Abstract
The sensitivity of KATP channels to high-affinity block by sulfonylureas and to stimulation by K+ channel openers and MgADP (PCOs) is conferred by the regulatory sulfonylurea receptor (SUR) subunit, whereas ATP inhibits the channel through interaction with the inward rectifier (Kir6.2) subunit. Phosphatidylinositol 4,5-bisphosphate (PIP2) profoundly antagonized ATP inhibition of KATP channels expressed from cloned Kir6.2+SUR1 subunits, but also abolished high affinity tolbutamide sensitivity. By stabilizing the open state of the channel, PIP2 drives the channel away from closed state(s) that are preferentially affected by high affinity tolbutamide binding, thereby producing an apparent loss of high affinity tolbutamide inhibition. Mutant KATP channels (Kir6.2[ΔN30] or Kir6.2[L164A], coexpressed with SUR1) also displayed an “uncoupled” phenotype with no high affinity tolbutamide block and with intrinsically higher open state stability. Conversely, Kir6.2[R176A]+SUR1 channels, which have an intrinsically lower open state stability, displayed a greater high affinity fraction of tolbutamide block. In addition to antagonizing high-affinity block by tolbutamide, PIP2 also altered the stimulatory action of the PCOs, diazoxide and MgADP. With time after PIP2 application, PCO stimulation first increased, and then subsequently decreased, probably reflecting a common pathway for activation of the channel by stimulatory PCOs and PIP2. The net effect of increasing open state stability, either by PIP2 or mutagenesis, is an apparent “uncoupling” of the Kir6.2 subunit from the regulatory input of SUR1, an action that can be partially reversed by screening negative charges on the membrane with poly-l-lysine.
Keywords: K+ current, sulfonylurea, MgADP, diazoxide, PIP2
Introduction
The 10 yr that followed the discovery of ATP-sensitive (KATP) channels (Noma 1983) led to the delineation of complex regulation by intracellular nucleotides and pharmacological agents (reviewed in Ashcroft 1988; Nichols and Lederer 1991; Terzic et al. 1994). The last 3 yr have seen a renewed interest in the regulation of ATP-sensitive potassium channels as a result of the cloning of the constituent subunits (Aguilar-Bryan et al. 1995; Inagaki et al. 1995, Inagaki et al. 1996). Uniquely, KATP channels are normally formed as a complex of sulfonylurea receptor (SURx)1 and inward rectifier (Kir6.x) subunits (Inagaki et al. 1995, Inagaki et al. 1997; Clement et al. 1997; Shyng and Nichols 1997). Recent studies demonstrate that the Kir6.x subunits form the pore, and control the hallmark inhibition by ATP (Shyng et al. 1997a; Tucker et al. 1997, Tucker et al. 1998; Drain et al. 1998), whereas the SURx subunit controls the sensitivity to the inhibitory sulfonylurea drugs, and to activating nucleotide diphosphates and potassium channel opening drugs (Aguilar-Bryan et al. 1995; Inagaki et al. 1996; Isomoto et al. 1996; Nichols et al. 1996; Shyng et al. 1997b; Gribble et al. 1997a,Gribble et al. 1997b; Schwanstecher et al. 1998).
Deletion of up to ∼36 amino acids from the COOH terminus of Kir6.2 results in the generation of ATP-sensitive channels in the absence of SURx subunits (Tucker et al. 1997; Zerangue et al. 1999), but these channels are not activated by MgADP or potassium channel openers (PCOs), nor are they inhibited at high affinity by sulfonylurea drugs, consistent with these agents acting through the SURx subunit (Gribble et al. 1997a). MgATP clearly binds to the nucleotide binding folds of SUR1 (Ueda et al. 1997), and ATP hydrolysis seems to be required for binding PCOs (Schwanstecher et al. 1998) and transduction (Nichols et al. 1996; Gribble et al. 1997b; Shyng et al. 1997a) of the stimulatory PCO signal to the channel. The physical nature of the coupling of SURx to Kir6.x subunits is essentially unknown at the present time, although intriguingly, Clement et al. 1997 demonstrated that Kir6.2 could be labeled with azido-glibenclamide only in the presence of SUR1 subunits, consistent with a tight physical association of the two subunits (Lorenz et al. 1998). In the present study, we have explored the functional coupling of SUR1 to Kir6.2 The results demonstrate that the pharmacological control of KATP channel function through SUR1 subunits can be “uncoupled” when the channel open-state stability is increased, either by mutation of the Kir6.2 subunit, or by manipulation of the phospholipid composition of the membrane. The results also suggest that nucleotide hydrolysis and PCO binding stimulate the channel activity by a convergent pathway with phosphatidylinositol 4,5-bisphosphate (PIP2). A preliminary report of these results was made to the Biophysical Society (annual meeting, Kansas City, MO, February, 1998).
methods
Molecular Biology
Kir6.2 mutations were prepared using PCR methods. Resulting PCR products were subcloned into the EcoRI-ClaI sites of the mammalian expression vector pCMV6b. SUR1 was cloned into the pECE expression vector. The nucleotide sequences of the mutant Kir6.2 constructs were verified by fluorescence-based cycle sequencing using AmpliTaq DNA polymerase, FS (Perkin-Elmer Cetus Corp.), and an ABI PRISM DNA sequencer (Perkin-Elmer Cetus Corp.).
Expression of KATP Channels in COSm6 Cells
COSm6 cells were plated at a density of ∼2.5 × 105 cells per well (30-mm six-well dishes) and cultured in Dulbecco's modified Eagle medium plus 10 mM glucose (DMEM-HG), supplemented with fetal calf serum (10%). The next day, cells were transfected by incubation for 4 h at 37°C in DMEM containing 10% nuserum, 0.4 mg/ml diethylaminoethyl-dextran, 100 μM chloroquine, and 5 μg each of pCMV6b-Kir6.2, pECE-SUR1, and pECE–green fluorescent protein cDNA. Cells were subsequently incubated for 2 min in HEPES-buffered salt solution containing DMSO (10%), and returned to DMEM-HG plus 10% FCS. Cells were assayed for KATP currents by patch-clamp measurements, 2–4 d after transfection.
Patch-Clamp Measurements
Patch-clamp experiments were made at room temperature, in an oil-gate chamber that allowed the solution bathing the exposed surface of the isolated patch to be changed rapidly. Micropipettes were pulled from thin-walled glass (WPI Inc.) on a horizontal puller (Sutter Instrument Co.). Electrode resistance was typically 0.5–1 MΩ when filled with K-INT solution (see below). Microelectrodes were “sealed” onto cells that fluoresced green under UV illumination by applying light suction to the rear of the pipette. Inside-out patches were obtained by lifting the electrode and then passing the electrode tip through the oil-gate. Membrane patches were voltage-clamped with an Axopatch 1B patch-clamp amplifier (Axon Inc.). The standard bath (intracellular) and pipette (extracellular) solution used in these experiments (K-INT) had the following composition: 140 mM KCl, 10 mM K-HEPES, 1 mM K-EGTA, pH 7.3. PIP2 was bath sonicated in ice for 30 min before use. PIP2 was obtained from Boehringer Mannheim. Tolbutamide, diazoxide, nucleotides, and poly-l-lysine (mol wt ∼ 1,000) were purchased from Sigma Chemical Co. Tolbutamide and diazoxide were dissolved as stock solutions in DMSO and diluted to <1% DMSO. All currents were measured at a membrane potential of −50 mV (pipette voltage = +50 mV). Inward currents at this voltage are shown as upward deflections. Data were normally filtered at 0.5–3 kHz, signals were digitized at 22 kHz (Neurocorder; Neurodata) and stored on video tape. Experiments were replayed onto a chart recorder, or digitized into a microcomputer using Axotape software (Axon Inc.). Off-line analysis was performed using Microsoft Excel programs. Wherever possible, data are presented as mean ± SEM. Microsoft Solver was used to fit data by a least-square algorithm.
results
High Affinity Sulfonylurea Sensitivity Is Lost After Kir6.2 NH2-Terminal Deletion
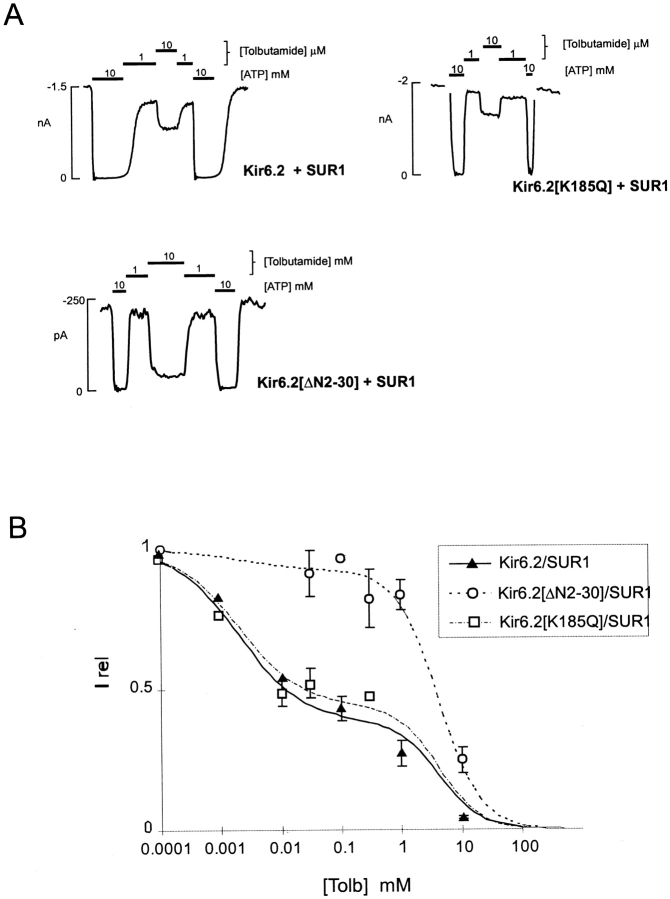
Gribble et al. 1997a reported that tolbutamide inhibition of Kir6.2+SUR1 coexpressed channels in Xenopus oocytes is biphasic, consisting of low and high affinity components. The mechanistic basis of the biphasic response to tolbutamide is presently unknown (see discussion), but it is clear that high affinity sulfonylurea interaction is with the SUR1 subunit (Aguilar-Bryan et al. 1995), whereas a low affinity action may occur through direct interaction with the Kir6.2 subunit (Gribble et al. 1997a). As shown in Fig. 1, similar biphasic dose–response curves are seen for both wild-type Kir6.2+SUR1 (WT+SUR1) channels and for Kir6.2[K185Q]+SUR1 channels expressed in COSm6 cells. The K185Q mutation in Kir6.2 reduces ATP sensitivity, possibly by altering ATP binding affinity, but does not affect the ATP-independent open probability (Tucker et al. 1997; Koster et al. 1999). In contrast, Kir6.2[ΔN2-30]+SUR1 channels also have a reduced ATP sensitivity, which in this case results from open-state stabilization that is reflected by near continuous bursting at the single channel level (Koster et al. 1999), and these channels show only low affinity inhibition by tolbutamide (Fig. 1 A). This raises alternate possibilities that high affinity tolbutamide block is lacking from Kir6.2[ΔN2-30] channels because the NH2 terminus is physically involved in “coupling” to the regulatory effects of SUR1, or because the high affinity inhibitory effect of tolbutamide depends on channel open state stability.
Figure 1.
Tolbutamide sensitivity of KATP currents from cells coexpressing Kir6.2, Kir6.2[ΔN2-30], or Kir6.2[K185Q] mutant subunits and SUR1. (A) Representative currents recorded from inside-out membrane patches containing wild-type or mutant KATP channels at −50 mV in Kint solution (see methods). Patches were exposed to differing [tolbutamide] or 10 mM ATP, as shown. (B) Steady state dependence of membrane current on [tolbutamide] [mean ± SEM, relative to current in zero tolbutamide (Irel)] for wild-type and mutant channels (from records such as those shown in Fig. 1 A). Data points represent the mean ± SEM (n = 3–8 patches). For all channels, the lines are fits of the sum of two Hill components (as in Gribble et al. 1997a), each of the form {Irel = 1/[1 + ([tolbutamide]/K 1/2)H]} with H fixed at 1.3 in each case, and the K 1/2 = 2 μM (high affinity) and 4.2 mM (low affinity). The relative fraction of each component was varied. The high-affinity component was 40, 35, and 7% for wild-type, Kir6.2[K185Q]/SUR1, and Kir6.2[K185Q]/SUR1 channels, respectively.
High Affinity Sulfonylurea Sensitivity Is Lost After PIP2 Treatment of Wild-Type Channels
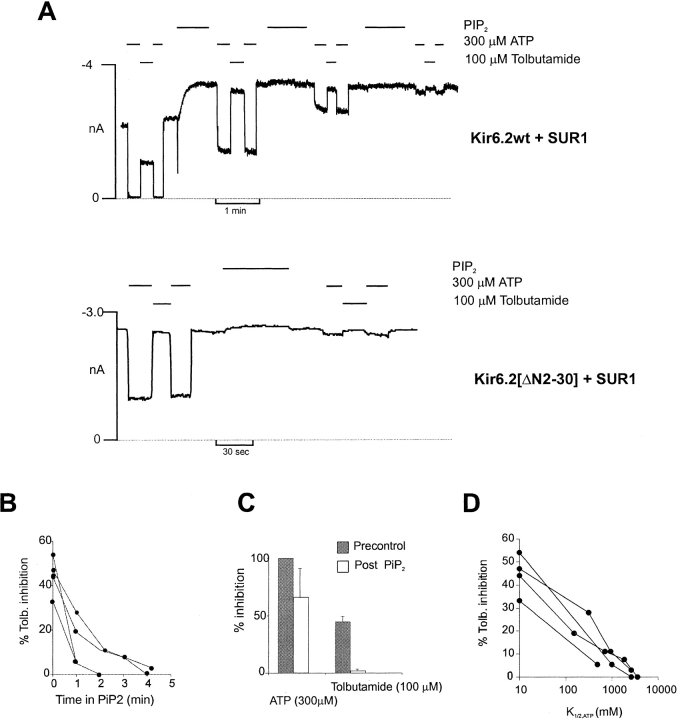
We can explore the correlation between tolbutamide sensitivity and open-state stability of the channel by applying PIP2. PIP2 increases the channel open probability by increasing bursting behavior of the single channel and decreases the sensitivity to ATP (Baukrowitz et al. 1998; Shyng and Nichols 1998). Although direct experimental proof is not available, both actions can be explained by models in which the action of PIP2 is to stabilize the channel open or bursting state, with ATP binding to, and stabilizing, the channel closed state (Shyng et al. 1997a). As shown in Fig. 2, treatment of wild-type Kir6.2+SUR1 channels with PIP2 leads to increased overall channel activity and loss of ATP sensitivity (Baukrowitz et al. 1998; Shyng and Nichols 1998). Concomitant with this increase in open-state stability, there is a gradual and complete loss of high affinity tolbutamide block (Fig. 2A and Fig. C). The rate of loss of both ATP sensitivity and high tolbutamide sensitivity (Fig. 2 B) are quite variable from patch to patch. However, there is a reasonable correlation between the tolbutamide inhibition and ATP sensitivity (Fig. 2 D).
Figure 2.
Pip2 abolishes high-affinity tolbutamide inhibition of Kir6.2+SUR1 channels. (A) Representative currents from inside-out patches containing wild-type (Kir6.2+SUR1) or Kir6.2 [ΔN2-30]+SUR1 channels. The patches were exposed to [tolbutamide], [ATP], or [Pip2] as indicated. The dashed line indicates zero current (determined in 5 mM ATP). (B) Percent tolbutamide inhibition versus time in Pip2 for four individual patches containing wild-type KATP channels. (C) Percent inhibition by ATP or tolbutamide before (Precontrol) or after (Post Pip2) application of Pip2 (2–4 min) from patches in B. (D) Plot of the change in percent tolbutamide inhibition versus change in K 1/2,ATP after application of Pip2 for patches from B.
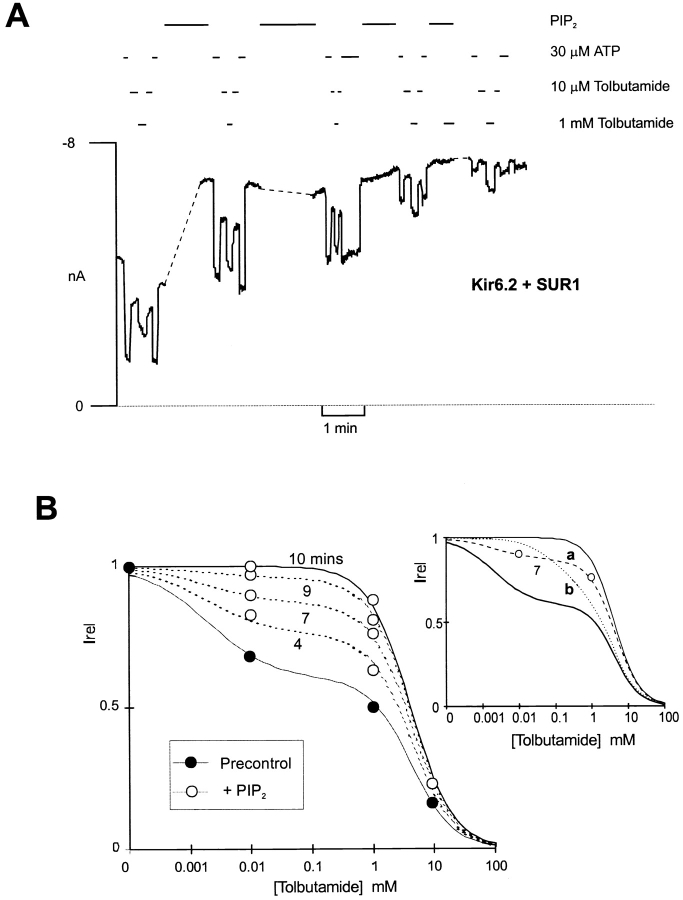
The loss of high affinity tolbutamide inhibition could occur because the high affinity component actually changes affinity (i.e., the real, or apparent, binding affinity of tolbutamide is reduced), or because high affinity binding fails to cause inhibition of channel activity. As shown in Fig. 3, the latter explanation is correct; with time after addition of PIP2, the dose–response relationship can be fit by assuming that the high affinity inhibition becomes a progressively smaller fraction of the [tolbutamide]-inhibition relationship. Data points at intermediate times cannot be fit by assuming a constant high affinity fraction, with reduced affinity. This is consistent with an effect of PIP2 on the coupling of high affinity binding to channel inhibition, not on modifying tolbutamide binding itself.
Figure 3.
Mechanism of the PIP2-induced loss of tolbutamide block. (A) Representative current recorded from inside-out membrane patches containing wild-type KATP channels exposed to differing [tolbutamide] or 30 μM ATP, as shown. The gaps in the record are 4.5, 2, and 1 min. The dashed line indicates zero current (determined in 5 mM ATP). (B) Plot of relative current (Irel) versus [tolbutamide] for trace segments shown in A, both before (•) and with time after (○) Pip2 application. Data at 10 mM tolbutamide are from control and 10-min time points. Fitted lines correspond to a least squares fit of the sum of two Hill equations as described in Fig. 1with the fraction of high affinity inhibition allowed to vary (37, 23, 13, 4, 0% at 0, 4.5, 7, 8 and 10 min) as indicated, after PIP2 application. The insert shows data only at 7 min. The dashed lines correspond to (A, dashed) the same curve as above, and (B, dotted) a least squares fit of the sum of two Hill equations, with the fraction of high affinity inhibition held constant at 37%, but with the affinity (i.e., K 1/2) allowed to vary. It is clear that high affinity block disappears because the high affinity fraction disappears, not because there is a change in the sensitivity of this component.
High Affinity Sulfonylurea Sensitivity Depends on the Channel Open-State Stability
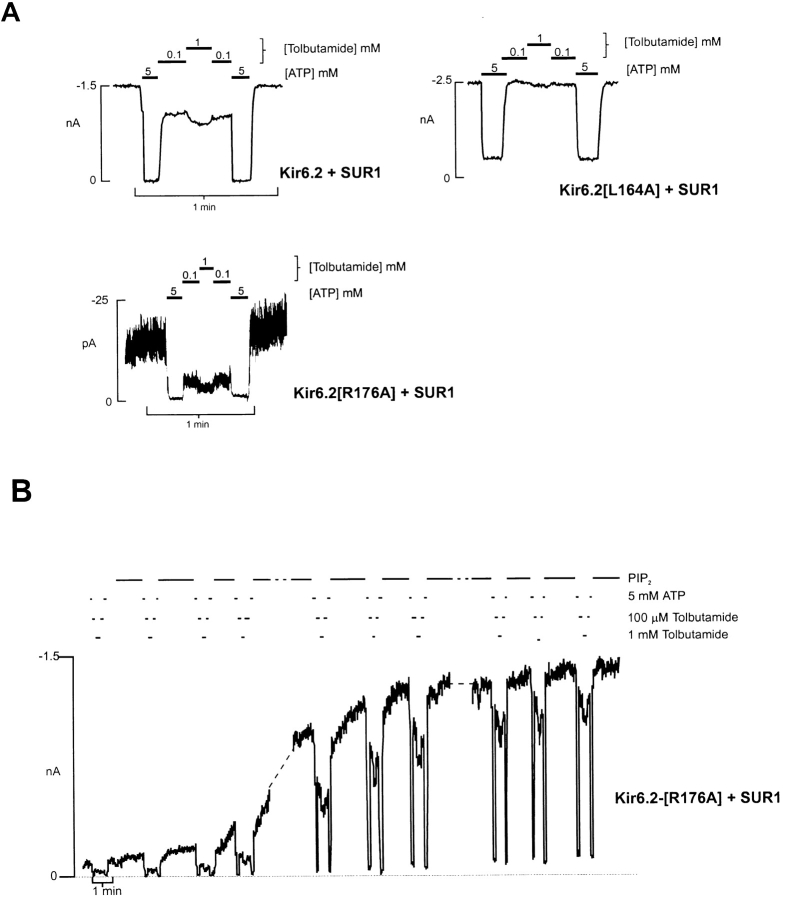
Since PIP2 and NH2-terminal deletion both increase the channel open state stability (Fan and Makielski 1997; Shyng and Nichols 1998; Koster et al. 1999), the loss of high affinity tolbutamide sensitivity in NH2-terminal truncated channels, and on wild-type channels treated with PIP2 suggests that the coupling of high affinity tolbutamide binding (at SUR1; Aguilar-Bryan et al. 1995) to channel inhibition may also depend on the open-state stability. To examine this possibility further, we have measured the tolbutamide sensitivity of channels expressed from Kir6.2[R176A]+SUR1, and Kir6.2[L164A+SUR1] channels, which have intrinsically very low, and high, open-state stabilities, respectively (Shyng and Nichols 1998). Kir6.2[R176A]+SUR1 channels have a much lower intrinsic open probability in the absence of ATP (P ozero < 0.1) than wild-type channels (P ozero ∼ 0.5), due to reduced PIP2 affinity (Fan and Makielski 1997; Huang et al. 1998; Shyng and Nichols 1998). As shown in Fig. 4 A, it is clear that these channels have a larger high affinity component of tolbutamide inhibition than wild-type channels, but which is again lost as P ozero increases after treatment with PIP2 (Fig. 4 B). In contrast, Kir6.2[L164A]+SUR1 channels have a very high P ozero (>0.85), corresponding to an intrinsic ATP sensitivity of K 1/2,ATP ∼ 1mM (data not shown), due to the open-state stabilizing effect of this mutation. As shown in Fig. 4 A, there is essentially no high affinity component of tolbutamide inhibition of Kir6.2[L164A]+SUR1 channels. On average, 100 μM tolbutamide inhibited wild-type Kir6.2+SUR1, Kir6.2[L164A]+SUR1, and Kir6.2[R176A]+SUR1 channels by 33 ± 3, 3 ± 2, and 77 ± 6%, respectively (n = 3 in each case).
Figure 4.
High affinity tolbutamide sensitivity depends on open-state stability. (A) Representative currents recorded from inside-out membrane patches containing wild-type or mutant KATP channels at −50 mV in Kint solution. Patches were exposed to differing [tolbutamide] or 5 mM ATP, as shown. (B) Current recorded from representative patch containing Kir6.2 [R176A]+SUR1 channels at −50 mV in Kint solution. The patch was exposed to differing [tolbutamide], ATP, or 5 μg/ml PIP2, as shown. The dashed lines represent 12- and 2-min gaps.
MgADP Stimulation and Diazoxide Stimulation of Channel Activity Disappears with PIP2 Stimulation
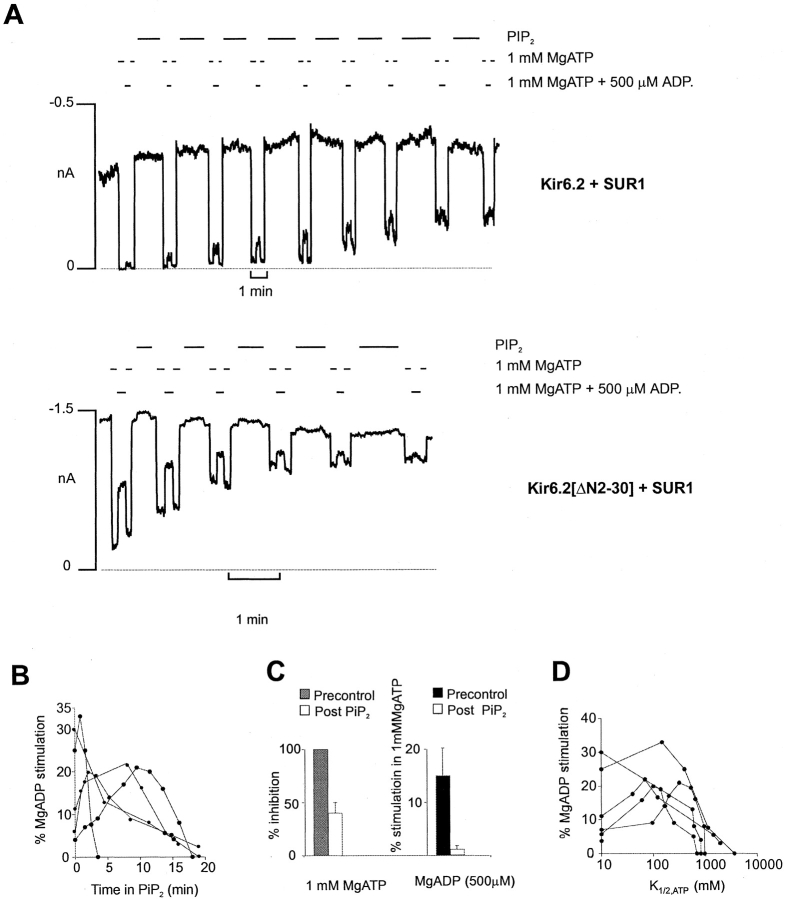
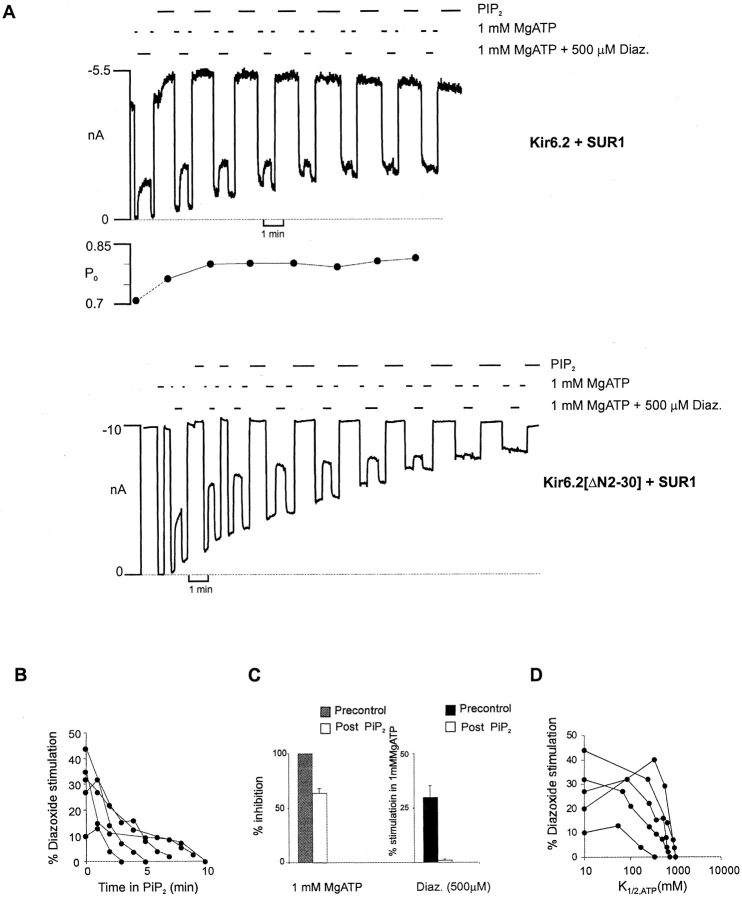
Activation of wild-type Kir6.2+SUR1 channels by MgADP and diazoxide, at a fixed [ATP], is quite variable from patch to patch (Fig. 5 B and 6 B). As shown in Fig. 5 A and 6 A, the ability of these agents to stimulate channel activity changes after PIP2 stimulation, and in a qualitatively similar way for both Kir6.2[ΔN2-30]+SUR1 and wild-type (Kir6.2+SUR1) channels. In each case, the stimulation tends to increase, but then gradually falls to zero with time after PIP2 application. The time course of this effect is also quite variable from patch to patch (Fig. 5 B and 6 B), but is reasonably well correlated with the accompanying change of ATP sensitivity (Fig. 5 D and 6 D). This result indicates that the stimulatory action of the PCOs, like ATP sensitivity itself, is not a fixed parameter of channel function, but is probably dependent on the open-state stability of the channel (Shyng and Nichols 1998). As PIP2 increasingly stabilizes the open state of the channel, sojourns in an ATP-accessible closed state become less and less frequent (Baukrowitz et al. 1998; Shyng and Nichols 1998). The present results are also consistent with PCOs acting by shifting the equilibrium between the open and closed states (see Shyng et al. 1997b), such that as the channel open-state stability approaches maximal, the stimulatory effect of the PCOs saturates.
Figure 5.
Pip2 effect on MgADP stimulation from cells expressing Kir6.2+SUR1 and Kir6.2[ΔN2-30]+SUR1 channels. (A) Representative currents from inside-out patches containing wild-type (Kir6.2+SUR1) or Kir6.2[ΔN2-30]+SUR1 channels. The patches were exposed to MgADP, MgATP, or Pip2 as indicated. The dashed line represents zero current determined in 5 mM ATP. (B) Plot of the percent MgADP stimulation versus time in Pip2 for five individual patches containing wild-type KATP channels. Percent MgADP stimulation was determined by calculating the increase in current in the presence of MgADP and MgATP relative to current in MgATP alone, and then expressing this value as a fraction of the maximal current observed in the absence of ATP. (C) Percent stimulation by MgADP and inhibition by ATP before (Precontrol) or after (Post Pip2) application of Pip2 (3–20 min) from patches in B. (D) Percent MgADP stimulation versus K 1/2,ATP after application of Pip2 to wild-type patches from B.
PIP2-induced Loss of Coupling Can Be Partially Restored with Poly-l-lysine Treatment
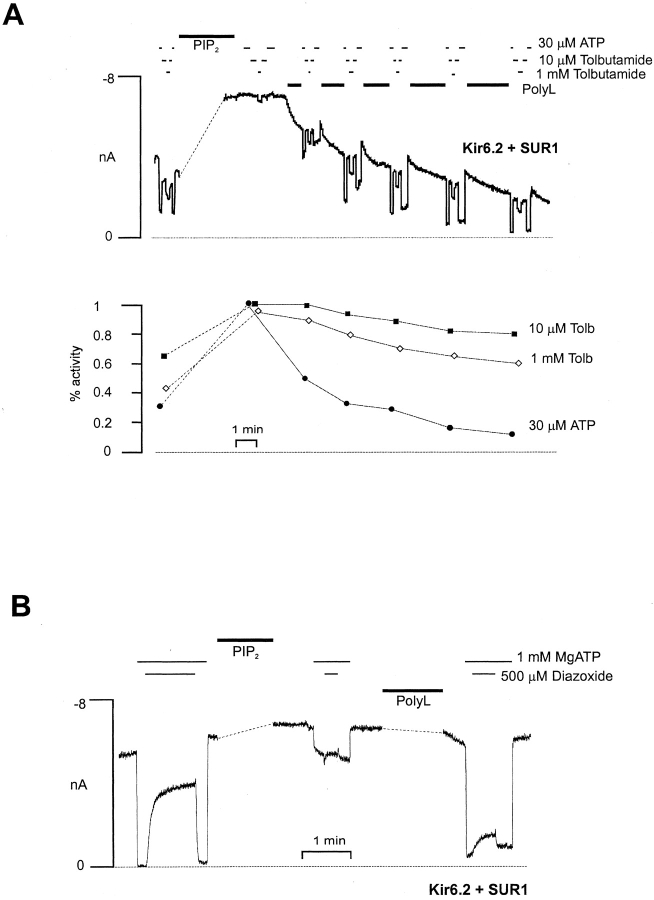
Treatment with polycations can reverse the stimulatory actions of PIP2 on open probability and ATP sensitivity (Deutsch et al. 1994; Shyng and Nichols 1998), probably by shielding the negative charges introduced by PIP2. As shown in Fig. 7, some reversal of both tolbutamide insensitivity and loss of PCO action is observed when patches are subsequently treated with poly-l-lysine. However, some irreversible loss of high affinity tolbutamide sensitivity, as well as of diazoxide and MgADP stimulation, also occurs after prolonged PIP2 treatment, such that poly-l-lysine may only partially restore the SUR1 coupling (e.g., Fig. 7 A), even though ATP sensitivity can be restored to, or beyond, control levels (see discussion).
Figure 7.
Pip2 effect on tolbutamide and PCO sensitivity is partially reversed by application of poly-l-lysine. (A) Current recorded from representative inside-out membrane patch containing wild-type channels at −50 mV in Kint solution. The patch was exposed to differing [tolbutamide] or ATP, as shown. The dashed line represents a 28-min gap in the trace, during which time Pip2 (5μg/ml) was applied. After Pip2 application, poly-l-lysine (10 μg/ml) was applied as indicated. ATP and tolbutamide sensitivity as a function of time is shown below. Sensitivity was assessed as the activity remaining in ATP or tolbutamide relative to maximal current in zero ATP, zero tolbutamide. (B) Current recorded from representative inside-out membrane patch containing wild-type channels at −50 mV in Kint solution. The patch was exposed to MgATP and diazoxide, as indicated. The dashed lines represent 18- and 13-min gaps in the trace, during which time Pip2 and poly-l-lysine, respectively, were applied.
Discussion
Loss of High Affinity Tolbutamide Sensitivity with Increased Open Probability
A biphasic dose–response relationship for tolbutamide inhibition of KATP channels was demonstrated by Gribble et al. 1997a, but the mechanistic basis was not made clear. When the high affinity component is saturated, there is an ∼40% reduction of wild-type currents. The high affinity binding of sulfonylureas is to the SUR1 subunit (Aguilar-Bryan et al. 1995; Gribble et al. 1997a), and channel inhibition results from an allosteric effect on the channel. By contrast, the low affinity inhibitory effect results from a direct interaction with the Kir6.2 subunit itself (Gribble et al. 1997a), and might be a pore-blocking action. The present results demonstrate that the high affinity, physiologically relevant, action can be abolished by increasing the open state stability (and hence P ozero) with PIP2, or by deleting the channel NH2 terminus. Kir6.2[ΔN2-30] channels, which have an intrinsically higher open state stability (Koster et al. 1999), show essentially no high affinity tolbutamide sensitivity (Fig. 1). Hence, although the high affinity sulfonylurea binding site is clearly on the SUR1 subunit (Aguilar-Bryan et al. 1995), the inhibitory effect on KATP channel activity will depend critically on the functional state and molecular nature of the Kir subunit. This prediction is dramatically borne out by the results (Fig. 4), which show that a mutant with even higher intrinsic open-state stability (Kir6.2[L164A]), is almost completely insensitive to tolbutamide, with no high-affinity inhibition. By contrast, in a mutant with intrinsically low open probability (Kir6.2[R176A]), putatively due to reduced PIP2 affinity, tolbutamide sensitivity is almost all high affinity, under ambient conditions after patch isolation. However, subsequent treatment with PIP2 still abolishes high affinity tolbutamide inhibition, as the open-state stability increases to, and beyond, that of the wild-type channel (i.e., the open probability increases and K 1/2,ATP decreases; Fig. 4 B).
The present findings are significant for understanding sulfonylurea sensitivity of KATP channels. They demonstrate that sulfonylurea sensitivity will depend critically on the open-state stability of the channels (manifested by open probability in the absence of ATP1). This can change dramatically in inside-out membrane patches as a consequence of “run down” and “run up.” Run down is a gradual, variable, and probably multifactorial, reduction of channel activity, often associated with decreased open probability and increased K 1/2,ATP (Thuringer and Escande 1989; Deutsch and Weiss 1993). A significant mechanism of run down is probably decreasing levels of phosphorylated phosphatidyl inositols in the cell membrane. Such run down can be reversed, and the channels run up, by application of exogenous PIP2 (Baukrowitz et al. 1998; Shyng and Nichols 1998), MgATP (Takano et al. 1990), and by application of MgUDP (Tung and Kurachi 1993; Terzic et al. 1994). Interestingly, Brady et al. 1998 reported an “operative condition-dependent response” of KATP channels to sulfonylureas, in which stimulation of channel activity by MgUDP led to a loss of glibenclamide sensitivity, but only if the channels had not previously run down. It is likely that in vivo variability of ATP sensitivity (Findlay and Faivre 1991) reflects cell-to-cell variability of the open state stability, resulting in turn from variability of membrane phospholipid levels. Similarly, in vivo variability of sulfonylurea sensitivity under different conditions (Findlay 1993; Venkatesh et al. 1991; Mukai et al. 1998) is also likely to reflect changes in open-state stability and accessibility of the closed channel.
PIP2 Activation Masks PCO Actions
It is clear that PIP2 activation of KATP channels and other inward rectifiers does not require the presence of a SUR1 subunit, and probably results from a direct interaction of PIP2 with the cytoplasmic portion of the channel protein itself (Hilgemann and Ball 1996; Fan and Makielski 1997; Baukrowitz et al. 1998; Huang et al. 1998; Shyng and Nichols 1998). The present results indicate that PCO sensitivity, like ATP sensitivity (Baukrowitz et al. 1998; Shyng and Nichols 1998) is a variable, dynamically dependent on membrane phospholipid levels rather than a fixed parameter. After PIP2 application to inside-out patches, there is generally first an increase in the stimulatory action of PCOs, and then a gradual disappearance of their action as the PIP2 stimulation saturates, such that, even though ATP inhibition is still observable at high concentrations, there is no relief of this inhibition by PCOs (Fig. 5 and Fig. 6). As discussed in Shyng and Nichols 1998 and Baukrowitz et al. 1998, it is likely that membrane phospholipid levels are variable from cell to cell, and that such variability accounts for the cell-to-cell variability of ATP sensitivity that is observed physiologically (Findlay and Faivre 1991). By the same reasoning, the variable stimulatory action of PCOs (see, e.g., Fig. 5 B and 6 B) might be a result of cell-to-cell variability of membrane phospholipid levels. The results raise the question: How does the membrane phospholipid level determine the PCO sensitivity? One possibility is that PIP2 affects ATP hydrolysis, or PCO binding to the SUR1 subunit. However, as we have previously suggested (Shyng et al. 1997b), it seems likely that PCOs act ultimately to stabilize the open state of the channel itself, just as the phospholipids do. Therefore, the lack of PCO effects after elevation of phospholipids, is likely to be a consequence of the convergent action of these two agents on the energetic stability of the open state relative to the ATP-accessible closed state.
Figure 6.
Pip2 effect on diazoxide stimulation from cells expressing Kir6.2+SUR1 and Kir6.2[ΔN2-30]+SUR1 channels. (A) Representative currents from inside-out patches containing wild-type (Kir6.2+SUR1) or Kir6.2[ΔN2-30]+SUR1 channels. The patches were exposed to diazoxide and MgATP, or Pip2 as indicated. The dashed line represents zero current determined in 5 mM ATP. (Top) Open probability, estimated from noise analysis of 10 s of current in zero ATP, is indicated. (B) Plot of the percent diazoxide stimulation versus time in Pip2 for five individual patches containing wild-type KATP channels. Percent diazoxide stimulation was determined by calculating the increase in current in the presence of diazoxide and MgATP relative to current in MgATP alone, and then expressing this value as a fraction of the maximal current observed in the absence of ATP. (C) Percent stimulation by diazoxide and inhibition by ATP before (Precontrol) or after (Post Pip2) application of Pip2 (3–20 min) from patches in B. (D) Percent diazoxide stimulation versus K 1/2,ATP after application of Pip2 to patches from B.
The Role of SUR Subunits in Controlling KATP Channel Function
It is now clear that the pore-forming (Kir6.2) subunits can generate ATP-sensitive K channels in the complete absence of expressed SUR subunits, even without truncation of the COOH terminus (Tucker et al. 1997; John et al. 1998; Mikhailov et al. 1998). So, what is the role of the SUR1 subunit? Clearly, there is evidence for a chaperoning action to bring the channel to the surface, and with which SUR1 remains in physical proximity (Clement et al. 1997; John et al. 1998; Zerangue et al. 1999). Moreover, the physiologically, and pharmacologically, important regulators of the channel seem to act through an interaction with the SUR1 subunit (Aguilar-Bryan et al. 1995; Schwanstecher et al. 1998). The balance of evidence suggests that ATP hydrolysis at the nucleotide binding folds activates the channel, and that this activation is stabilized by binding of MgADP and other PCOs to the SUR1 subunit (Nichols et al. 1996; Gribble et al. 1997b; Shyng et al. 1997a,Shyng et al. 1997b; Schwanstecher et al. 1998). High-affinity sulfonylurea binding is to the SUR subunit (Aguilar-Bryan et al. 1995), and this effect is then transduced to inhibition of channel activity. The physical nature of the coupling between SURx and the Kir6.x subunits and the interacting regions of each subunit remain unknown. The present results show that deletion of the NH2 terminus of Kir6.2 can functionally uncouple the high affinity tolbutamide sensitivity from the channel. However, it is clear that PCO actions on the channel remain for the NH2-terminus truncated channel so that a physical coupling is still intact.
High affinity sulfonylurea sensitivity and PCO sensitivity is conferred by the SUR1 subunit, and is absent for Kir6.2 channels expressed in the absence of SUR1 (Gribble et al. 1997a; Tucker et al. 1997), which begs the question whether the effect of PIP2 is to cause a physical, or functional, uncoupling of Kir6.2 from SUR1. A physical uncoupling seems unlikely based on the observation that treatment with polylysine leads to (a) some reversal of the PIP2 abolition of pharmacological regulation, and (b) full restoration of the SUR1-dependent K 1/2,ATP of ∼10 μM. Nevertheless, we cannot exclude the possibility that the PIP2 action physically interrupts the transduction of the inhibitory signal from SUR1 to Kir6.2.
Conclusions
High affinity tolbutamide inhibition seems, like ATP inhibition, to be the result of a closed state stabilization, but, unlike ATP inhibition, is not likely to be a direct binding to the closed channel. Stabilizing the open state and raising the channel open probability, either by mutation or by application of PIP2, reduces high affinity tolbutamide sensitivity. Similarly, PCOs act on SUR1 to stabilize the channel in the open state, convergent with PIP2 action, such that PIP2 treatment leads to channel activation without further activation in the presence of PCOs. Treatment with polylysine causes at least partial reversal of the uncoupling actions of PIP2 effect, restoring some high affinity tolbutamide sensitivity and PCO stimulation. These results indicate that, in native cells, the pharmacological and physiological control of channel activity by the SUR1 subunit will be critically dependent on the open-state stability, itself determined by the phospholipid content of the membrane.
Acknowledgments
We thank Jeremiah Shepard and Rebecca Dunlap for technical assistance with these experiments. We are grateful to Dr. S. Seino for providing us with the Kir6.2 clone.
This work was supported by a grant from the National Institutes of Health (NIH) (HL45742 to C.G. Nichols), a Career Development Award from the American Diabetes Association (S.-L. Shyng, the Washington University Diabetes Research and Training Center (NIH Training grant support of J.C. Koster, and reagents), and the Washington University Cardiovascular NIH Training Grant (fellowship support for Q. Sha).
Footnotes
1used in this paper: Kir, inward rectifier; PCO, potassium channel opener; PIP2, phosphatidylinositol 4,5-bisphosphate; SUR, sulfonylurea receptor
The open state stability is the stability of the “bursting” state relative to a longer closed state that is accessible to ATP. As the open state stability increases, the open probability increases towards a saturating level of ∼0.9 (i.e., the intraburst open probability), and the K 1/2,ATP increases continually (Shyng et al. 1997a).
The increase in P o that occurs after PIP2 application is followed by a variable, very slow, loss of channel activity over many minutes (“terminal rundown”). Such rundown occurs in the presence or absence of PIP2. This terminal rundown may occur by channels terminally disappearing from the patch, the open probability estimated by noise analysis (i.e., the open probability of channels that remain functional) does not decline during this process, as quantified for the record in Fig. 6 A.
References
- Aguilar-Bryan L., Nichols C.G., Wechsler S.W., Clement J.P., IV, Boyd A.E., III, Gonzalez G., Herrera Sosa H., Nguy K., Bryan J., Nelson D.A. The β cell high affinity sulfonylurea receptora regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Schulte U., Oliver D., Herlitze S., Krauter T., Tucker S.J., Ruppersberg J.P., Fakler B. PIP2 and PIP as determinants for ATP inhibition of K-ATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Brady P.A., Alekseev A.E., Terzic A. Operative condition-dependent response of cardiac ATP-sensitive K+ channels toward sulfonylureas. Circ. Res. 1998;82:272–278. doi: 10.1161/01.res.82.2.272. [DOI] [PubMed] [Google Scholar]
- Clement J.P., IV, Kunjilwar K., Gonzalez G., Schwanstecher M., Panten U., Aguilar-Bryan L., Bryan J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- Deutsch N., Matsuoka S., Weiss J.N. Surface charge and properties of cardiac ATP-sensitive K+ channels. J. Gen. Physiol. 1994;104:773–800. doi: 10.1085/jgp.104.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch N., Weiss J.N. ATP-sensitive K+ channel modification by metabolic inhibition in isolated guinea-pig ventricular myocytes. J. Physiol. 1993;465:163–179. doi: 10.1113/jphysiol.1993.sp019671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P., Li L.H., Wang J. K-ATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C-terminus of the pore-forming subunit. Proc. Natl. Acad. Sci. USA. 1998;95:13953–13958. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Makielski J.C. Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Findlay I. Sulphonylurea drugs no longer inhibit ATP-sensitive K+ channels during metabolic stress in cardiac muscle. J. Pharmacol. Exper. Ther. 1993;266:456–467. [PubMed] [Google Scholar]
- Findlay I., Faivre J.F. ATP-sensitive K channels in heart muscle. Spare channels. FEBS Lett. 1991;279:95–97. doi: 10.1016/0014-5793(91)80259-6. [DOI] [PubMed] [Google Scholar]
- Gribble F.M., Tucker S.J., Ashcroft F.M. The interaction of nucleotides with tolbutamide block of cloned ATP-sensitive K channel currents expressed in Xenopus oocytes—a reinterpretation J. Physiol. 504 1997. 35 45a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble F.M., Tucker S.J., Ashcroft F.M. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide EMBO (Eur. Mol. Biol. Organ.) J 16 1997. 1145 1152b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W., Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2 . Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Huang C.L., Feng S.Y., Hilgemann D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by G-beta-gamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J.P., IV, Namba N., Inazawa J., Gonzales G., Aguilar-Bryan L., Seino S., Bryan J. Reconstitution of IKATPan inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J.P., IV, Wang C.Z., Aguilar-Bryan L., Bryan J., Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Seino S. Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K+ channel. FEBS Lett. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- Isomoto S., Kondo C., Yamada M., Matsumoto S., Higashiguchi O., Horio Y., Matsuzawa Y., Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- John S.A., Monck J.R., Weiss J.N., Ribalet B. The sulfonylurea receptor SUR1 regulates ATP-sensitive mouse Kir6.2 K+ channels linked to green fluorescent protein in human embryonic kidney cells (HEK 293) J. Physiol. 1998;510:333–345. doi: 10.1111/j.1469-7793.1998.333bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster J.C., Sha Q., Shyng S.-L., Nichols C.G. ATP inhibition of KATP channelscontrol of nucleotide sensitivity by the N-terminal domain of the Kir6.2 subunit. J. Physiol. 1999;515:19–30. doi: 10.1111/j.1469-7793.1999.019ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E., Alekseev A.E., Krapivinsky G.B., Carrasco A.J., Clapham D.E., Terzic A. Evidence for direct physical association between a K+ channel (Kir6.2) and an ATP-binding cassette protein (SUR1) which affects cellular distribution and kinetic behavior of an ATP-sensitive K+ channel. Mol. Cell. Biol. 1998;18:1652–1659. doi: 10.1128/mcb.18.3.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov M.V., Proks P., Ashcroft F.M., Ashcroft S.J. Expression of functionally active ATP-sensitive K-channels in insect cells using baculovirus. FEBS Lett. 1998;429:390–394. doi: 10.1016/s0014-5793(98)00640-1. [DOI] [PubMed] [Google Scholar]
- Mukai E., Ishida H., Kato S., Tsuura Y., Fujimoto S., Ishida-Takahashi A., Horie M., Tsuda K., Seino Y. Metabolic inhibition impairs ATP-sensitive K+ channel block by sulfonylurea in pancreatic beta-cells. Am. J. Physiol. 1998;274:E38–E44. doi: 10.1152/ajpendo.1998.274.1.E38. [DOI] [PubMed] [Google Scholar]
- Nichols C.G., Lederer W.J. ATP-sensitive potassium channels in the cardiovascular system. Am. J. Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Nichols C.G., Shyng S.-L., Nestorowicz A., Glaser B., Clement J., IV, Gonzalez G., Aguilar-Bryan L., Permutt A.M., Bryan J.P. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Schwanstecher M., Sieverding C., Dorschner H., Gross I., Aguilar-Bryan L., Schwanstecher C., Bryan J. Potassium channel openers require ATP to bind to and act through sulphonylurea receptors. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S.-L., Ferrigni T., Nichols C.G. Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit J. Gen. Physiol. 110 1997. 141 153a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S.-L., Ferrigni T., Nichols C.G. Regulation of KATP channel activity by diazoxide and MgADP:distinct functions of the two nucleotide binding folds of the sulfonylurea receptor J. Gen. Physiol. 110 1997. 643 654b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S.L., Nichols C.G. Octameric stoichiometry of the KATP channel complex. J. Gen. Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S.-L., Nichols C.G. Phosphatidyl inositol phosphates control of nucleotide-sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Takano M., Qin D.Y., Noma A. ATP-dependent decay and recovery of K+ channels in guinea pig cardiac myocytes. Am. J. Physiol. 1990;258:H45–H50. doi: 10.1152/ajpheart.1990.258.1.H45. [DOI] [PubMed] [Google Scholar]
- Terzic A., Findlay I., Hosoya Y., Kurachi Y. Dualistic behavior of ATP-sensitive K+ channels toward intracellular nucleoside diphosphates. Neuron. 1994;12:1049–1058. doi: 10.1016/0896-6273(94)90313-1. [DOI] [PubMed] [Google Scholar]
- Thuringer D., Escande D. Apparent competition between ATP and the potassium channel opener RP 49356 on ATP-sensitive K+ channels of cardiac myocytes. Mol. Pharmacol. 1989;36:897–902. [PubMed] [Google Scholar]
- Tucker S.J., Gribble F.M., Zhao C., Trapp S., Ashcroft F.M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Tucker S.J., Gribble F.M., Proks P., Trapp S., Ryder T.J., Haug T., Reimann F., Ashcroft F.M. Molecular determinants of K-ATP channel inhibition by ATP. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:3290–3296. doi: 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung R.T., Kurachi Y. On the mechanism of nucleotide diphosphate activation of the ATP-sensitive K+ channel in ventricular cell of guinea-pig. J. Physiol. 1993;437:239–256. doi: 10.1113/jphysiol.1991.sp018593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Inagaki N., Seino S. MgADP antagonism of Mg2+-dependent ATP binding of the sulfonylurea receptor. J. Biol. Chem. 1997;272:22983–22986. doi: 10.1074/jbc.272.37.22983. [DOI] [PubMed] [Google Scholar]
- Venkatesh N., Lamp S.T., Weiss J.N. Sulfonylureas, ATP-sensitive K+ channels, and cellular K+ loss during hypoxia, ischemia, and metabolic inhibition in mammalian ventricle. Circ. Res. 1991;69:623–637. doi: 10.1161/01.res.69.3.623. [DOI] [PubMed] [Google Scholar]
- Zerangue N., Schwappach B., Jan Y.N., Jan L.Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K-ATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]