Abstract
We have applied the perforated patch whole-cell technique to β cells within intact pancreatic islets to identify the current underlying the glucose-induced rhythmic firing of action potentials. Trains of depolarizations (to simulate glucose-induced electrical activity) resulted in the gradual (time constant: 2.3 s) development of a small (<0.8 nS) K+ conductance. The current was dependent on Ca2+ influx but unaffected by apamin and charybdotoxin, two blockers of Ca2+-activated K+ channels, and was insensitive to tolbutamide (a blocker of ATP-regulated K+ channels) but partially (>60%) blocked by high (10–20 mM) concentrations of tetraethylammonium. Upon cessation of electrical stimulation, the current deactivated exponentially with a time constant of 6.5 s. This is similar to the interval between two successive bursts of action potentials. We propose that this Ca2+-activated K+ current plays an important role in the generation of oscillatory electrical activity in the β cell.
Keywords: insulin, pancreas, Ca2+-activated K+ channel, Ca2+, membrane potential
INTRODUCTION
Stimulation of insulin secretion by glucose is linked to the generation of electrical activity in pancreatic β cells. This electrical activity, first documented more than 30 yr ago (Dean and Matthews 1968), consists of oscillations in membrane potential between depolarized plateaus, on which action potentials are superimposed, and repolarized electrically silent intervals (bursting pattern; for review see Henquin and Meissner 1984). The cytoplasmic Ca2+ concentration ([Ca2+]i) varies in synchrony with electrical activity and correlates with pulsatile insulin secretion (Bergsten and Hellman 1993; Barbosa et al. 1998). Patch-clamp experiments on isolated β cells have enabled the characterization of many of the membrane conductances participating in glucose-induced electrical activity (Ashcroft and Rorsman 1989). The processes that underlie the bursting pattern remain undetermined, although a number of hypotheses have been proposed (for review see Satin and Smolen 1994; Sherman 1996). These include activation of large-conductance Ca2+-activated K+ channels (Atwater et al. 1983), inactivation of the voltage-gated Ca2+ current (Cook et al. 1991), cell–cell coupling (Andreu et al. 1997), cyclic activation of a store-activated cation conductance (Worley et al. 1994), and opening of Ca2+-activated K+ channels by mobilization of intracellular Ca2+ (Ämmälä et al. 1991). However, it has not been possible to establish which of these mechanisms (if any) participates in the generation of the glucose-induced oscillations in membrane potential. This is because dissociated β cells maintained in tissue culture, the standard preparation for patch-clamp recordings, only rarely exhibit oscillatory electrical activity reminiscent of that seen in the intact islets (Smith et al. 1989; Kinard et al. 1999). We have now applied the patch-clamp technique to β cells within intact islets. These cells retain the bursting pattern of action potential firing. Here we describe a Ca2+-activated K+ current that activates gradually during electrical activity and exhibits several features consistent with a role in the regulation of the rhythmic firing of action potentials.
MATERIALS AND METHODS
Preparation of Pancreatic Islets and β Cells
Unless otherwise indicated, the electrophysiological experiments were carried out on β cells in intact islets. NMRI mice were purchased from a commercial breeder (Moellegaard). The mice were stunned by a blow against the head and killed by cervical dislocation and the pancreas quickly removed. Collagenase (2 mg) was dissolved in Hank's buffer and injected into the pancreatic duct. Pancreatic islets were isolated by gentle collagenase digestion (25 min, 37°C). Islets thus isolated were subsequently maintained in short-term tissue culture (<16 h) in RPMI 1640 containing 5 mM glucose and 10% (vol/vol) fetal calf serum (Flow Laboratories) and supplemented with 100 μg/ml streptomycin and 100 IU/ml penicillin (both from Northumbria Biologicals, Ltd.). The experiments in Fig. 1C and some of those displayed in Fig. 5 were carried out on dispersed β cells. These were prepared by shaking islets in Ca2+-free solution. The resultant cell suspension was plated on glass cover slips (diameter: 22 mm) or Nunc plastic petri dishes and maintained in tissue culture for up to 48 h using the tissue culture medium mentioned above.
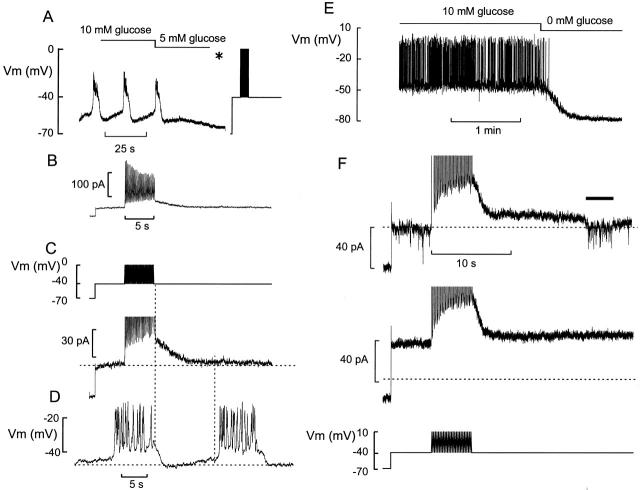
Figure 1.
Train of action potentials elicits an outward current in β cells. (A) Membrane potential oscillations in a β cell exposed to 10 mM glucose. The glucose concentration was lowered to 5 mM as indicated above the voltage trace. *The amplifier was switched from the current-clamp into the voltage-clamp mode, the membrane potential held at −70 mV, and the command voltage varied as indicated. (B) The membrane currents elicited by the pulse train (C, top). Note time-dependent decline of outward current. (C, bottom) Change of holding current displayed on an expanded vertical scale. Note gradual development of a holding current. Same experiment as in B. (D) Membrane potential recording from the same cell as in C before lowering the glucose concentration. In C and D, the vertical line marks the temporal relationship between cessation of stimulation and the onset of rapid repolarization (left) and the onset of rapid depolarization during the subsequent burst (right). The horizontal lines indicate (from top to bottom) the steady state holding current at −40 mV, the plateau potential from which the cell repolarizes upon termination of the burst, and the most negative membrane potential attained between two bursts. (E) Membrane potential recording from an isolated (dispersed) β cell maintained in tissue culture. The glucose concentration was changed from 10 to 0 mM as indicated above the voltage trace. (F) Currents elicited by the train of depolarizations (bottom) in the presence of 10 (top) and 5 (middle) mM glucose. The inward current (indicated by the horizontal line above the current trace) is due to a burst of action potentials generated in a neighboring β cell. Note that step current elicited when stepping from −70 to −40 mV is larger at 5 than at 10 mM glucose, reflecting greater activity of the KATP channels.
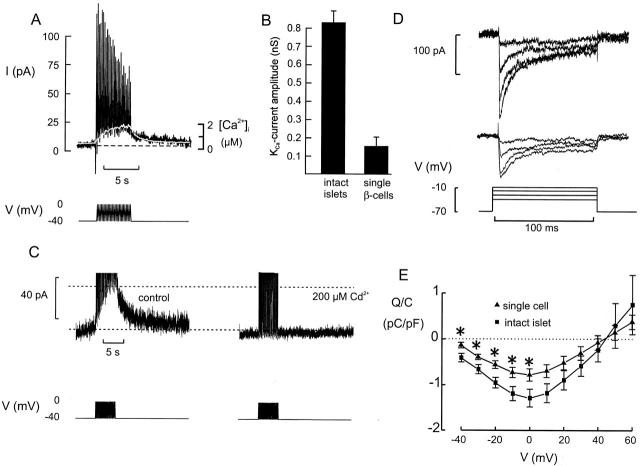
Figure 5.
Association between [Ca2+]i and Kslow current activation. (A) Membrane current (top) elicited by a train of depolarizations (bottom) and the associated changes of the cytoplasmic [Ca2+]i in an isolated cell (white trace superimposed on current trace). The horizontal line indicate steady state current and [Ca2+]i. (B) The current amplitude measured at the end of the train in isolated cells and in β cells within intact islets. *P < 0.001. (C) Kslow current (top) elicited by the train of depolarizations (bottom) under control conditions and in the presence of 200 μM Cd2+. (D) Voltage-gated Ca2+ currents recorded during 100-ms depolarizations to 0 mV from a holding potential of −70 mV in β cells in intact islets (top) and in dispersed β cells (middle). (E) Charge entry normalized to cell capacitance (Q/C)–voltage (V) relationships of the Ca2+ current recorded in isolated β cells (▴) and in β cells within intact islets (▪). Mean values ± SEM of 8 (▪) and 15 (▴) experiments. *P < 0.05.
Electrophysiology
Pancreatic islets were immobilized by a wide-bore (diameter: 50–100 μm) suction pipette. The measurements were performed using an EPC-9 patch-clamp amplifier (HEKA Electronics) and the software pulse (version 6.2 and later). Patch pipettes were pulled from borosilicate glass (tip resistance: 3–7 ΜΩ when filled with the pipette solution). Pancreatic β cells were functionally identified by the generation of oscillatory electrical activity in the presence of 10 mM glucose. Cells thus identified exhibited electrophysiological characteristics similar to those previously described for β cells maintained in tissue culture (Ashcroft and Rorsman 1989). Using these criteria, the β cells can safely be distinguished from the α and δ cells; the latter cell types being equipped with a large voltage-gated Na+ (Göpel, S.O., T. Kanno, S. Barg, J. Galvanovskis, and P. Rorsman, manuscript submitted for publication).
Stimulation
In the intact islet, the β cells are electrically coupled and electrical activity in neighboring cells spreads into the voltage-clamped cell via the gap junctions (Mears et al. 1995; Fig. 1 D and 3). To allow voltage-clamp measurements without interference by currents originating from the neighboring cells, the glucose concentration was usually lowered to 5 mM to suppress glucose-induced electrical activity. Electrical activity was then simulated by application of a sequence of voltage-clamp pulses. This consisted of depolarization from −70 to −40 mV for 5 s, followed by a series of 26 simulated “action potentials.” The latter consisted of a voltage ramp between −40 and 0 mV (100 ms) followed by a ramp from 0 to −40 mV (100 ms). The action potential waveform was applied at a frequency of 5 Hz. This voltage range, frequency, and duration approximate the β cell action potential. Subsequent to the train of voltage-clamp pulses, the cell was held at −40 mV for 10 or 20 s (except in Fig. 2 A and 3 D) to facilitate the observation of K+ currents. The interval between two successive stimulation series was normally 0.5–2 min to allow complete recovery from inactivation. All experiments were carried out using the perforated patch whole-cell configuration (Lindau and Fernandez 1986; Horn and Marty 1988) and were conducted at 30–32°C. During the experiments, the islet was continuously superfused with extracellular medium at a rate of 1–2 ml/min.
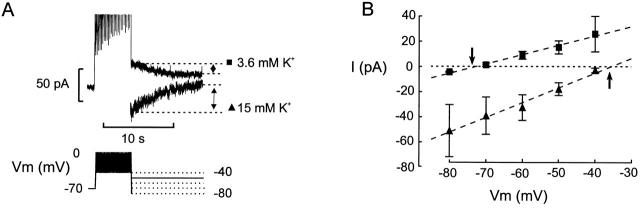
Figure 2.
Kslow current is K+ selective. (A, bottom) After the train of depolarizations, the membrane potential was held for 10 s at voltages between −40 and −80 mV as indicated schematically by the pulse protocol. (A, top) Membrane currents recorded at −50 mV after the train when the extracellular medium contained 3.6 or 15 mM K+ as indicated. Note that the currents go in opposite directions. (B) The peak tail currents recorded at membrane potentials between −80 and −40 mV before and after elevation of extracellular K+ from 3.6 mM (▪) to 15 mM (▴). The amplitude of the current was measured as illustrated in B. The arrows indicate the reversal potentials recorded at low and high extracellular K+.
Solutions
The standard extracellular medium consisted of (mM): 140 NaCl, 3.6 KCl, 2 NaHCO3, 0.5 NaH2PO4, 0.5 MgSO4, 5 HEPES, pH 7.4 with NaOH, 2.5 CaCl2, and d-glucose at the indicated glucose concentration. The pipette solution was composed of (mM): 76 K2SO4, 10 NaCl, 10 KCl, 1 MgCl2, and 5 mM HEPES, pH 7.35 with KOH. Whole-cell Ca2+ currents were recorded with the same solutions with the exception that K2SO4 in the pipette-filling solution was replaced by an equimolar amount of Cs2SO4. In all recordings, electrical contact with the cell interior was established by addition of the pore-forming antibiotic amphotericin B (Rae et al. 1991) to the pipette solution (Sigma Chemical Co.). Perforation required a few minutes and the voltage-clamp was considered satisfactory when Gseries exceeded 20 nS. Charybdotoxin and apamin were from Alomone and dissolved in water. Tolbutamide was obtained from Sigma Chemical Co., and nifedipine was supplied by Research Biochemicals, Inc. Whenever DMSO was used as the solvent of the test compounds, the final concentration in the experimental solution was ≤0.1%.
Measurements of [Ca2+]i
The [Ca2+]i measurements were made using an Axiovert 135 inverted microscope equipped with a Plan-Neofluar 100×/1.30 objective (Carl Zeiss, Inc.) and an fluorescence imaging system (Ionoptix) as described elsewhere (Bokvist et al. 1995). Excitation was effected at 340 and 380 nm and emitted light recorded at 510 nm with a video camera synchronized to the excitation light source and a computer interface. The experiments were conducted using the perforated-patch whole-cell configuration using the pipette-filling solution specified above. Before the experiments, the cells were loaded for 20 min with 0.2 μM of fura-2/AM (Molecular Probes, Inc.). Calibration of the fluorescence ratios was performed by using the standard whole-cell configuration to infuse fura-2 with different of Ca2+-EGTA mixtures of known [Ca2+]i.
Data Analysis
Data are presented as mean values ± SEM. Statistical significances were evaluated using Student's t test.
RESULTS
Electrical Activity Activates an Outward Current in β Cells
Fig. 1 A shows a patch-clamp recording of the membrane potential in a β cell within an intact pancreatic islet. In the presence of 10 mM glucose, the β cell exhibited regenerative electrical activity consisting of bursts of action potentials. Confocal microscopy reveals that ≈30% of the superficial cells are insulin-containing β cells (not shown, but see Göpel et al. 2000). The number of cells exhibiting the characteristic glucose-induced electrical activity, a hallmark of pancreatic β cells (Henquin and Meissner 1984), amounted to 52 cells from total of >200 during a period of 2 yr. In 21 randomly selected cells exposed to 10 mM glucose, the duration of the depolarized plateau and the silent interval between two bursts averaged 7.8 ± 0.8 and 14.2 ± 2.1 s, respectively.
To identify the current that terminates the burst, the glucose concentration was lowered to 5 mM and the amplifier switched into the voltage-clamp mode (Fig. 1 A, *). Electrical activity was then simulated by a pulse train of voltage-clamp depolarizations. The voltage pulses evoke inward as well as outward currents. The latter reflect activation of delayed rectifying K+ channels and, in agreement with previous results (Rorsman and Trube 1986), exhibited significant inactivation during the train (Fig. 1 B). However, close inspection of the current responses revealed that electrical stimulation was also associated with the gradual development of an outward holding current (Fig. 1 C). The activation of the current could in most cases be described by a single exponential. The time constant of activation (τa) was determined as 2.3 ± 0.3 s (n = 27). In a series of 30 experiments, the amplitude of this current measured at the end of the train amounted to 28 ± 2 pA. Fig. 1 D shows a representative example of two bursts of action potentials on an expanded time base. The membrane potential was maximally negative immediately after termination of the burst, and then gradually returned towards the threshold potential from which a new burst of action potentials originated. Comparison of the mean values of τa (2.3 s) and the burst duration (7.8 s for glucose-induced electrical activity or 5 s for the train of simulated action potentials) suggests that the current has reached ≥90% of its maximal amplitude at the end of the burst. After cessation of stimulation, the current deactivated and returned to the prestimulatory level within 10 s. The correlation between the deactivation of the current and the depolarization between two bursts was analyzed in a subset of 18 cells in which the current returned to the baseline. In these cells, the time constant of current deactivation (τd) averaged 6.4 ± 1.8 s and the interval between two bursts in the same cells amounted to 13 ± 3 s (n = 18). During the latter period, the measured deactivation of the current amounted to 94 ± 2% (n = 18), close to the 87% predicted from τd.
Consistent with previous reports (Smith et al. 1989; Ämmälä et al. 1991; Larsson et al. 1996), electrical activity in intact islets differs from that recorded from β cell clusters maintained in short-term (24 h) tissue culture. Electrical activity in the latter preparation consists of very long bursts (often lasting several minutes) of action potentials. Fig. 1 E shows an example of electrical activity evoked by 10 mM glucose in a small cluster of cultured β cells. It can be seen that action potential firing continues uninterruptedly for at least 2 min, and that removal of glucose leads to termination of electrical activity.
It is unlikely that the current in Fig. 1 C is attributable to the induction of regenerative electrical activity in neighboring β cells. As shown in Fig. 1 F, action potential firing in neighboring unclamped β cells in an islet exposed to 10 mM glucose gives rise to an inward current, whereas the current elicited by the train of simulated action potentials is outward. Also shown in Fig. 1 F is the current recorded in the same cell after lowering the glucose concentration to 5 mM. This suppresses electrical activity in the neighboring cells and allows the slowly activating and deactivating current to be studied in isolation. It is clear that reduction of the glucose concentration from 10 to 5 mM is associated with a 50% increase in the resting (KATP) membrane conductance (compare step current responses when stepping the membrane potential from −70 to −40 mV). It is also evident, however, that the current elicited by the train of depolarizations has the same amplitude, activation, and deactivation properties at both glucose concentrations.
Ion Selectivity
We next determined the ionic selectivity of the current elicited by the train of action potentials. Fig. 2 A shows the currents recorded after the train when the membrane potential was subsequently clamped at −50 mV in the presence of 3.6 and 15 mM extracellular K+ ([K+]o). It is clear that whereas the current is outward at normal [K+]o, it becomes inward at the supraphysiological concentration. Fig. 2 B summarizes the relationship between membrane potential and peak current amplitude. With 3.6 mM extracellular K+, the current reversed at a membrane potential of −73 ± 1 mV (n = 4). After elevation of [K+]o to 15 mM, the reversal was observed at −36 ± 2 mV (n = 3). This shift of 37 mV for a 4.2-fold change of [K+]o is precisely that predicted by the Nernst equation for a K+-selective conductance. Because the current activates and deactivates slowly and flows through K+-selective channels, we will henceforward refer to it as the Kslow current.
Cell Coupling Does Not Contribute to the Kslow Current Responses
Fig. 3 shows recording of glucose-induced electrical activity in a β cell in an intact islet (A) and the variations of the holding current subsequently measured in the same cell under voltage-clamp conditions (B, top). It can be observed that the holding current oscillates in a way reminiscent of inverted bursts of action potentials. This is because the electrical activity in the neighboring cells spreads into the voltage-clamped cells via the gap junctions, giving rise to oscillations in the holding current (Mears et al. 1995). Voltage pulses (±10 mV, 200-ms long, 2 Hz) were applied to monitor changes in the membrane conductance (B, bottom). The input conductance was the same during the silent intervals and the periods of action potential firing (Fig. 3 C, gray shaded area) and averaged 1.0 nS. When the same series of pulses were applied after a train of depolarizations, the membrane conductance (measured in the same β cell after lowering of the glucose concentration from 10 to 5 mM) was greatest at the end of the train and subsequently declined to a new steady state level (Fig. 3 D), from a starting value of >2.1 nS to a steady state value of ≈1.3 nS (Fig. 3 E). The latter value is higher than that measured in the presence of 10 mM glucose because the KATP conductance increased when the concentration of the sugar was lowered to 5 mM.
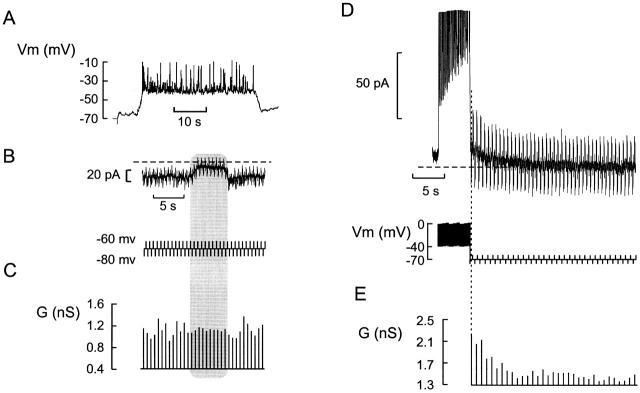
Figure 3.
Cell coupling does not account for Kslow current. (A) Membrane potential recording from a β cell in an intact islet. (B) Voltage-clamp recording at a holding potential of −70 mV. Changes of the cell conductance (G) were determined from the current responses (top) elicited by application of ±10-mV voltage pulses (200-ms duration, 2 Hz frequency; bottom). (C) Cell conductance calculated from the ±10-mV voltage steps in B. Note that the cell conductance is stable and amounts to ≈1 nS. In B and C, the shaded area indicated the silent interval between two successive bursts. (D) Current responses (top) elicited by a train of depolarizations followed by a series of ±10-mV voltage pulses applied from a holding potential of −70 mV to monitor the cell conductance (bottom). (E) Cell conductance (G) calculated from the ±10-mV voltage pulses. Note that the conductance is greatest (2.1 nS) immediately after the train, but subsequently declines to the steady state value (1.3 nS). The same cell was used in A–E.
Collectively, the data presented in Fig. 3 suggest that, whereas electrical activity in the neighboring β cells spreads into the voltage-clamped cells via the gap junctions and thus gives rise to oscillations of the holding current, activation of ion channels or passive charging of the membrane in the unclamped cells does not contribute to the measured membrane conductance measured in the voltage-clamped cell.
Pharmacology
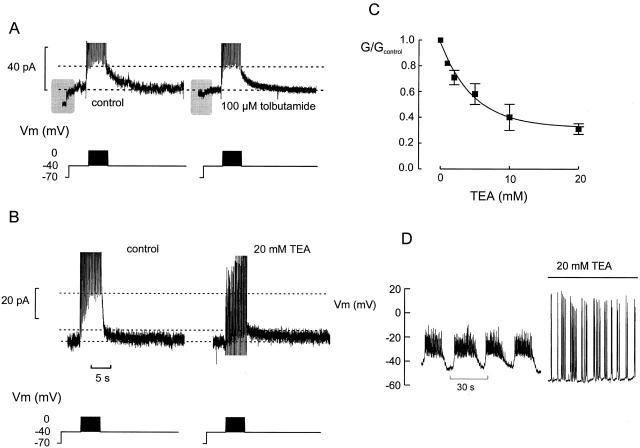
Tolbutamide, an inhibitor of KATP channels (Ashcroft and Rorsman 1989), had no effect on the current (n = 3; Fig. 4 A), but reduced the current step obtained when stepping the membrane potential from −70 to −40 mV. The latter observation indicates that some KATP channels remained active in 5 mM glucose. The Kslow current was likewise unaffected by both charybdotoxin (100 nM) and apamin (1 μM), blockers of large- and small-conductance Ca2+-activated K+ channels, respectively (not shown). By contrast, the broad spectrum K+ channel blocker tetraethylammonium (TEA)1 reduced the amplitude of the Kslow current in a concentration-dependent manner. Fig. 4 B shows an example where 20 mM TEA reduced the slowly deactivating current by ≈70%. The concentration dependence of the inhibition is summarized in Fig. 4 C. Inhibition was half-maximal at ≈5 mM TEA and ≈30% of the current was resistant to TEA. The effect of TEA on the amplitude of the current evoked by the train was associated with dramatic changes of the action potential firing pattern and the bursts of action potential were replaced by large overshooting action potentials that were either generated singly or as groups of 5–10 spikes (Fig. 4 D).
Figure 4.
Pharmacological characterization of Kslow. (A) Failure of tolbutamide (100 μM) to affect current amplitude. Note that the current response observed when stepping from −70 to −40 mV is reduced by tolbutamide reflecting closure of KATP channels (shaded areas). The horizontal lines indicate the peak amplitude and steady state current, respectively. (B) Effects of TEA (20 mM). The horizontal dotted lines indicate (from top to bottom) the peak current amplitude, under control conditions and in the presence of TEA, and the steady state current, respectively. (C) Concentration dependence of inhibitory action of TEA. The Kslow conductance (G) is expressed as the fractional current using the current amplitude in TEA-free solution as unity (Gcontrol). Note that inhibition is half-maximal at 5 mM and that 30% of the current is resistant to TEA. (D) Electrical activity evoked by 15 mM glucose in the same cell before and after addition of TEA.
Relationship between Kslow Current Activation and [Ca2+]i
We next investigated the relationship between Kslow current activation and [Ca2+]i. These experiments had to be conducted on isolated β cells rather than intact islets as it is not possible to voltage-clamp an entire islet with the patch electrode. Moreover, fluorescence originating from the unclamped neighboring β cells an be expected to contribute to the overall signal and obscure that derived from the voltage-clamped cell. Fig. 5 A shows simultaneous recordings of [Ca2+]i and Kslow current. It is clear that both the activation and deactivation of the current reasonably correlate with the observed changes of [Ca2+]i. In this particular cell, the Kslow current amplitude at the end of the train was 14 pA. This is considerably larger than the typical value and the average, determined without intracellular fura-2, was 4 ± 1 pA (n = 18). This amplitude in the isolated β cells is only 15% of the Kslow current observed in intact islets (28 ± 2 pA; Fig. 5 B).
Given the correlation between [Ca2+]i and Kslow current activation, we tested the effects of inhibiting the voltage-gated Ca2+ channels. The Ca2+ channel antagonists nifedipine (10 μM, not shown), Co2+ (5 mM, not shown), and Cd2+ (0.2 mM, Fig. 5 C) all prevented activation of the Kslow current. Because the Kslow current is dependent on Ca2+ influx, and its activation and deactivation echoes changes of [Ca2+]i, it seems plausible that it flows through some sort of Ca2+-activated K+ channel.
We next considered the possibility that the Kslow current is smaller in dispersed cultured β cells because the Ca2+ current is reduced in these cells. Indeed, the Ca2+ current elicited by 100-ms depolarizations was significantly (40%) smaller in dispersed cells than in β cells within intact islets at all voltages ≤0 mV (Fig. 5, D–E).
Comparison with KATP Conductance
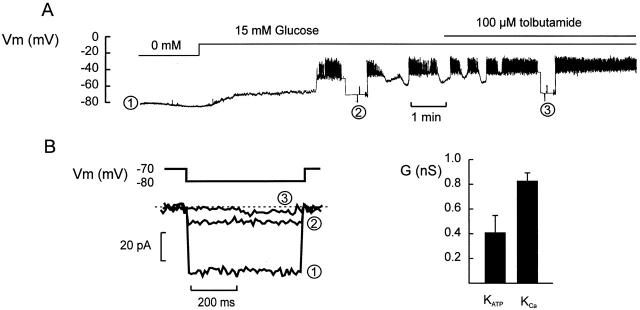
We finally compared the amplitude of the Kslow current with the changes of the KATP conductance associated with the transition from oscillatory into uninterrupted electrical activity. Fig. 6 shows parallel recordings of membrane potential and KATP conductance in a β cell within an islet. In the absence of glucose, the membrane potential was approximately −80 mV. The membrane conductance under these conditions (determined by switching the amplifier into the voltage-clamp mode, holding at −70 mV, and applying ±10-mV voltage pulses) exceeded 5 nS (Fig. 6 B, 1). Addition of 15 mM glucose resulted in membrane depolarization and the induction of oscillatory electrical activity. This was associated with a >75% reduction in resting membrane conductance, which fell to ≈1.2 nS (Fig. 6 B, 2). Subsequent addition of 100 μM tolbutamide, to inhibit remaining KATP channel activity, evoked continuous spiking and a further reduction of the membrane conductance to ≤1 nS (Fig. 6, Fig. 3). In a series of six experiments, the membrane conductance measured in the absence of glucose averaged 3.9 ± 1.0 nS. In the presence of 10 (five cells) or 15 (four cells) mM glucose (i.e., when the β cells generated oscillatory electrical activity), the membrane conductance dropped to 1.4 ± 0.2 nS (n = 9; P < 0.001 vs. that observed in the glucose-free solution). The corresponding value in the simultaneous presence of 10 or 15 mM glucose and 100 μM tolbutamide was 1.0 ± 0.1 nS (n = 7). The decrease in membrane conductance obtained by addition of tolbutamide (100 μM) to islets already exposed to 10 or 15 mM glucose thus amounted to 0.4 ± 0.1 nS (n = 7; P < 0.025). This value is smaller than the 0.8 ± 0.1 (n = 30) that can be derived for the Kslow current from its amplitude and reversal potential (28 ± 2 pA at −40 and −73 mV, respectively).
Figure 6.
Parallel recordings of glucose-induced changes of the membrane potential and membrane conductance in a β cell within an intact islet. (A) Membrane potential recorded in the absence of glucose, after elevation of glucose to 15 mM, and in the simultaneous presence of 15 mM glucose and 100 μM tolbutamide. At the times indicated (1–3), the amplifier was switched from the current-clamp into the voltage-clamp mode and the membrane conductance was monitored by application of ±10-mV voltage pulses (duration: 500 ms; frequency: 0.5 Hz). (B) Membrane currents measured during the voltage steps to −80 mV. The current responses shown in A–C were taken as indicated in A. (C) Net change of whole-cell KATP produced by 0.1 mM tolbutamide added in the presence of 10 or 15 mM glucose and the amplitude of the Kslow current elicited by a train of depolarizations.
DISCUSSION
We describe here a Ca2+-activated K+ current that turns on gradually during electrical activity (Kslow current). A number of considerations argue that the Kslow current is recorded from single β cells in the islet and that it cannot be attributed to regenerative electrical activity in neighboring unclamped cells. First, the measured cell capacitance for β cells in the intact islets is only 40% larger than that of the isolated cells (7.4 vs. 5.3 pF; Göpel et al. 2000). Second, the amplitude of the KATP conductance in intact islets is close to that measured in isolated cells (4 vs. 5 nS, Fig. 6; compare Smith et al. 1989). Third, regenerative electrical activity in neighboring cells does not give rise to detectable changes of the membrane conductance in the voltage-clamped cell (Fig. 3). Fourth, a current with similar properties can be recorded from a small fraction of dispersed β cells (Fig. 5).
We considered the possibility that depolarization of the voltage-clamped cell influences the membrane potential of neighboring cells and that the slowly deactivating current we observe reflects the reversal of this process. The time constant (τ) of the latter process would be represented by the product R j C m, where R j is the gap junction resistance and C m is the cell capacitance. The gap-junction resistance has been estimated as 0.7 GΩ and the cell capacitance is ≈7 pF (Göpel et al. 2000). These values predict that the time constant for passive charging of the voltage (and its reversal) is ≈5 ms; i.e., three orders of magnitude from the value of τ actually observed. This argument is simplistic in assuming that the value of R j reflects the coupling to a single cell. Nevertheless, these considerations make it obvious that the voltage-clamped β cell needs to be directly coupled to ≥1,000 cells to give rise to the experimentally observed time constant of current decay that is clearly unreasonable.
Function of the Kslow Current
The slowly activating current is small, but it may nevertheless play an important role in the shaping of oscillatory electrical activity in the β cell. For example, the gradual decline in action potential frequency and slight repolarization of the plateau potential that is observed during the burst can be attributed to the gradual turn-on of a repolarizing K+ current. The time course of the deactivation of the current is similar to that of the pacemaker depolarization between two bursts and may echo the gradual return of [Ca2+]i to the resting concentration.
The amplitude of the whole-cell Kslow conductance (GK,slow) attained at the end of the burst is equivalent to 0.8 nS. This is 20% of the whole-cell KATP conductance (GK,ATP) in the absence of glucose. It is important to emphasize, however, that the magnitude of GK,slow is actually larger than the decrease in GK,ATP when the tolbutamide is added to β cells already exposed to 10 or 15 mM glucose; i.e., when the β cell goes from oscillatory to continuous electrical activity (Fig. 6). This serves to illustrate that small changes in the whole-cell conductance are capable of exerting marked effects on the pattern of action potential firing (see also Kinard et al. 1999).
The fact that the Kslow current we now describe is not affected by selective inhibitors of large- and small-conductance Ca2+-activated K+ channels such as charybdotoxin and apamin makes it difficult to unequivocally demonstrate that it is responsible for the repolarization terminating the burst of action potentials. However, it is of interest that TEA at high concentrations (≥10 mM) reduces the current by ≈70% and suppresses the normal oscillatory pattern (Fig. 4 D, and Atwater et al. 1979; Henquin 1990). The interpretation of the effects of TEA is naturally complicated by the nonselective properties of this compound and TEA affects all K+ conductances characterized in the β cell, including delayed rectifying K+ channels and large-conductance Ca2+-activated K+ channels (Bokvist et al. 1990a,Bokvist et al. 1990b; Fatherazi and Cook 1991). The finding that TEA abolishes the normal oscillatory pattern can therefore not be used to argue that the Ca2+-dependent K+ current we describe here (the Kslow current) participates in the generation of the bursting pattern. Indeed, the fact that the action potentials remain grouped in the presence of TEA seemingly contradict such a role, but we point out that a fraction (>30%) is resistant to TEA. The overshooting and long-lasting action potentials generated in the presence of TEA are associated with massive Ca2+ influx and large elevations of [Ca2+]i (Rorsman et al. 1992) that can be expected to result in great activation of the Kslow current. It is therefore possible that 30% of the current activated by the large action potentials in terms of the absolute current amplitude is greater than that activated by a normal burst of action potentials. The failure of high concentrations of TEA to completely inhibit the current may be explained if it flows through two types of K+ channel, one of which is resistant to TEA. KATP channels show little sensitivity to TEA (Bokvist et al. 1990b) and it is possible that the Ca2+-influx taking place during repetitive stimulation lowers the ATP/ADP ratio sufficiently to produce some activation of the KATP channels (Detimary et al. 1998). However, ≈70% of the current is TEA sensitive and the Kslow current is unaffected by tolbutamide, making the contribution of the KATP channels questionable. As to the molecular identity, a Ca2+-activated K+ channel clearly represents an attractive candidate despite the failure of both charybdotoxin and apamin to be effective. However, we point out that the slow activation and deactivation kinetics as well as resistance to apamin conform with the properties of SK1 channels (Bond et al. 1999). With regard to the future pharmacological and molecular characterization of the Kslow current, it is of interest that a Ca2+-activated K+ current, which seemingly shares many properties with the Kslow current in β cells, has been documented in clonal βTC3 cells (Kozak et al. 1998).
Why Don't Cultured β Cells Exhibit Rapid Oscillations in Membrane Potential?
The finding that dispersed β cells generate an atypical electrical activity (Fig. 1, and Smith et al., 1990; Larsson et al. 1996) combined with the observation that the Kslow current is much reduced in such cells (Fig. 5) provide circumstantial evidence for its involvement in the termination of the bursts of action potentials. Interestingly, rapid membrane potential oscillations can be induced in isolated β cells under experimental conditions associated with InsP3-dependent mobilization of Ca2+ from intracellular stores (Ämmälä et al. 1991). The associated increase in [Ca2+]i leads to the activation of a Ca2+-activated K+ current that, like the Kslow current described in this study, is resistant to both apamin and charybdotoxin (Ämmälä et al. 1991), and is little affected by TEA at concentrations ≤5 mM. Given these similarities, it seems possible that it is identical to the Kslow current we now describe in situ. The whole-cell conductance of the current measured with physiological ionic gradients in dispersed cells is 0.25 nS (Ämmälä et al. 1993b); i.e., ≈30% of the Kslow current amplitude in the islet. It remains to be determined why this current is not activated by the Ca2+ influx associated with a train of action potentials (or voltage-clamp depolarizations). However, it may be of relevance that the amplitude of the Ca2+ current in dispersed cells is only 60% of that observed in β cells within intact islets (Fig. 5, D–E). Another possibility is that the architecture of the β cell changes during cell isolation so that the Kslow and Ca2+ channels become separated from each other. The domain of elevated Ca2+ beneath the Ca2+ channel may consequently not extend sufficiently to activate the Kslow channels. The latter possibility is suggested by the observation that although they could not be activated by Ca2+ influx during action potential firing, they remained activatable by InsP3-dependent mobilization of intracellular Ca2+ (Ämmälä et al. 1991, Ämmälä et al. 1993b).
Kslow Current and Glucose-induced Electrical Activity
We propose that control of the membrane potential oscillations by two distinct K+ conductances, one regulated by glucose metabolism (KATP channels) and one determined by electrical activity via increases in [Ca2+]i (Kslow channels), provides the β cell with the means of responding to glucose in a graded fashion. The generation of oscillatory β cell electrical activity can be explained in the following way: increasing the glucose concentration from 0 to 10 mM produces a decrease in GK,ATP from 4 nS to ≈1.5 nS (Fig. 6). This results in membrane depolarization and induction of electrical activity. The Ca2+ entry associated with electrical activity leads to a gradual increase in Kslow channel activity (GK,slow) that echoes the change of [Ca2+]i. When the total K+ conductance is sufficient to overcome the depolarizing influence of the voltage-gated Ca2+ current, the β cell starts repolarizing, leading to regenerative closure of the voltage-gated Ca2+ channels and a reduction of [Ca2+]i. The decrease in [Ca2+]i in turn leads to the deactivation of GK,slow. In this model, the background GK,ATP defines how much GK,slow may increase before initiating membrane repolarization. The concentration-dependent glucose-induced decrease in GK,ATP thus allows for progressively greater activation of the Kslow current before repolarization occurs and the duration of the burst accordingly increases. Continuous electrical activity is elicited at glucose concentrations approaching 20 mM when GK,ATP is sufficiently suppressed to allow electrical activity to continue even when GK,slow is maximally activated. This model is compatible with the observation that tolbutamide converts oscillatory electrical activity to continuous action potential firing (Henquin 1988) and the latter observation should not be used as an argument that the KATP channels are directly involved in the burst repolarization. We acknowledge that an increase in K+ conductance may not be the sole mechanism participating in the generation of oscillatory electrical activity in the β cell. For example, inactivation of the voltage-gated Ca2+ current has also been implicated in the process (Cook et al. 1991)
The scenario outlined above predicts that [Ca2+]i should increase throughout the burst of action potentials, reaching a peak at the time repolarization commences (compare Ämmälä et al. 1993a; Gilon et al. 1999). However, not all studies have documented such behavior; for example, Santos et al. 1991 report that [Ca2+]i quickly rises to a plateau at the beginning of the burst of action potentials but subsequently remains fairly stable. It should be kept in mind, however, that the relationship between [Ca2+]i and indo-1 fluorescence (the dye used in the above study) is nonlinear and does not increase much at concentrations >1 μM (Ämmälä et al. 1993a). In this context, it should also be pointed out that the Ca2+ channels and the Kslow channels are likely to be spatially separated. This is suggested by the fact that glucose-induced electrical activity consists of bursts of action potentials. If the two types of ion channels were situated in the immediate vicinity of each other, then the β cell would be expected to exhibit fast afterhyperpolarizations after every action potential as documented in, for example, hippocampal neurons (Marrion and Tavalin 1998). The finding that such afterhyperpolarizations are not observed instead argues that Ca2+ needs to diffuse over some distance within the β cell to activate the Kslow channels. During this process, the Ca2+ signal may be subject to various modulatory influences. For example, it may be amplified by Ca2+ release from intracellular stores (Liu et al. 1996; Islam et al. 1998). This scenario is supported by the finding that exposure of pancreatic islets to thapsigargin, an inhibitor of the Ca2+-ATPase in smooth endoplasmic reticulum, abolishes the bursting pattern and results in uninterrupted action potential firing (Worley et al. 1994). A recent study also demonstrates that a train of voltage-clamp depolarizations evoke a slowly decaying component of [Ca2+]i increase that disappears after pretreatment with thapsigargin (Gilon et al. 1999). The time constant of this [Ca2+]i component was reported to be 13 s, not too different from the 6.5 s we observe for the deactivation of the Kslow current and very close to the 13-s interval between two successive bursts. It is therefore of interest that preliminary evidence suggests that the Kslow current is sensitive to thapsigargin and that its amplitude is reduced after pretreatment with this ATPase inhibitor (Göpel and Rorsman 1998).
Acknowledgments
We thank Novo Nordisk A/S, Bagsvaerd for the loan of some of the equipment used in the experiments described in this paper.
This study was supported by grants from the Swedish Diabetes Association, the Swedish Medical Research Council (8647 and 13147), the Juvenile Diabetes Foundation, the Wallenberg Foundation, the Crafoord Foundation, the National Board for Planning and Coordination of Research, the Segerfalk Foundation, the Aage and Louise Hansen Memorial Foundation, and the Novo Nordisk Foundation.
Footnotes
TEA, tetraethylammonium
References
- Ämmälä C., Bokvist K., Larsson O., Berggren P.O., Rorsman P. Demonstration of a novel apamin-insensitive calcium-activated K+ channel in mouse pancreatic B cells Pflügers Arch. 422 1993. 443 448b [DOI] [PubMed] [Google Scholar]
- Ämmälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F.M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells J. Physiol. 472 1993. 665 688a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ämmälä C., Larsson O., Berggren P.O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol triphosphate–dependent periodic activation of a Ca2+-activated K+ conductance in glucose-stimulated pancreatic β-cells. Nature. 1991;353:849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Andreu E., Soria B., Sanchez-Andres J.V. Oscillation of gap junction electrical coupling in the mouse pancreatic islets of Langerhans. J. Physiol. 1997;498:753–761. doi: 10.1113/jphysiol.1997.sp021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M., Rorsman P. Electrophysiology of the pancreatic β-cell. Prog. Biophys. Res. Commun. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Mouse pancreatic β-cellstetraethylammonium blockage of the potassium permeability increase induced by depolarization. J. Physiol. 1979;288:561–574. [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Rosario L., Rojas E. Properties of the Ca-activated K+-channel in pancreatic β-cells. Cell Calc. 1983;4:451–461. doi: 10.1016/0143-4160(83)90021-0. [DOI] [PubMed] [Google Scholar]
- Barbosa R.M., Silva A.M., Tome A.R., Stamford J.A., Santos R.M., Rosario L.M. Control of pulsatile 5-HT/insulin secretion from single mouse pancreatic islets by intracellular calcium dynamics. J. Physiol. 1998;510:135–143. doi: 10.1111/j.1469-7793.1998.135bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten P., Hellman B. Glucose-induced cycles of insulin release can be resolved into distinct periods of secretory activity. Biochem. Biophys. Res. Commun. 1993;192:1182–1188. doi: 10.1006/bbrc.1993.1541. [DOI] [PubMed] [Google Scholar]
- Bokvist K., Eliasson L., Ämmälä C., Renström E., Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokvist K., Rorsman P., Smith P.A. Effects of external tetraethylammonium ions and quinine on delayed rectifying K+ channels in mouse pancreatic β-cells J. Physiol 423 1990. 311 325a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokvist K., Rorsman P., Smith P.A. Block of ATP-regulated and Ca2+-activated K+ channels in mouse pancreatic β-cells by external tetraethylammonium and quinine J. Physiol. 423 1990. 327 342b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C.T., Maylie J., Adelman J.P. Small-conductance calcium-activated potassium channels. Ann. NY Acad. Sci. 1999;868:370–378. doi: 10.1111/j.1749-6632.1999.tb11298.x. [DOI] [PubMed] [Google Scholar]
- Cook D.L., Satin L.S., Hopkins W.F. Pancreatic B cells are bursting, but how? Trends Neurosci. 1991;14:411–414. doi: 10.1016/0166-2236(91)90033-q. [DOI] [PubMed] [Google Scholar]
- Dean P.M., Matthews E.K. Electrical activity in pancreatic islet cells. Nature. 1968;219:389–390. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- Detimary P., Gilon P., Henquin J.C. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratioa feedback control mechanism in mouse pancreatic islets. Biochem. J. 1998;333:269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatherazi S., Cook D.L. Specificity of tetraethylammonium and quinine for three K channels in insulin-secreting cells. J. Membr. Biol. 1991;120:104–114. doi: 10.1007/BF01872393. [DOI] [PubMed] [Google Scholar]
- Gilon P., Arredouani A., Gailly P., Gromada J., Henquin J.C. Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+-concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. J. Biol. Chem. 1999;274:20197–20205. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- Göpel S., Kanno T., Barg S., Galvanovskis J., Rorsman P. Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J. Physiol. 2000;In press doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpel S.O., Rorsman P. Activation of a Ca2+-activated K+-conductance terminates the burst of action potentials in insulin-secreting pancreatic B-cells. Diabetologia. 1998;41:540. [Google Scholar]
- Henquin J.C. ATP-sensitive K+ channels may control glucose-induced electrical activity in pancreatic B-cells. Biochem. Biophys. Res. Commun. 1988;156:769–775. doi: 10.1016/s0006-291x(88)80910-0. [DOI] [PubMed] [Google Scholar]
- Henquin J.C. Role of voltage- and Ca2+-dependent K+ channels in the control of glucose-induced electrical activity in pancreatic B-cells. Pflügers Arch. 1990;416:568–572. doi: 10.1007/BF00382691. [DOI] [PubMed] [Google Scholar]
- Henquin J.C., Meissner H.P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia (Basel). 1984;40:1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.S., Leibiger I., Leibiger B., Rossi D., Sorrentino V., Ekström T.J., Westerblad H., Andrade F.H., Berggren P.O. In situ activation of the type 2 ryanodine receptor in pancreatic β cells requires cAMP-dependent phosphorylation. Proc. Natl. Acad. Sci. USA. 1998;95:6145–6150. doi: 10.1073/pnas.95.11.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinard T.A., de Vries G., Sherman A., Satin L.S. Modulation of the bursting properties of single mouse pancreatic β-cells by artificial conductances. Biophys. J. 1999;76:1423–1435. doi: 10.1016/S0006-3495(99)77303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak J.A., Misler S., Logothetis D.E. Characterization of a Ca2+-activated K+ current in insulin-secreting murine βTC-3 cells. J. Physiol. 1998;509:355–370. doi: 10.1111/j.1469-7793.1998.355bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O., Kindmark H., Brandström R., Fredholm B., Berggren P.O. Oscillations in KATP channel activity promote oscillations in cytoplasmic free Ca2+ concentration in pancreatic β cell. Proc. Natl. Acad. Sci. USA. 1996;93:5161–5165. doi: 10.1073/pnas.93.10.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Fernandez J.M. IgE-mediated degranulation of mast cells does not require opening of ion channels. Nature. 1986;319:150–153. doi: 10.1038/319150a0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Grapengiesser E., Gylfe E., Hellman B. Crosstalk between the cAMP and inositol triphosphate–signalling pathways in pancreatic β-cells. Arch. Biochem. Biophys. 1996;334:295–302. doi: 10.1006/abbi.1996.0458. [DOI] [PubMed] [Google Scholar]
- Marrion N.V., Tavalin S.J. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Mears D., Sheppard N.F., Atwater I., Rojas E. Magnitude and modulation of pancreatic β-cell gap junction electrical conductance in situ. J. Membr. Biol. 1995;146:163–176. doi: 10.1007/BF00238006. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J. Neurosci. Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Ämmälä C., Berggren P.-O., Bokvist K., Larsson O. Cytoplasmic calcium transients due to single action potentials and voltage-clamp depolarizations in mouse pancreatic B-cells. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:2877–2884. doi: 10.1002/j.1460-2075.1992.tb05356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and potassium currents in mouse pancreatic β-cells under voltage-clamp conditions. J. Physiol. 1986;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.M., Rosario L.M., Nadal A., Garcia-Sancho J., Soria B., Valdeolmillos M. Widespread synchronos (Ca2+)i oscillations due to bursting electrical activity in single pancreatic islets. Pflügers Arch. 1991;418:417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Satin L.S., Smolen P.D. Electrical bursting in β-cell of the pancreatic islets of Langerhans. Endocrine. 1994;2:677–687. [Google Scholar]
- Sherman A. Contributions of modeling to understanding stimulus-secretion coupling in pancreatic β-cells. Am. J. Physiol. 1996;271:E362–E372. doi: 10.1152/ajpendo.1996.271.2.E362. [DOI] [PubMed] [Google Scholar]
- Smith P.A., Ashcroft F.M., Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K+-currents in isolated mouse pancreatic β-cells. FEBS Lett. 1989;261:187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- Worley J.F., McIntyre M.S., Spencer B., Mertz R.J., Roe M.W., Dukes I.D. Endoplasmic reticulum calcium store regulates membrane potential in mouse islet β-cells. J. Biol. Chem. 1994;269:14359–14362. [PubMed] [Google Scholar]