scholar]
- [25].Vidugiriene J, Sharma DK, Smith TK, Baumann NA, Menon AK. Segregation of glycosylphosphatidylinositol biosynthetic reactions in a subcompartment of the endoplasmic reticulum. J. Biol. Chem. 1999;274:15203–15212. doi: 10.1074/jbc.274.21.15203. [DOI] [PubMed] [Google Scholar]
- [26].Ardail D, Gasnier F, Lerme F, Simonot C, Louisot P, Gateau-Roesch O. Involvement of mitochondrial contact sites in the subcellular compartmentalization of phospholipid biosynthetic enzymes. J. Biol. Chem. 1993;268:25985–25992. [PubMed] [Google Scholar]
- [27].Franke WW, Kartenbeck J. Outer mitochondrial membrane continuous with endoplasmic reticulum. Protoplasma. 1971;73:35–41. doi: 10.1007/BF01286409. [DOI] [PubMed] [Google Scholar]
- [28].Ardail D, Lerme F, Louisot P. Involvement of contact sites in phosphatidylserine import into liver mitochondria. J. Biol. Chem. 1991;266:7978–7981. [PubMed] [Google Scholar]
- [29].Cascarano J, Montisano DF, Pickett CB, James TW. Rough endo-plasmic reticulum-mitochondrial complexes from rat liver. An extramito-chondrial succinic dehydrogenase associated with this rough endoplasmic reticulum. Exp. Cell Res. 1982;139:39–50. doi: 10.1016/0014-4827(82)90316-0. [DOI] [PubMed] [Google Scholar]
- [30].Lewis JA, Tata JR. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J. Cell Sci. 1973;13:447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- [31].Meier PJ, Spycher MA, Meyer UA. Isolation and characterization of rough endoplasmic reticulum associated with mitochondria from normal rat liver. Biochim. Biophys. Acta. 1981;646:283–297. doi: 10.1016/0005-2736(81)90335-7. [DOI] [PubMed] [Google Scholar]
- [32].Shore GC, Tata JR. Two fractions of rough endoplasmic reticulum from rat liver. I. Recovery of rapidly sedimenting endoplasmic reticulum in association with mitochondria. J. Cell Biol. 1977;72:714–725. doi: 10.1083/jcb.72.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mannella CA, Buttle K, Rath BK, Marko M. Electron microscopy tomography of rat-liver mitochondria and their interactions with the endoplasmic reticulum. BioFactors. 1998;8:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- [34].Lewin TM, Van Horn CG, Krisans SK, Coleman RA. Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes, and mitochondrial-associated membrane. Arch. Biochem. Biophys. 2002;404:263–270. doi: 10.1016/s0003-9861(02)00247-3. [DOI] [PubMed] [Google Scholar]
- [35].Daum G, Vance JE. Import of lipids into mitochondria. Prog. Lipid Res. 1997;36:103–130. doi: 10.1016/s0163-7827(97)00006-4. [DOI] [PubMed] [Google Scholar]
- [36].Voelker DR. Bridging gaps in phospholipid transport. Trends Biochem. Sci. 2005;30:396–404. doi: 10.1016/j.tibs.2005.05.008. [DOI] [PubMed] [Google Scholar]
- [37].Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J. Biol. Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- [38].Schwer B, Ren S, Pietschmann T, Kartenbeck J, Kaehlcke K, Bartenschlager R, Yen TS, Ott M. Targeting of hepatitis C virus core protein to mitochondria through a novel C-terminal localization motif. J. Virol. 2004;78:7958–7968. doi: 10.1128/JVI.78.15.7958-7968.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shin DH, Paulauskis JD, Moustaid N, Sul HS. Transcriptional regulation of p90 with sequence homology to Escherichia coli glycerol-3-phosphate acyltransferase. J. Biol. Chem. 1991;266:23834–23839. [PubMed] [Google Scholar]
- [40].Broadway NM, Pease RJ, Birdsey G, Shayeghi M, Turner NA, Saggerson ED. The liver isoform of carnitine palmitoyltransferase 1 is not targeted to the endoplasmic reticulum. Biochem. J. 2003;370:223–231. doi: 10.1042/BJ20021269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Truscott KN, Brandner K, Pfanner N. Mechanisms of protein import into mitochondria. Curr. Biol. 2003;13:R326–R337. doi: 10.1016/s0960-9822(03)00239-2. [DOI] [PubMed] [Google Scholar]
- [42].Rapaport D. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J. Cell Biol. 2005;171:419–423. doi: 10.1083/jcb.200507147. [DOI] [PMC free article] [PubMed] [Google Scholar]


