Abstract
Many psychological disorders are characterized by anxiety and alterations in social interactions. Recent studies demonstrate that the chemical messenger nitric oxide (NO) can regulate both anxiety and social behaviours. We tested whether an enzyme that produces NO in the brain, neuronal nitric oxide synthase (nNOS), serves as an interface between social interactions and anxiety-like behaviour. Several investigators have observed that mice increase anxiety-like responses in the elevated plus-maze after pair housing. nNOS gene deletion and 3-Bromo-7-Nitroindazole were used to inhibit the production of neuronal NO. Similar to previous studies, pair housing reduced open arm exploration in the elevated plus-maze. Pair housing also increased corticotropin-releasing hormone (CRH) immunoreactive cells in the paraventricular nucleus (PVN) of the hypothalamus. Inhibition of NO production increased open arm exploration in pair-housed mice but decreased open arm exploration in individually-housed mice. These results suggest that the effect of nNOS inhibition on anxiety-like responses is context dependent and that behavioural responses to social housing are altered after nNOS inhibition. This research suggests that NO may play an important role in mediating the effect social interactions have on anxiety.
Keywords: elevated plus-maze, corticotropin-releasing hormone, paraventricular nucleus, nNOS, NOS-1, mice
1. Introduction
Many psychological disorders are characterized by anxiety and alterations in social interactions. Social interactions influence a wide array of affective behaviours. In humans, social isolation is a risk factor for suicide [19]. In rhesus monkeys (Macaca mulatta), anxiety- and depressive-like behaviours increase in response to social isolation [24]. Although social isolation in primates has a negative influence on affect, individual housing has the opposite effect in male mice. Individually housed male mice have reduced anxiety-like responses compared to male mice housed in pairs [1,28,33,44]. This difference in affective responses may reflect species differences in social structure. Primates are highly social animals and isolation from conspecifics has negative consequences in terms of affective and stress responses [18,38]. In contrast, socially-housed male house mice form dominance hierarchies and territories [2,25] which may contribute to the reduced exploratory behavior. Also, strain-specific anxiety-like responses have been reported in rodents. For example, male Wistar rats, in common with mice, increase anxiety-like responses after pair housing [40] whereas social isolation appears to be anxiogenic in male Sprague-Dawley rats [48]. The biological substrates of social interactions, anxiety, and their interaction remain unclear. One chemical system that may be of interest is nitric oxide (NO) because inhibition of neuronal nitric oxide alters social [13,29] and anxiety-like behaviours [9,11,35,45].
NO is a gas that can act as a neurotransmitter in the brain [17,30,31]. NO is labile, with a half-life of <5 sec at body temperature; consequently, many studies manipulate NO indirectly by affecting its synthetic enzyme, NO synthase (NOS), that transforms arginine into NO and citrulline. Three distinct isoforms of NOS have been discovered: (1) eNOS (NOS-3) in the endothelial tissue of blood vessels, (2) iNOS (NOS-2) in macrophages as an inducible form, and (3) nNOS (NOS-1) in neurons, which is expressed constitutively [21].
NO has broad effects on social behaviours. Most research on the role of NO in social behaviours has focused on aggression [5,13,29,31,41]. Male mice lacking the gene that encodes nNOS (nNOS-/-) are highly aggressive and do not relent in response to submissive postures of wild-type (WT) males [29]. nNOS-/- mice also persist in mounting anestrous WT females despite signs of disinterest [29]. The persistence of these behaviours in nNOS-/- mice may reflect alterations in brain areas that are important for the processing of social cues.
Recent research suggests that the effect of nNOS inhibition on behaviour is dependent on social context. Although nNOS-/- mice are more aggressive toward WT intruders, suppression of nNOS with the selective nNOS inhibitor 3-Bromo-7-Nitroindazole (3-Br-7-NI) reduces maternal aggression in response to an intruder relative to untreated dams [13]. In addition, single-housed mice treated with 3-Br-7-NI also reduce social investigation in a barrier test and a habituation-dishabituation test relative to vehicle-treated mice but this effect was eliminated when mice were pair housed [41]. Together, these results suggest that deficits in NO signaling alter behavioural responses to social interactions.
Studies on NO signaling and affective behaviours have yielded mixed results. Pharmacological inhibition of NO by administration of either NG-nitro-l-arginine methyl ester (l-NAME) or NG-nitro-l-arginine (l-NOARG), two nonspecific NOS inhibitors, increases anxiety-like responses [7,8,26,35,42]. l-NOARG also reverses anxiolysis associated with benzodiazepines [36] and nitrous oxide (N2O) [4] suggesting that NO is anxiolytic. In contrast to these results, administration of l-NAME, l-NOARG, or 7-nitroinazole (7-NI) decreases anxiety-like responses in the elevated plus-maze [9-11,15,35,45]. The nonselective properties of l-NAME and l-NOARG may be an important contributing factor in the disparate results that have been reported across studies, as these drugs influence both neuronal and endothelial NOS [47]. Furthermore, these drugs may increase anxiety-like responses through nonspecific changes such as vasoconstriction or reduced locomotor activity [7,35]. However, studies using the nNOS inhibitor 7-NI report decreases in anxiety-like responses [9,11,35,45].
NO dysfunctions may alter hormones and neurotransmitters to influence anxiety-like responses. NO is intimately involved with the hypothalamic-adrenal-pituitary (HPA) axis and is located (although not exclusively) within the paraventricular nucleus (PVN) [30]. The PVN is a major source of corticotropin-releasing hormone (CRH) which induces adrenocorticotropic hormone (ACTH) release from the pituitary which stimulates cortisol (or corticosterone) release from the adrenal cortex [32]. Although cortisol has been implicated in playing a major role in the development of affective disorders, the central effects of CRH itself may be important in anxiety and anxiety-like responses [16]. CRH terminals, cell bodies and receptors are located throughout the brain including the central nucleus of the amygdala [43], which plays an integral role in the autonomic and behavioural responses to fearful stimuli [20,46]. Although many studies have investigated the effects of NO inhibition on anxiety-like responses, less attention has been paid to changes that occur in brain areas that are associated with fear and anxiety. In addition, many of the aforementioned studies do not consider the effect of social housing conditions on behaviour.
We examined the influence of nNOS inhibition on anxiety-like responses and CRH immunoreactivity in the PVN and central nucleus of the amygdala (CeA) of single-and pair-housed mice. We hypothesized that pair housing of WT mice would reduce open arm exploration in the elevated plus-maze. Furthermore, we expected that nNOS inhibition would eliminate the effect of pair housing on anxiety-like responses. Using nNOS-/- mice and a selective nNOS inhibitor we tested whether NO function interacts with social housing conditions to influence anxiety-like responses.
2. Methods
2.1 Animals
Adult wild-type (WT) mice (B6; 129SF1/SImJ, Stock Number 002633) and nNOS-/- mice (B6;129S4/SvJae-Nos1tm1Plh/J, Stock Number 101045) were obtained from Jackson Laboratory (Bar Harbor, ME, USA) for Experiment 1. Adult WT mice (B6;129SF1/SImJ, Stock Number 002633) were again obtained from Jackson Laboratory at a later date and used in Experiment 2. The nNOS-/- mutation is on a C57B6 × 129S background. The WT mice are also a C57B6 × 129S cross that is an appropriate match to the background of the nNOS-/- line. Although not an exact match, this strain has been used previously as a WT control strain as well as in pharmacological manipulations [41]. Mice were housed in polypropylene cages (dimensions: 27.8 × 17.5 × 13 cm). All mice had unlimited access to food (Harlan Teklad 8640 rodent diet, Indianapolis, IN) and filtered tap water. The light cycle was 16L:8D with the dark phase beginning at 1500 h EST. All procedures were approved by the Ohio State Institutional Animal Care and Use Committee prior to the study and meet guidelines published in the National Institutes of Health (1986) Guide for the Care and Use of Laboratory Animals.
2.2 Experiment 1
Upon arrival, WT and nNOS-/- mice were randomly assigned to either individual-or pair-housing with a mouse of the same genotype (WT single house, n = 5, WT pair housed, n = 7, nNOS-/- single housed, n = 6, nNOS-/- pair housed, n = 6). Two weeks after arrival, mice underwent behavioural testing in the open field chamber and elevated plus maze on consecutive days during the dark phase.
2.2.1 Open Field
The test chamber for open field was enclosed in a sound and light attenuating cabinet and consisted of a 60 cm3 clear Plexiglas arena lined with corncob bedding. The arena was surrounded by a series of infrared lights that tracked the movement of the mouse in three dimensions. Mice were allowed to acclimate to the testing room for 15 min before testing began. The test chamber was rinsed thoroughly with a 70% ethanol solution and the bedding changed between each test. Each test session was 30 min in duration. The results were generated online by the PAS software package (San Diego Instruments, San Diego, CA, USA). The total locomotor activity (number of beam breaks) served as the dependent measure.
2.2.2 Elevated Plus-Maze
The maze was ~1 m above the floor and consisted of two open arms bisected by two arms enclosed with dark-tinted acrylic. Testing began approximately 45 min after lights-out and mice were allowed to acclimate to the testing room for 15 min before testing began. The room was illuminated with a dim red light. Mice were placed into a closed arm and recorded on video for 5 min. The maze was wiped with 70% EtOH between tests. An open arm entry was scored when the two forepaws had entered an open arm. Latency to enter an open arm, total time spent in the open arms, and open arm entries were scored by a blind observer using Observer software (Noldus Corp., Leesburg, VA).
2.2.3 Tissue Collection
Two days after testing in the elevated plus-maze, mice were anesthetized with isoflurane and decapitated. Brains were quickly removed and fixed overnight in 5% acrolein. Brains were then transferred to 30% sucrose for 24 h and frozen at -80° C.
2.2.4 CRH Immunohistochemistry
Brains were sectioned at 40 μm on a cryostat and free-floating sections were processed for CRH immunohistochemistry as previously described [23]. Sections were washed three times in phosphate-buffered saline (PBS), and then incubated in 1% sodium borohydride in PBS for 10 min. Sections were then rinsed in 20% normal goat serum and 0.3% hydrogen peroxide in PBS for 20 min. Sections were incubated in primary CRH antibody (1:20,000, gift of Dr. Wylie Vale, Salk Institute, San Diego, CA) in 1% normal goat serum in 0.5% Triton-X PBS (PBS + TX) at 4 °C for 48 h. Next, sections were rinsed three times in PBS, and incubated for 90 min with biotinylated goat-anti-rabbit antibody (1:500, Vector Laboratories, Burlingame, CA) in PBS + TX. The sections were rinsed three times in PBS and then incubated for 30min in avidin–biotin complex (ABC Elite kit, Vector Laboratories, Burlingame, CA). After three rinses in PBS, the sections were developed in diaminobenzidine for 2 min. Sections were mounted, dehydrated, and coverslipped with Permount. Sections containing the PVN and CeA were identified using a mouse brain atlas [12]. Images were captured using a Nikon E800 microscope. Images of the PVN were taken at -0.70 to -0.94 bregma. Positively stained cells in the PVN were distinct and considered positive if they showed any staining. Images of the CeA were taken at -1.46 to -1.58 bregma. The CeA did not show positively stained cells. To quantify staining in this area, the percentage of staining was calculated using the image analysis software ImageJ, 1.38x (NIH). Control sections in which primary antibodies were not added showed no staining.
2.3 Experiment 2
Mice were randomly assigned to receive Silastic implants containing the selective neuronal nitric oxide synthase inhibitor 3-Bromo-7-Nitroindazole (3-Br-7-NI) [13,41]. Silastic tubing (1.98 mm I.D. × 3.18 mm O.D.; Dow Corning, Midland, MI, USA) was packed with 2 mm of 3-Br-7-NI and then implanted subcutaneously between the scapulae (empty capsule single-housed, n = 5, empty capsule pair-housed, n = 9, 3-Br-7-NI capsule single-housed, n = 5, 3-Br-7-NI capsule pair-housed, n = 5). The optimal length of the capsule was determined in a pilot study of the effects of 1, 2, 3 mm of 3-Br-7-NI on citrulline staining. One week after surgery, mice underwent behavioural testing as previously described in Experiment 1.
2.4 Statistical Analysis
Data from Experiment 1 were analysed with two-way ANOVAs, testing for the effects of genotype, housing environment and their interaction. Data from Experiment 2 were analysed with two-way ANOVAs, testing for the effects of 3-Br-7-NI, housing environment and their interaction. In both experiments, planned comparisons were used to compare the effects of genotype or implant type within housing environment and the effects of housing environment within either genotype or implant type. In the elevated plus-maze data of Experiment 1, two mice were removed from the single-housed, WT condition because they entered an open arm and did not move for the remainder of the test. ANOVAs and planned comparisons were considered statistically significant if p < 0.05.
3. Results
3.1 Experiment 1
nNOS-/- mice entered the open arms sooner than WT mice regardless of housing environment (F1,18 = 25.428, p < 0.05). There was a significant interaction between genotype and housing for time spent in the open arm (F1,18 = 5.6, p < 0.05; Fig 1A). Among WT mice, pair housing decreased time spent in the open arms (p < 0.05; Fig 1A). This effect was reversed, however, in the nNOS-/- mice; pair-housed nNOS-/- mice spent more time in the open arms compared with single-housed nNOS-/- mice (p < 0.05; Fig 1A). Among single-housed mice, nNOS-/- mice significantly reduced the amount of time in the open arm compared with WT mice (p < 0.05; Fig 1A). Genotype and housing interacted to affect number of open arm entries (F1,18 = 13.621, p < 0.05; Fig 1B). Pair-housed nNOS-/- mice entered the open arms significantly more than pair-housed WT and single-housed nNOS-/- mice (p < 0.05 in both cases; Fig 1B). There was a significant genotype by housing environment interaction on the number of CRH-positive cells in the PVN (F1,20 = 5.433; p < 0.05; Fig 2 & 3). In WT mice, pair housing significantly increased the number of CRH-positive cells (p < 0.05; Fig 2). In nNOS-/- mice, housing did not alter CRH-positive cells in the PVN (p > 0.05; Fig 2). Among single-housed mice, nNOS-/- mice had significantly more CRH-positive cells in the PVN compared with WT mice (p < 0.05; Fig 2). There was no effect of housing or genotype on CRH staining in the CeA (p > 0.05).
Figure 1.
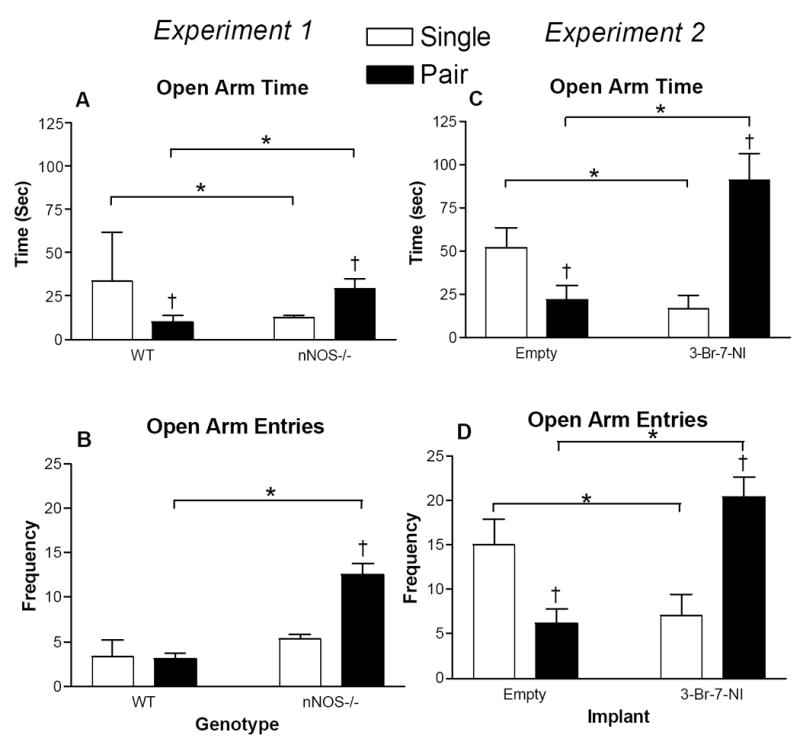
Mean ± SEM time (A and C) spent in the open arms and frequency (B and D) of entries into the open arms of an elevated plus maze in nNOS-/- or 3-Br-7-NI-treated mice compared to wild-type or empty- capsule control animals, respectively. Open bars represent single housed mice. Black bars represent pair housed mice. * (Comparison within housing environment) = p < 0.05; † (Comparison within genotype or implant) = p < 0.05.
Figure 2.
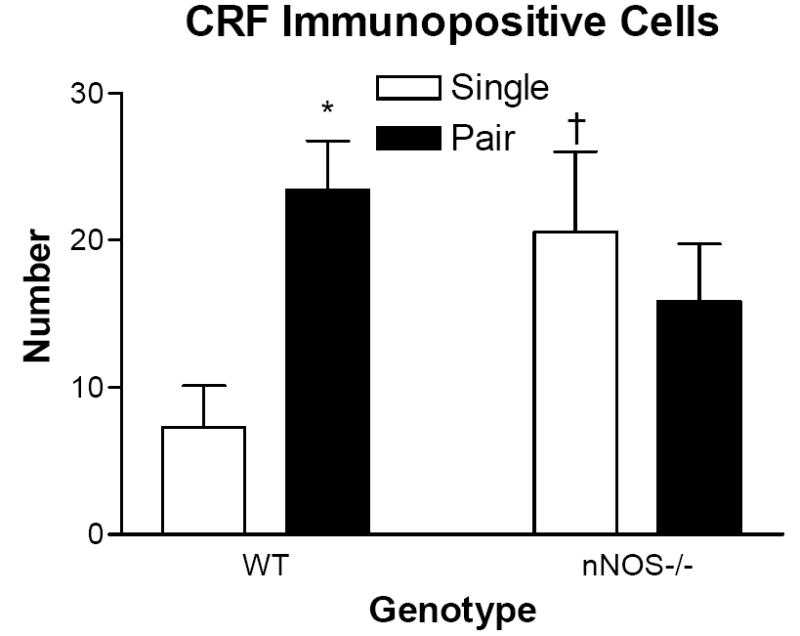
Mean ± SEM of number of cells stained positively for corticotropin releasing factor (CRH) in the paraventricular nucleus (PVN). Open bars represent single housed mice. Black bars represent pair housed mice. * (Comparison within housing environment) = p < 0.05; † (Comparison within genotype) = p < 0.05.
Figure 3.
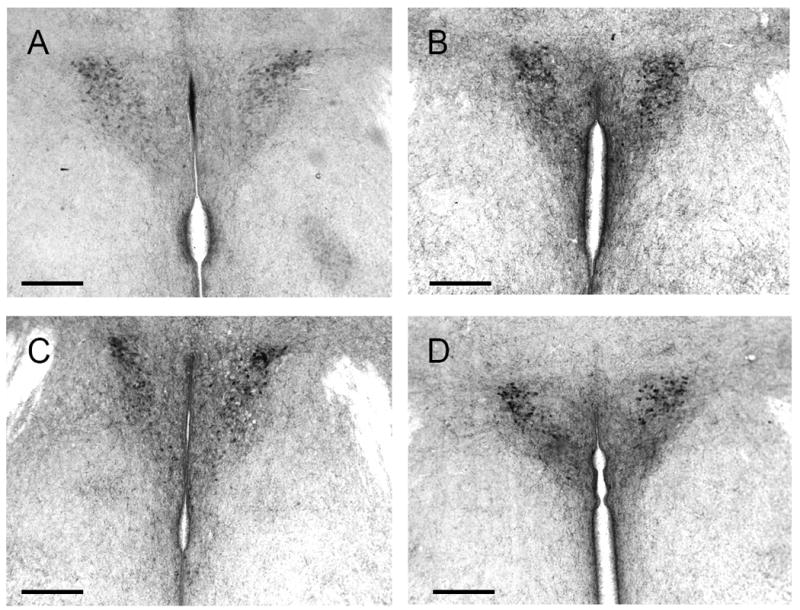
Corticotropin-releasing hormone staining in the paraventricular nucleus. A) wild-type single housed B) wild-type pair housed C) nNOS -/- single housed D) nNOS -/- pair housed. Scale bars = 180 μm.
Genotype and housing did not interact to affect total locomotor activity in the open field (F1,20 = 0.122, p > 0.05; Table 1). Neither housing environment nor genotype alone affected total locomotor activity in the open field chamber (p > 0.05 in both cases; Table 1).
Table 1.
Average Beam Breaks ± SEM after 30 min in the Locomotor Chamber
| Treatment | Single | Paired |
|---|---|---|
| WT | 4366±169 | 4918±473 |
| nNOS-/- | 4813±474 | 5738±669 |
| Empty Implant | 4388±579 | 3519±290 |
| 3-Br-7-NI Implant | 4142±413 | 4318±525 |
3.2 Experiment 2
Housing condition and 3-Br-7-NI-treatment interacted to affect latency to enter an open arm (F1,20 = 6.294; p <0.05; Fig 4). Among mice implanted with empty capsules, pair-housed mice significantly increased the latency to enter an open arm compared with single-housed mice (p < 0.05; Fig 4). Among pair-housed mice, treatment with 3-Br-7-NI significantly reduced the latency to enter an open arm compared with empty capsules (p < 0.05; Fig 4). Pair housing reduced time in the open arms of the mice implanted with empty capsules, (p < 0.05; Fig 1C). Treatment with 3-Br-7-NI reversed this effect: pair housed mice treated with 3-Br-7-NI spent significantly more time in the open arms compared with pair-housed mice with empty capsules (p < 0.05; Fig 1C). Among single-housed mice, 3-Br-7-NI-treatment reduced the time spent in the open arms (p < 0.05; Fig 1C). Housing condition and treatment with 3-Br-7-NI interacted to affect number of open arm entries (F1,20 = 9.858, p < 0.05; Fig 1D). Of mice with empty capsules, pair housing significantly reduced number of open arm entries (p < 0.05; Fig 1D). Treatment with 3-Br-7-NI reversed this effect: pair-housed mice with 3-Br-7-NI capsules significantly increased open arm entries compared with paired mice implanted with empty capsules (p < 0.05; Fig 1D). Among single-housed mice, 3-Br-7-NI-treatment reduced the number of open arm entries (p < 0.05; Fig 1D). There was a significant interaction between 3-Br-7-NI treatment and housing environment on the number of closed arm entries (F1,20 = 6.489, p < 0.05). This effect was largely attributable to the difference between pair- and single-housed mice within the control group: pair-housed mice with empty capsules reduced entries into closed arms compared with single-housed mice with empty capsules (p <0.05). The amount of time spent in the center of the elevated plus-maze was unaffected by 3-Br-7-NI treatment and housing environment (F1,20 = 0.975, p > 0.05).
Figure 4.
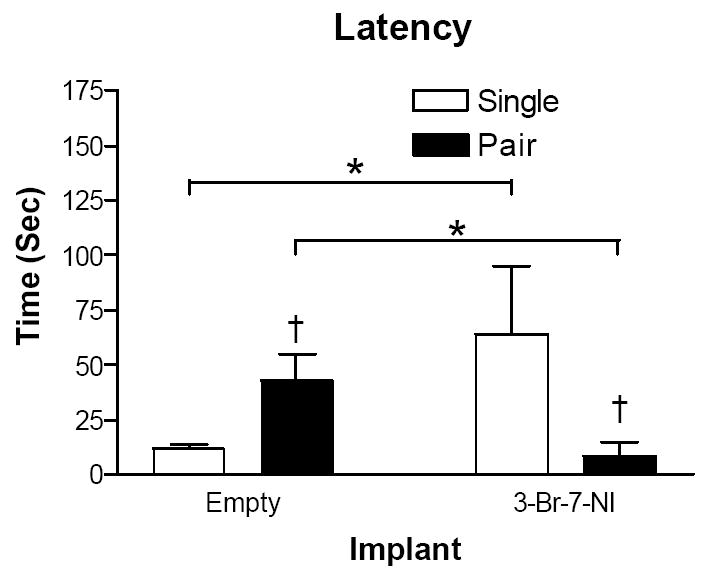
Mean ± SEM of latency (sec) to enter an open arm in the elevated plus-maze. Open bars represent single housed mice. Black bars represent pair housed mice. * (Comparison within housing environment) = p < 0.05; † (Comparison within implant type) = p < 0.05.
In the open field, housing environment and 3-Br-7-NI-treatment did not interact to affect total locomotor activity (F1,20 = 1.413, p > 0.05; Table 1). Neither housing environment nor 3-Br-7-NI-treatment alone affected total locomotor activity in the open field chamber (p > 0.05 in both cases; Table 1).
4. Discussion
These data indicate that nNOS inhibition alters the effect of pair housing on anxiety-like responses. WT, pair-housed mice reduced open arm exploration in the elevated plus-maze. nNOS inhibition, however, reversed this effect. Further, nNOS inhibition (either by genetic or pharmacological manipulation) decreased open arm exploration of single housed-mice in the elevated plus-maze, but increased open arm exploration in pair-housed mice.
In this study, pair housing increased anxiety-like responses in male mice in the elevated plus-maze without altering basal locomotor activity. This confirms previous research establishing that, in mice, social housing increases anxiety-like responses [1,28,33,44]. This is a unique phenomenon that may be attributable to the social structure of mice. Socially housed male mice tend to form dominance hierarchies [22] which may explain the decreased exploratory behavior of pair-housed mice. Subordinate mice tend to reduce exploratory behaviour when compared with dominant mice [34]. More study is warranted, however, to determine if reduced exploratory behavior in paired mice is a result of social dominance hierarchies or simply a result of fighting between cage mates. One possible mechanism for this effect is an increase in CRH production, as we observed that pair housing increased the number of CRH-positive cells in the PVN (Fig 2 & 3).
Inhibition of nNOS either by gene deletion or treatment with 3-Br-7-NI reduced open arm exploration in single-housed mice; an effect that was more pronounced in 3-Br-7NI-treated mice than nNOS-/- mice (Fig 1). This observation suggests that NO reduces anxiety in single-housed mice. However, nNOS inhibition in pair-housed mice increases open arm exploration. Given these results, nNOS inhibition appears to play a role in the ability to respond behaviourally to social stimuli. Research on aggressive behaviours and nNOS inhibition also supports this conclusion. Typically, a supine posture establishes dominance in an agonistic encounter and will cause the attacker to discontinue. nNOS-/- mice, however, do not cease attacks when the WT counterpart submits [29]. In addition, nNOS inhibition via 3-Br-7-NI reduces maternal aggression in response to an intruder [13]. Lastly, nNOS inhibition does not seem to impair the ability to identify social cues (evident in a habituation-dishabituation test). Instead, nNOS deficient mice appear less motivated to investigate social stimuli [41]. Collectively, these data suggest that behavioural responses to social stimuli are impaired after nNOS inhibition.
In our study, nNOS inhibition did not simply impair the behavioural response to pair housing but had the opposite effect on behaviour. We observed that single housed, nNOS-/- mice and pair housed, WT had more CRH-positive cells in the PVN relative to WT, single-housed mice (Fig 2 & 3). This increase in CRH staining is associated with a reduction in open arm exploration. Nitric oxide is colocalized with CRH in a subset of cells in the PVN [39,49] and also modulates the release of CRH in vitro [6]. Further evidence indicates that NO can modulate the effects of CRH on exploratory behavior. Pharmacological inhibition of nNOS reverses the behavioral effects of exogenous CRH [37]. If pair housing alters behavior through CRH activity, then it is possible that deficient NO signaling can reverse these behaviors by altering CRH activity. Pair housing, however, did not significantly reduce CRH staining of nNOS-/- mice but did significantly increase open arm exploration, indicating that CRH may not be solely responsible for altered anxiety-like responses in nNOS-/- mice. Alternatively, pair housing and nNOS disruption may interact to effect serotonin which ultimately can affect anxiety-like responses. Both social housing {Rilke, 1998 #41} and nNOS gene deletion {Chiavegatto, 2001 #47} alter serotonin turnover.
Our results from pair-housed mice are consistent with previous research indicating that nNOS inhibition is anxiolytic in group-housed male mice [35,45]. These studies used 7-NI, which is an inhibitor of nNOS and is less selective than the nNOS inhibitor we used (3-Br-7-NI). Although substantial variability exists in reports of the effects of NOS inhibitors on anxiety-like responses, the type of NOS inhibitor may explain much of the variability in the effects of NOS inhibition on anxiety-like responses. Both l-NAME and l-NOARG may alter anxiety-like behaviours though vasoconstriction or hypertension by affecting eNOS [47], whereas NOS inhibitors selective for the neuronal isoform only affect NO signaling through nNOS [3,27].
Our results suggest that future studies on the effects of nNOS on affective behaviours should carefully consider the impact of social environment. The results of this research may also provide insight into psychological disorders that are characterized by increases in anxiety.
Acknowledgments
We thank L.B. Martin and K.J. Navara for helpful comments on an earlier version of this manuscript and M. Weber and K. McCarthy for technical assistance. This research was supported by NIH grants MH 57535 to RJN and MH 076313 to BCT and NSF grant 04-16897.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramov U, Raud S, Koks S, Innos J, Kurrikoff K, Matsui T, Vasar E. Targeted mutation of CCK(2) receptor gene antagonises behavioural changes induced by social isolation in female, but not in male mice. Behav Brain Res. 2004;155:1–11. doi: 10.1016/j.bbr.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PK, Hill JL. Mus musculus: experimental induction of territory formation. Science. 1965;148:1753–1755. doi: 10.1126/science.148.3678.1753. [DOI] [PubMed] [Google Scholar]
- 3.Babbedge RC, Blandward PA, Hart SL, Moore PK. Inhibition of Rat Cerebellar Nitric-Oxide Synthase by 7-Nitro Indazole and Related Substituted Indazoles. Brit J Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caton PW, Tousman SA, Quock RM. Involvement of nitric oxide in nitrous oxide anxiolysis in the elevated plus-maze. Pharmacol Biochem Behav. 1994;48:689–692. doi: 10.1016/0091-3057(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 5.Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa A, Trainer P, Besser M, Grossman A. Nitric-Oxide Modulates the Release of Corticotropin-Releasing Hormone from the Rat Hypothalamus Invitro. Brain Res. 1993;605:187–192. doi: 10.1016/0006-8993(93)91739-f. [DOI] [PubMed] [Google Scholar]
- 7.Czech DA, Jacobson EB, LeSueur-Reed KT, Kazel MR. Putative anxiety-linked effects of the nitric oxide synthase inhibitor L-NAME in three murine exploratory behavior models. Pharmacol Biochem Behav. 2003;75:741–748. doi: 10.1016/s0091-3057(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 8.DeOliveira CL, DelBel EA, Guimaraes FS. Effects of L-NOARG on plus-maze performance in rats. Pharmacol Biochem Be. 1997;56:55–59. doi: 10.1016/S0091-3057(96)00156-6. [DOI] [PubMed] [Google Scholar]
- 9.Dunn RW, Reed TA, Copeland PD, Frye CA. The nitric oxide synthase inhibitor 7-nitroindazole displays enhanced anxiolytic efficacy without tolerance in rats following subchronic administration. Neuropharmacology. 1998;37:899–904. doi: 10.1016/s0028-3908(98)00076-8. [DOI] [PubMed] [Google Scholar]
- 10.Faria MS, Muscara MN, Moreno H, Junior, Teixeira SA, Dias HB, De Oliveira B, Graeff FG, De Nucci G. Acute inhibition of nitric oxide synthesis induces anxiolysis in the plus maze test. Eur J Pharmacol. 1997;323:37–43. doi: 10.1016/s0014-2999(97)00027-7. [DOI] [PubMed] [Google Scholar]
- 11.Forestiero D, Manfrim CM, Guimaraes FS, de Oliveira RM. Anxiolytic-like effects induced by nitric oxide synthase inhibitors microinjected into the medial amygdala of rats. Psychopharmacology (Berl) 2006;184:166–172. doi: 10.1007/s00213-005-0270-6. [DOI] [PubMed] [Google Scholar]
- 12.Franklin KBJ, Paxinos G. The Mouse Brain: in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- 13.Gammie SC, Olaghere-da Silva UB, Nelson RJ. 3-bromo-7-nitroindazole, a neuronal nitric oxide synthase inhibitor, impairs maternal aggression and citrulline immunoreactivity in prairie voles. Brain Res. 2000;870:80–86. doi: 10.1016/s0006-8993(00)02404-5. [DOI] [PubMed] [Google Scholar]
- 14.Guimaraes FS, Beijamini V, Moreira FA, Aguiar DC, de Lucca ACB. Role of nitric oxide in brain regions related to defensive reactions. Neurosci Biobehav R. 2005;29:1313–1322. doi: 10.1016/j.neubiorev.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Guimaraes FS, Deaguiar JC, Delbel EA, Ballejo G. Anxiolytic Effect of Nitric-Oxide Synthase Inhibitors Microinjected into the Dorsal Central Grey. Neuroreport. 1994;5:1929–1932. doi: 10.1097/00001756-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Heinrichs SC, Lapsansky J, Lovenberg TW, DeSouza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Peptides. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- 17.Jaffrey SR, Snyder SH. Nitric oxide: a neural messenger. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP. The biobehavioral consequences of psychogenic stress in a small, social primate (Callithrix jacchus jacchus) Biol Psychiatry. 1996;40:317–337. doi: 10.1016/0006-3223(95)00397-5. [DOI] [PubMed] [Google Scholar]
- 19.Joiner TE, Jr, Brown JS, Wingate LR. The psychology and neurobiology of suicidal behavior. Annu Rev Psychol. 2005;56:287–314. doi: 10.1146/annurev.psych.56.091103.070320. [DOI] [PubMed] [Google Scholar]
- 20.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- 22.Mackintosh J. Territory Formation by Laboratory Mice. Anim Behav. 1970;18:177. [Google Scholar]
- 23.Martin LB, Trainor BC, Finy MS, Nelson RJ. HPA activity and neotic and anxiety-like behavior vary among Peromyscus species. Gen Comp Endocrinol. 2007;151:342–350. doi: 10.1016/j.ygcen.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mineka S, Suomi SJ. Social separation in monkeys. Psychol Bull. 1978;85:1376–1400. [PubMed] [Google Scholar]
- 25.Mondragon R, Mayagoitia L, Lopez-Lujan A, Diaz JL. Social structure features in three inbred strains of mice, C57Bl/6J, Balb/cj, and NIH: a comparative study. Behav Neural Biol. 1987;47:384–391. doi: 10.1016/s0163-1047(87)90500-0. [DOI] [PubMed] [Google Scholar]
- 26.Monzon ME, Varas MM, De Barioglio SR. Anxiogenesis induced by nitric oxide synthase inhibition and anxiolytic effect of melanin-concentrating hormone (MCH) in rat brain. Peptides. 2001;22:1043–1047. doi: 10.1016/s0196-9781(01)00439-9. [DOI] [PubMed] [Google Scholar]
- 27.Moore PK, Babbedge RC, Wallace P, Gaffen ZA, Hart SL. 7-Nitro Indazole, an Inhibitor of Nitric-Oxide Synthase, Exhibits Antinociceptive Activity in the Mouse without Increasing Blood-Pressure. Brit J Pharmacol. 1993;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moragrega I, Carmen Carrasco M, Redolat R. Effects of housing and nicotine on shuttle-box avoidance in male NMRI mice. Behav Brain Res. 2005;164:178–187. doi: 10.1016/j.bbr.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 30.Nelson RJ, Kriegsfeld LJ, Dawson VL, Dawson TM. Effects of nitric oxide on neuroendocrine function and behavior. Front Neuroendocrinol. 1997;18:463–491. doi: 10.1006/frne.1997.0156. [DOI] [PubMed] [Google Scholar]
- 31.Nelson RJ, Trainor BC, Chiavegatto S, Demas GE. Pleiotropic contributions of nitric oxide to aggressive behavior. Neurosci Biobehav Rev. 2006;30:346–355. doi: 10.1016/j.neubiorev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Owens MJ, Nemeroff CB. Physiology and Pharmacology of Corticotropin-Releasing Factor. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- 33.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 34.Parmigiani S, Pasquali A. Exploratory and aggressive behaviour of isolated, dominant and subordinated mice. Acad Sci Lett. 1980;114:3–9. [Google Scholar]
- 35.Pokk P, Vali M. The effects of the nitric oxide synthase inhibitors on the behaviour of small-platform-stressed mice in the plus-maze test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:241–247. doi: 10.1016/s0278-5846(01)00261-5. [DOI] [PubMed] [Google Scholar]
- 36.Quock RM, Nguyen E. Possible involvement of nitric oxide in chlordiazepoxide-induced anxiolysis in mice. Life Sci. 1992;51:PL255–260. doi: 10.1016/0024-3205(92)90119-a. [DOI] [PubMed] [Google Scholar]
- 37.Reddy DS, Kulkarni SK. Inhibition of neuronal nitric oxide synthase (n-cNOS) reverses the corticotrophin-induced behavioral effects in rats. Mol Cell Biochem. 1998;183:25–38. doi: 10.1023/a:1006815125689. [DOI] [PubMed] [Google Scholar]
- 38.Reimers M, Schwarzenberger F, Preuschoft S. Rehabilitation of research chimpanzees: stress and coping after long-term isolation. Horm Behav. 2007;51:428–435. doi: 10.1016/j.yhbeh.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Siaud P, Mekaouche M, Ixart G, Balmefrezol M, Givalois L, Barbanel G, Assenmacher I. A Subpopulation of Corticotopin-Releasing Hormone Neurosecretory-Cells in the Paraventricular Nucleus of the Hypothalamus Also Contain Nadph-Diaphorase. Neurosci Lett. 1994;170:51–54. doi: 10.1016/0304-3940(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 40.Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Trainor BC, Workman JL, Jessen R, Nelson RJ. Impaired nitric oxide synthase signaling dissociates social investigation and aggression. Behav Neurosci. 2007;121:362–369. doi: 10.1037/0735-7044.121.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vale AL, Green S, Montgomery AM, Shafi S. The nitric oxide synthesis inhibitor L-NAME produces anxiogenic-like effects in the rat elevated plus-maze test, but not in the social interaction test. J Psychopharmacol. 1998;12:268–272. doi: 10.1177/026988119801200306. [DOI] [PubMed] [Google Scholar]
- 43.Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of bat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 44.Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 45.Volke V, Soosaar A, Koks S, Bourin M, Mannisto PT, Vasar E. 7-Nitroindazole, a nitric oxide synthase inhibitor, has anxiolytic-like properties in exploratory models of anxiety. Psychopharmacology (Berl) 1997;131:399–405. doi: 10.1007/s002130050309. [DOI] [PubMed] [Google Scholar]
- 46.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YX, Lim SL, Pang CCY. Increase by N-G-Nitro-L-Arginine Methyl-Ester (L-Name) of Resistance to Venous Return in Rats. Brit J Pharmacol. 1995;114:1454–1458. doi: 10.1111/j.1476-5381.1995.tb13369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Yamada K, Emson P, Hokfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;10:295–316. doi: 10.1016/0891-0618(96)00133-0. [DOI] [PubMed] [Google Scholar]


