Abstract
The exocyst is an octameric protein complex implicated in the tethering of post-Golgi secretory vesicles to the plasma membrane before fusion. The function of individual exocyst components and the mechanism by which this tethering complex is targeted to sites of secretion are not clear. In this study, we report that the exocyst subunit Exo70 functions in concert with Sec3 to anchor the exocyst to the plasma membrane. We found that the C-terminal Domain D of Exo70 directly interacts with phosphatidylinositol 4,5-bisphosphate. In addition, we have identified key residues on Exo70 that are critical for its interaction with phospholipids and the small GTPase Rho3. Further genetic and cell biological analyses suggest that the interaction of Exo70 with phospholipids, but not Rho3, is essential for the membrane association of the exocyst complex. We propose that Exo70 mediates the assembly of the exocyst complex at the plasma membrane, which is a crucial step in the tethering of post-Golgi secretory vesicles for exocytosis.
Keywords: PI(4,5)P2; Exo70; exocyst; exocytosis; Rho
Introduction
Membrane traffic and protein cargo delivery in eukaryotic cells require accurate recognition and fusion of transport vesicles with their cognate acceptor membranes. A number of proteins and protein complexes function to tether different classes of transport vesicles to their target membranes at various stages of traffic before SNARE-mediated membrane fusion (Pfeffer, 1999; Guo et al, 2000; Waters and Hughson, 2000; Whyte and Munro, 2002). For exocytosis, the tethering of post-Golgi secretory vesicles to the plasma membrane is mediated by the evolutionarily conserved exocyst complex, which is composed of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84 (for review see Guo et al, 2000; Hsu et al, 2004; Munson and Novick, 2006). In the budding yeast Saccharomyces cerevisiae, the exocyst complex is localized to the growing tip of the daughter cell (the ‘bud tip'), which is the site of active exocytosis and cell surface expansion. The Rab protein, Sec4, directly interacts with the exocyst and promotes the assembly of the exocyst complex at the plasma membrane (Guo et al, 1999).
In order to elucidate the molecular mechanism of vesicle tethering, we must first understand how the vesicle tethering proteins themselves are targeted to the acceptor membranes. For the exocyst complex, previous studies in budding yeast suggest that Sec3 subunit functions as a spatial landmark for polarized exocytosis (Finger et al, 1998). Biochemical studies have shown that Sec3 directly interacts with members of the Rho family of small GTP-binding proteins, which are key regulators that coordinate the actin cytoskeleton and polarized cell growth (Guo et al, 2001; Zhang et al, 2001). However, studies have also shown that Sec3 may not be the sole landmark for exocytosis. In a sec3 mutant strain, in which the N-terminal Rho-binding domain of Sec3 is deleted (sec3ΔN), the localization of the other exocyst components is normal and there is no block in secretion (Guo et al, 2001). Furthermore, cells with SEC3 deleted are still viable and the other exocyst subunits remain polarized (Wiederkehr et al, 2003; Zhang et al, 2005a). These observations suggest that, in addition to Sec3, another exocyst component also participates in targeting the exocyst to the plasma membrane. A likely candidate is Exo70. Using fluorescence recovery after photobleaching (FRAP) analyses and immunoelectron microscopy (EM), Boyd and co-workers have shown that the yeast exocyst subunits Sec5, Sec6, Sec8, Sec10, Sec15 and Exo84 associate with the exocytic vesicles, and rely on actin cables for their delivery to the bud tip. On the other hand, a portion of Exo70, like Sec3, is stably localized to the bud tip and remains polarized even when the actin cables are disrupted (Boyd et al, 2004). Studies have also shown that Exo70 is an effecter of the Rho GTPase Rho3, which plays regulatory roles in exocytosis and actin organization in yeast (Adamo et al, 1999; Robinson et al, 1999).
Here, we report that Exo70 directly binds to phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in the plasma membrane. A subset of positively charged residues at the C-terminus of Exo70, most of which are evolutionarily conserved from yeast to mammals, are essential for this interaction. We have designed a genetic assay, in which the significance of the Exo70–PI(4,5)P2 or Exo70–Rho3 interaction can be assessed. Using this assay, we demonstrated that Exo70 and Sec3 function together to mediate the association of the exocyst to the plasma membrane for exocytosis. We also found that disruption of the Exo70–lipid interaction caused mislocalization of the exocyst and a block in secretion. On the other hand, disruption of the Exo70–Rho3 interaction did not lead to any detectable defects. Our study reveals a molecular mechanism by which the exocyst is targeted to the plasma membrane, and sheds light on our understanding of vesicle tethering during exocytosis.
Results
Exo70 interacts with phosphatidylinositol 4,5-bisphosphate
The recently solved crystal structure of Exo70 revealed that this protein forms a 160 Å-long rod principally composed of α-helices that fold into four domains (named Domains A, B, C and D) (Dong et al, 2005; Hamburger et al, 2006). Domain D is the most evolutionarily conserved region in Exo70. It contains a number of basic residues that cluster into an electropositive patch on the surface of the C-terminus. It is known that a number of proteins interact with the negatively charged phosphoinositides in the membrane via clusters of basic residues (for reviews see McLaughlin et al, 2002; McLaughlin and Murray, 2005; Balla, 2005). We thus speculated that Domain D of Exo70 is involved in a direct interaction with PI(4,5)P2 in the plasma membrane.
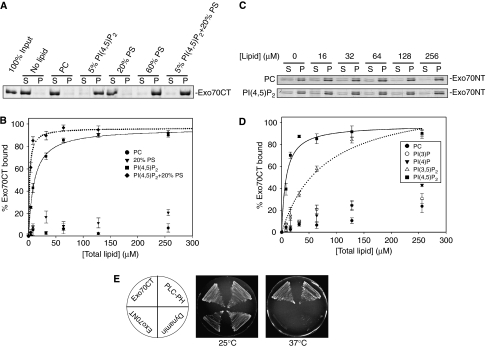
To test this possibility, we examined the ability of the Exo70 C-terminus recombinant protein (Exo70CT, aa 323–623) to bind phospholipids by co-sedimentation assays using reconstituted large unilamellar vesicles (LUVs) containing different types of phospholipids. As shown in Figure 1A, GST-Exo70CT fusion protein bound strongly to LUVs containing 5% PI(4,5)P2. It also bound to LUVs containing a high concentration (60%) of phosphatidylserine (PS). GST-Exo70CT did not bind to 20% PS. However, it bound strongly to LUVs composed of both 5% PI(4,5)P2 and 20% PS that mimic the biological membranes. As controls, GST-Exo70CT did not bind to the neutrally charged phosphatidylcholine (PC); and GST alone did not bind to LUVs of any lipid composition (data not shown).
Figure 1.
The C-terminus of Exo70 interacts with phospholipids. (A) LUVs containing 100% PC, 5% PI(4,5)P2, 20% PS, 60% PS or a combination of 5% PI(4,5)P2 and 20% PS were incubated with 300 nM GST-Exo70CT purified from bacteria. After centrifugation, proteins in the supernatant (S) and precipitate (P) were subjected to SDS–PAGE and visualized by SYPRO-red staining. Exo70 bound to vesicles that contain 5% PI(4,5)P2, and more strongly to vesicles containing both 5% PI(4,5)P2 and 20% PS. It also bound to vesicles containing 60% PS. (B) Exo70CT (300 nM) was incubated with various concentrations of LUVs composed of 100% PC, 20% PS, 5% PI(4,5)P2 or 5% PI(4,5)P2+20% PS. The solid and dotted curves are the Best-fit results of the 5% PI(4,5)P2 and 5% PI(4,5)P2+20% PS data to a single rectangular hyperbolae equation using SigmaPlot. The binding affinity (Kd) was calculated from the Best-fit curves. The error bars designate the standard deviation (s.d.); n=3. (C) Exo70NT (150 nM) was incubated with various concentrations of LUVs composed of 100% PC or 5% PI(4,5)P2. The binding of Exo70NT with 5% PI(4,5)P2 was indistinguishable from its nonspecific binding with 100% PC. (D) Exo70CT (300 nM) was incubated with various concentrations of LUVs composed of 100% PC, 5% PI(3)P, 5% PI(4)P, 5% PI(3,5)P2 or 5% PI(4,5)P2. The solid and dotted curves are the Best-fit results of the 5% PI(4,5)P2 and 5% PI(3,5)P2 data to hyperbolae, respectively. The binding affinity (Kd) was calculated from the Best-fit curves. The error bars designate the s.d.; n=3. (E) Analysis of Exo70–plasma membrane interaction using the yeast Ras-rescue assay. The cdc25ts mutant was transformed with plasmids, in which RasQ61L was fused to the N-terminus (NT) or C-terminus (CT) of Exo70. Transformants were streaked on plates and incubated at the permissive (25°C) or restrictive (37°C) temperature for 3 days. The growth of the cdc25ts yeast at the non-permissive temperature (37°C) suggests that Exo70CT binds to phospholipids and brings the RasQ61L to the plasma membrane. The PH domains from PLC and dynamin were used as positive and negative controls, respectively, in this assay.
To measure the affinity of the Exo70–phospholipid interaction, we examined the binding of Exo70 to LUVs with different lipid concentrations. The bound Exo70 was quantified and plotted against the lipid concentration according to the following equation: Kd=[P][L]/[C] (Kd is the dissociation constant; [P], [L] and [C] represent the concentrations of the protein, lipid and bound complex, respectively). As shown in Figure 1B, Exo70CT barely bound to 20% PS even at the highest lipid concentration tested. In contrast, Exo70CT bound to 5% PI(4,5)P2 with a Kd of 10.2 μM. Combining 5% PI(4,5)P2 with 20% PS in LUVs further increases the affinity between Exo70CT and the LUV (Kd=1.8 μM). As a positive control, a fragment of N-WASP (aa 178–274) also bound to 5% PI(4,5)P2, albeit with lower affinity (Supplementary Figure 1; Papayannopoulos et al, 2005). We also tested the binding of the Exo70 N-terminus recombinant protein (Exo70NT, aa 1–300) to LUVs. As shown in Figure 1C, the binding of Exo70NT with LUV containing 5% PI(4,5)P2 was indistinguishable from its nonspecific binding with 100% PC, suggesting that Exo70NT does not bind to PI(4,5)P2. Taken together, these results suggest that Exo70 directly interacts with PI(4,5)P2 via its C-terminus.
To determine the potential selectivity of Exo70 for phosphoinositides, we tested the binding of Exo70CT to three additional phosphoinositides, PI(3)P, PI(4)P and PI(3,5)P2. In S. cerevisiae, PI(3)P, PI(4)P and PI(4,5)P2 are the most abundant phosphoinositides. PI(3)P is mainly localized on intralumenal vesicles of endosomes and vacuoles (Gillooly et al, 2000), whereas PI(4)P and PI(4,5)P2 are concentrated on the Golgi apparatus and the plasma membrane, respectively (Stefan et al, 2002). Similar to that in mammals, PI(4,5)P2 comprises more than 90% of the bis-phosphoinositides in yeast (Bonangelino et al, 2002). PI(3,5)P2, the only detectable stereoisomer of PI(4,5)P2 in yeast, is distributed to the late endosomes and vacuoles (Rudge et al, 2004). No PI(3,4)P2 or PI(3,4,5)P3 can be detected in yeast under normal conditions (Desrivieres et al, 1998; Emr SD, personal communication). As shown in Figure 1D, PI(3,5)P2 bound to Exo70CT with affinity 4–5-fold lower than PI(4,5)P2. PI(3)P and PI(4)P only weakly bound to Exo70CT, with affinity more than 20-fold lower than PI(4,5)P2. Given that PI(4,5)P2 is much more abundant in the plasma membrane, we believe that PI(4,5)P2 is the physiological ligand for Exo70 in cells.
In addition to the above in vitro binding experiments, we were also able to detect the interaction of Exo70 with the plasma membrane using the Ras-rescue assay. The Ras-rescue assay has been used to identify proteins that strongly bind to PI(4,5)P2 in the inner leaflet of the plasma membrane in yeast (Isakoff et al, 1998). The assay takes advantage of a temperature-sensitive mutant of CDC25 (cdc25ts), which encodes the Ras guanine nucleotide exchange factor in yeast. Cdc25 activates Ras at the plasma membrane and is essential for the survival of the yeast. Expressing a fusion of an activated form of Ras (RasQ61L) with proteins that can target RasQ61L to the plasma membrane will rescue cdc25ts. Therefore, the ability of a protein to bind to the plasma membrane can be detected if the fusion protein can rescue cdc25ts at the restrictive temperature. As shown in Figure 1E, expression of the RasQ61L-Exo70CT fusion, but not the Exo70NT fusion, fully rescued cdc25ts at 37°C. As positive control, RasQ61L fused to PLCδ-PH, which is known to strongly bind to PI(4,5)P2 (Lemmon et al, 1995), was able to rescue cdc25ts. As a negative control, a fusion protein containing the dynamin-PH domain, which has a weaker affinity for PI(4,5)P2 (Klein et al, 1998), failed to rescue the mutant. These results further suggest that the C-terminal domain of Exo70 binds to the plasma membrane in yeast cells.
Localization and membrane association of the exocyst in the mss4 mutant
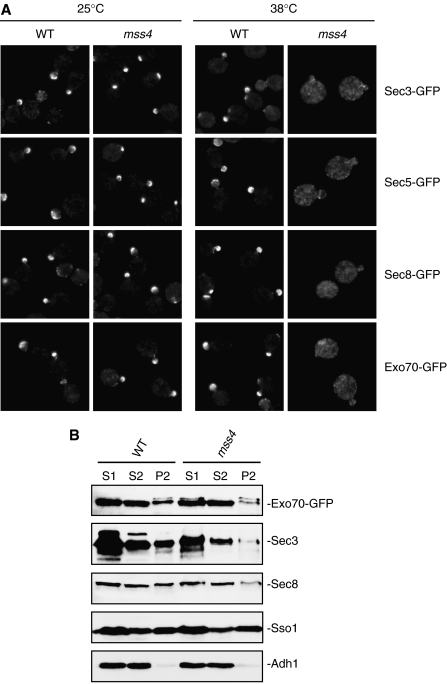
Mss4 is a PI4P 5-kinase that catalyzes the production of PI(4,5)P2 at the plasma membrane in yeast cells (Desrivieres et al, 1998; Stefan et al, 2002). In mss4 temperature-sensitive mutants, PI(4,5)P2 synthesis is reduced at the restrictive temperature of 38°C (Desrivieres et al, 1998; Stefan et al, 2002). We therefore examined the localization of Exo70 and other exocyst subunits in mss4 mutant cells. As shown in Figure 2A, at 25°C, the exocyst components were well polarized to the bud tip membrane. After shifting to the restrictive temperature, the exocyst components were completely diffused in the mss4 mutant. As a control, the exocyst proteins remain polarized in the wild-type cells at 38°C. As mss4 mutations may also affect the polarity of several other proteins in yeast (Desrivieres et al, 1998; Audhya et al, 2004), we further carried out membrane fractionation experiments to directly test whether the exocyst is physically associated with the plasma membrane in mss4 mutants. Cell lysates were centrifuged successively at 500 g and 13 000 g to generate S1, P1 and S2, P2 as previously described (Goud et al, 1988). Previous analyses of various membrane markers had shown that the plasma membrane is mostly distributed in the P2 fraction (Goud et al, 1988). Therefore, the association of the exocyst with the plasma membrane can be revealed by examining its presence in the P2 fraction. Due to the lack of sensitive anti-Exo70 antibodies, we performed membrane fractionation experiments using strains in which the endogenous Exo70 was tagged with GFP. We also examined other exocyst components including Sec3 and Sec8. As shown in Figure 2B, for parental wild-type cells, Exo70-GFP, Sec3 and Sec8 were present in the P2 fraction. In contrast, only diminutive amounts of these proteins were detected in the P2 fraction of the mss4 mutant cells. Together, the fluorescence microscopy and membrane fractionation results are consistent with the notion that plasma membrane targeting of Exo70 is mediated by its direct interaction with PI(4,5)P2.
Figure 2.
Localization and plasma membrane association of the exocyst subunits in the mss4 temperature-sensitive mutant cells. (A) Wild type and mss4 cells expressing the GFP-tagged exocyst components under the control of their endogenous promoters were grown to early log phase at 25°C and then either remained at 25°C or shifted to 38°C for 3 h before processed for fluorescence microscopy. Members of the exocyst (Sec3, Sec5, Sec8 and Exo70) were polarized at the bud tip membrane in the mss4 mutant cells at 25°C, but were mostly diffused at 38°C. As a control, the exocyst remained polarized at the bud tip in the wild-type cells at 38°C. (B) Wild type and mss4 cells, in which the endogenous Exo70 was tagged with GFP, were processed for membrane fractionation after the temperature shift. Equal volumes of samples from S1, S2 and P2 were subjected to SDS–PAGE and analyzed by Western blot using the anti-GFP, anti-Sec3 or anti-Sec8 antibody. As controls, the anti-Sso1 (the plasma membrane t-SNARE) and anti-Adh1 antibodies were used to detect the plasma membrane (P2) and cytosol (S2) fractions. The association of Exo70, Sec3 and Sec8 with the P2 fraction was greatly reduced in the mss4 mutant cells.
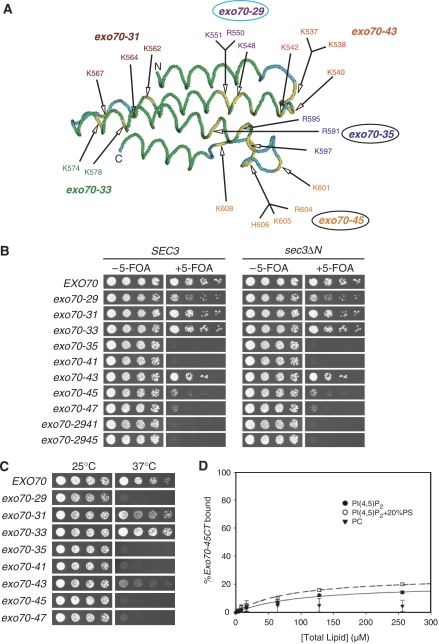
Synthetic growth defects of sec3ΔN with Exo70 Domain D mutants
The binding of Exo70 with PI(4,5)P2 provides a possible mechanism by which the exocyst is anchored to the plasma membrane. If this were the case, disrupting the Exo70–membrane interaction would be expected to block exocytosis and affect yeast cell growth. As clusters of basic residues have often been implicated in PI(4,5)P2 interaction (McLaughlin et al, 2002; McLaughlin and Murray, 2005; Balla, 2005), we performed extensive mutagenesis on the basic residues in Domain D of Exo70, and tested the effect of these mutations on cell growth. Surprisingly, most of the mutants were indistinguishable from the wild-type cells in growth. Only a few mutants showed mildly defective growth at temperatures equal or below 25°C (Table I Class III mutants). The exocyst subunits in these mutants are still associated with the plasma membrane and are localized to the bud tip (see Figures 4 and 5 below). These results suggest that disrupting the Exo70–phospholipid interaction alone is not sufficient to abolish the plasma membrane targeting of the exocyst.
Table 1.
Exo70 Domain D mutants
| Mutants | Mutation sites | Growth of exo70 | Growth of exo70 sec3ΔN |
|---|---|---|---|
| Class I mutants | |||
| exo70-31 | K562, K564, K567 | Normal | Normal |
| exo70-33 | K574, K578 | Normal | Normal |
| exo70-43 | K537, K538, K540, K542 | Normal | Normal |
| Class II mutants | |||
| exo70-29 | K548, R550, K551 | Normal | Defective (⩽25°C) |
| exo70-47 | R595 | Normal | Severely defective |
| exo70-45 | K601, R604, K605, H606, K608 | Normal | Severely defective |
| exo70-41 | R595, K597 | Normal | Near lethal |
| Class III mutants | |||
| exo70-2945 | K548, R550, K551, K601, R604, K605, H606, K608 | Mildly defective (⩽25°C) | Lethal |
| exo70-35 | R591, R595, K597 | Defective (⩽25°C) | Lethal |
| exo70-2941 |
K548, R550, K551, R595, K597 |
Defective (⩽25°C) |
Lethal |
| *The growth defects of exo70-29 sec3ΔN, exo70-2945, exo70-2941 and exo70-35 strains are more readily observed at low temperatures (⩽25°C). | |||
| **Please note that exo70-41 and exo70-35 differ by one amino acid. | |||
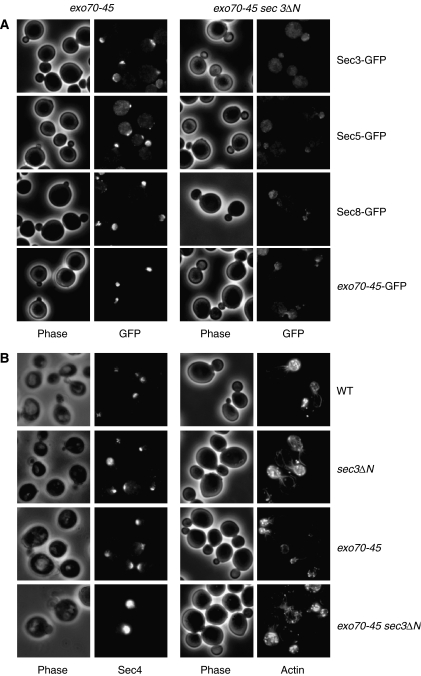
Figure 4.
Localization of the exocyst, Sec4 and actin in the exo70 sec3ΔN double mutant. (A) exo70-45 or exo70-45 sec3ΔN cells expressing the GFP-tagged exocyst components under the control of their endogenous promoters were grown to early log phase at 34°C, and then shifted to 18°C for 6 h. Cells were then fixed for fluorescence microscopy. Members of the exocyst including Sec3 (or sec3ΔN in the exo70-45 sec3ΔN cells), Sec5, Sec8 and the mutant exo70-45 protein remained polarized at the bud tip in the exo70-45 single mutant, but were largely diffused in the mother cell or in the daughter cell cytosol in the exo70-45 sec3ΔN double mutant cells. (B) The localization of the Rab protein Sec4 in exo70-45 and exo70-45 sec3ΔN cells was detected with anti-Sec4 antibody. Sec4 was polarized but diffused in the exo70-45 sec3ΔN double mutant. The cells were also stained with Alex488-phaloidin for F-actin. Actin cables and cortical patches remained polarized in the exo70-45 sec3ΔN double mutant.
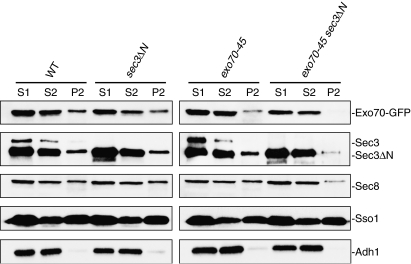
Figure 5.
The exocyst failed to associate with the plasma membrane in the exo70-45 sec3ΔN double mutant. Wild type, sec3ΔN, exo70-45 and exo70-45 sec3ΔN cells were grown to mid-log phase at 34°C, and then shifted to 18°C for 6 h. Cell lysates were prepared and centrifuged successively at 500 g and 13 000 g to generate S1, P1 and S2, P2, respectively. Equal volumes of samples were subjected to SDS–PAGE and analyzed by Western blot, using antibodies against the exocyst components Sec3 and Sec8. For Exo70, the endogenous copy of Exo70 or exo70-45 was tagged by GFP and an anti-GFP antibody was used to detect the Exo70-GFP or Exo70-45-GFP fusion protein. As controls, the anti-Sso1 (the plasma membrane t-SNARE) and anti-Adh1 antibodies were used to detect the plasma membrane and cytosol fractions. The level of Exo70-45, Sec3 and Sec8 associated with the P2 fraction was greatly reduced in the exo70-45 sec3ΔN double mutant. Note that the full-length Sec3 is mostly degraded to a major band of approximately 110 kDa as previously described (TerBush and Novick, 1995), which has molecular weight similar to that of the Sec3N protein.
It was previously shown that Exo70 and another exocyst component, Sec3, associate with the bud tip membrane more stably than the other exocyst components, and that their polarization to the bud tip is independent of actin cables (Boyd et al, 2004). Therefore Exo70 and Sec3 may function in concert to mediate the anchorage of the exocyst complex to the plasma membrane, which may explain the apparent lack of growth defect in exo70 Domain D mutants or the sec3ΔN mutant, in which the N-terminal membrane targeting domain of Sec3 is deleted (Guo et al, 2001). We hypothesize that disrupting membrane targeting of both Exo70 and Sec3 would affect the exocyst function in cells. To test this hypothesis, we introduced a number of exo70 Domain D mutants into the sec3ΔN background, and tested the double mutants for their growth (Figure 3). These exo70 mutants are categorized in Table I according to their growth phenotypes. The Class I mutants had normal growth and showed no synthetic growth defect with sec3ΔN. The Class II mutants grew normally by themselves, but were defective in growth or nearly lethal (exo70-41) when combined with sec3ΔN. Mutants in Class III are defective in growth at 25°C or lower temperatures, and are synthetic lethal with sec3ΔN. Next, we tested whether the membrane association of the Class II and III exo70 mutants were affected using the Ras-rescue assay. As shown in Figure 3C, these mutations indeed affected or abolished the ability of Exo70 to rescue cdc25ts at the restrictive temperature. As controls, the membrane association of the Class I exo70 mutants was either not affected (exo70-31, exo70-33) or only mildly affected (exo70-43). Meanwhile, we also performed LUV co-sedimentation assay to test whether mutations in one of the Class II exo70 mutants, exo70-45, affect the binding of Exo70 with PI(4,5)P2. Consistent with the results of the Ras-rescue assay, the binding of Exo70-45 with PI(4,5)P2 was greatly impaired (Figure 3D). Based on the study of the phenotypes of the various exo70 mutants, we found that all alleles that showed severe synthetic growth effects with sec3ΔN harbor mutations at the C-terminal end of the tertiary structure of Exo70. Especially, residues in the last two α-helices and their connecting loop (see exo70-35 and exo70-45) are critical. On the other hand, mutations in other α-helices or loops have less (exo70-29) or no effects (exo70-31, exo70-33 and exo70-43). We speculate that the C-terminal end of the folded Exo70 is directly involved in the interaction of Exo70 with PI(4,5)P2.
Figure 3.
exo70 alleles with the positively charged residues in Domain D mutated have synthetic growth defect with sec3ΔN. (A) The structure of Exo70 Domain D is illustrated with the green and blue colors specifying α-helices and loop regions in this domain. The yellow color indicates the positively charged residues mutated to alanine in different exo70 mutant alleles. The exo70-35 and exo70-45 alleles (with black circles) with mutations located at the last two α-helices and their connecting loop showed synthetic growth defect with sec3ΔN. The exo70-29 allele (with blue circles) showed a lesser extent of synthetic growth defect with sec3ΔN. The other exo70 alleles did not have any synthetic growth defect with sec3ΔN. (B) Various exo70 mutant alleles were introduced into the sec3ΔN strain, in which the chromosomal copy of SEC3 was replaced by sec3ΔN with intact SEC3 promoter. The exo70 mutants were expressed behind EXO70 promoters in a CEN TRP1 plasmid. The sec3ΔN exo70 double mutants supplemented with a CEN URA3 EXO70 balancer were streaked out on plates in the presence or absence of 5-FOA, and incubated for 5 days at 25°C. The exo70 single mutants (the SEC3 panel, left) were used as controls. Exo70 harbor different mutations in Domain D were tested for growth after losing the EXO70 balancer on 5-FOA plates. A selective set of mutants (exo70-29, exo70-41, exo70-35, exo70-45, exo70-47, exo70-2941, exo70-2945) showed synthetic growth defect or lethality with sec3N. The summary and categorization of all the tested exo70 mutant alleles are listed in Table I. (C) Analysis of the interaction between exo70 mutants and the plasma membrane using the Ras-rescue assay. The rescue of cdc25-ts at the restrictive temperature (37°C) is shown on the right. (D) GST-tagged Exo70-45CT (150 nM) was incubated with various concentrations of LUVs composed of 100% PC, 5% PI(4,5)P2 or 5% PI(4,5)P2+20% PS. The solid and dashed curves are the Best-fit results of the 5% PI(4,5)P2 and 5% PI(4,5)P2+20% PS data to a hyperbolae, respectively. The error bars designate the s.d.; n=3.
The exocyst failed to associate with bud tip membrane in the exo70 sec3ΔN double mutants
If Exo70 and Sec3 function in concert to mediate the membrane association of the exocyst to sites of exocytosis, members of the exocyst would no longer be localized to the bud tip if the association of both Exo70 and Sec3 with the plasma membrane is disrupted. To test this hypothesis, we first examined the localization of the GFP-tagged exocyst subunits in the exo70-45 sec3ΔN double mutant. The exo70-45 mutant harbors mutations on several positively charged residues at the C-terminal end of Exo70 (K601A, R604A, K605A, H606A, K608A). While several exo70 mutants with disruption of membrane binding are synthetic lethal with sec3ΔN (Table I), the exo70-45 sec3ΔN double mutant is defective in growth, and this growth defect is exacerbated at temperatures below 25°C. The viability of this double mutant allows us to carry out further cell biological characterization. As shown in Figure 4A, the exocyst subunits were well polarized to the bud tip as concentrated patches or crescents in the exo70-45 or sec3ΔN single mutant. However, in the exo70-45 sec3ΔN double mutant, the exocyst subunits were either completely depolarized or diffused inside or in the vicinity of the daughter cells, probably as a result of a failure in anchorage to the bud tip membrane.
We have also examined the Rab protein, Sec4, and actin in these cells. Sec4 and actin were mostly polarized in the exo70-45 sec3ΔN double mutant (Figure 4B). However, the Sec4 signal in the daughter cells was diffused rather than forming concentrated foci at the bud tip. This result indicates that mutations in Exo70 and Sec3 do not affect the directional transport of the post-Golgi secretory vesicles to the daughter cell. However, with the exocyst function impaired, these vesicles cannot be properly tethered to the bud tip membrane for subsequent fusion.
We have also examined the physical association of the exocyst with the plasma membrane in the double mutant by membrane fractionation. As shown in Figure 5, approximately 20–30% of Sec3 or Sec8 were present in the P2 fraction of wild type, sec3ΔN and exo70-45 single mutants, which is comparable to the amounts previously reported in cells of the same strain background (Bowser et al, 1992). In contrast, in the sec3ΔN exo70-45 double mutant, the amount of Sec3 or Sec8 in P2 was greatly reduced. We have also examined the membrane association of Exo70 in strains with Exo70 or Exo70-45 tagged with GFP. Approximately 25% of Exo70-GFP was present in the P2 fraction from wild type or sec3ΔN single mutant. In contrast, only diminutive amount of Exo70-45-GFP could be detected in P2 from the exo70-45 sec3ΔN double mutant (Figure 5). As controls, the distribution of the plasma membrane t-SNARE Sso1 and the cytosolic enzyme Adh1 between S2 and P2 are comparable in all the strains tested. This result, together with the localization study of the exocyst components (Figure 4), demonstrates that Exo70 plays a pivotal role in targeting the exocyst to the bud tip membrane. In addition, these data further suggest that Exo70 and Sec3 function in concert in targeting the exocyst to the plasma membrane.
The exo70-45 sec3ΔN double mutant is defective in secretion
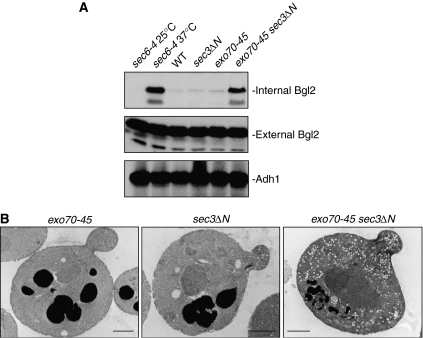
As the target of the exocyst to the plasma membrane is impaired in the exo70-45 sec3ΔN double mutant, we expect that exocytosis is affected in these mutant cells. We have recently shown that Exo70 mediates the secretion of the exocytic cargo containing Bgl2, the endo-glucanase that functions in cell wall remodeling during daughter cell growth in yeast (He et al, 2007). We therefore examined the secretion of Bgl2 in exo70-45, sec3ΔN and exo70-45 sec3ΔN. As shown in Figure 6A, the exo70-45 sec3ΔN double mutant exhibited pronounced accumulation of Bgl2, whereas neither exo70-45 nor sec3ΔN single mutants accumulated Blg2 internally. To directly visualize the secretion defect in cells, we examined the accumulation of post-Golgi secretory vesicles in sec3ΔN, exo70-45 and sec3ΔN exo70-45 mutants by thin-section transmission EM. As shown in Figure 6B, neither sec3ΔN nor exo70-45 accumulated vesicles. In contrast, the sec3ΔN exo70-45 double mutant cells accumulated a number of secretory vesicles 80–100 nm in diameter. Therefore, cells harboring mutations in the exo70 sec3 double mutant are defective in secretion.
Figure 6.
The exo70-45 sec3ΔN double mutant is defective in secretion. (A) The exo70-45 sec3ΔN double mutant blocks the secretion of Bgl2. For exo70-45 and exo70-45 sec3ΔN, cells were cultured at 34°C, and then shifted to 18°C for 6 h. For sec6-4, cells were cultured at 25°C and shifted to 37°C for 1 h. The internal and external pools of Bgl2 were separated and subjected to Western blot with anti-Bgl2 antibody. (B) Thin-section electron microscopic analysis of exo70-45, sec3ΔN and exo70-45 sec3ΔN cells. Cells were grown to early log phase at 34°C, and then shifted to 18°C for 6 h before being processed for EM. Bars, 1 μm.
Cell morphology defects in the exo70-45 sec3ΔN double mutant
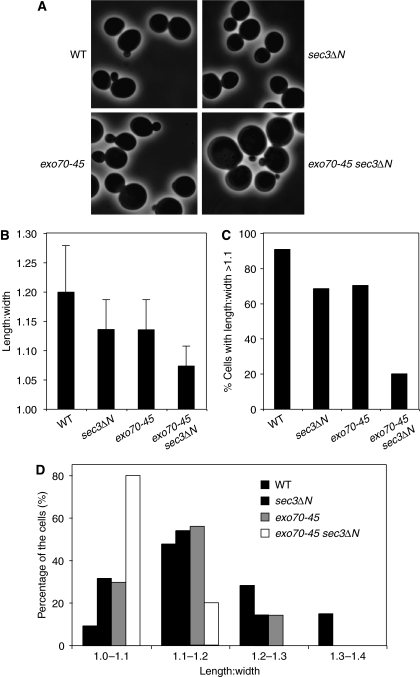
Polarized growth of yeast cells requires accurate targeting and fusion of the exocytic vesicles at defined areas of the plasma membrane, which in turn relies on the precise localization of the exocyst to the bud tip membrane. Here we examined whether the impaired association of the exocyst with the plasma membrane in the exo70-45 sec3ΔN mutant affects yeast morphology. As shown in Figure 7A, compared with the exo70-45 or sec3ΔN cells, the exo70-45 sec3ΔN double mutant cells were significantly larger and rounder than the wild-type cells, which are normally ellipsoid in shape. This is further reflected by the quantification of the length:width radio of the wild-type and mutant cells. As indicated in Figure 7B–D, most of the exo70-45 sec3ΔN double mutant cells have a much reduced length:width radio than the wild-type or the single mutant cells. We speculate that, when the exocyst–plasma membrane interaction is affected, the incoming vesicles cannot be precisely tethered to the appropriate sites (the ‘bud tip') even though they can still be delivered to the daughter cells (see Sec4 immunofluorescence and actin staining in Figure 4). This results in isotropic membrane fusion along the entire daughter cell membrane rather than at the bud tip. After generations of growth, the cells are ‘rounder' in shape for both mother and daughter cells. It is important to note that the above experiments were carried out with the exo70-45 sec3ΔN cells, whereas cells with more severe exo70 mutations (Class III mutants) are inviable in sec3ΔN background.
Figure 7.
Cell morphology of the exo70-45 sec3ΔN double mutant. (A) Wild type, sec3ΔN, exo70-45 or exo70-45 sec3ΔN cells were cultured at 34°C to early log phase, and then shifted to 18°C for 6 h before processed for morphological analysis under microscope. The phase-contrast images show that the exo70-45 sec3ΔN cells are larger and rounder than the wild type, sec3ΔN or exo70-45 cells. (B) Comparison of the mother cell length:width ratio of each indicated strains. The difference between the wild type and each single mutant, or between the single mutant and the double mutant was calculated by Student's t-test (n=160; P<0.001). (C) The percentage of cells with length:width ratio >1.1 in each strain. (D) Distribution of cells of different length:width ratios in the wild type and mutant strains.
The exo70 mutants defective in binding Rho3 have normal cell growth
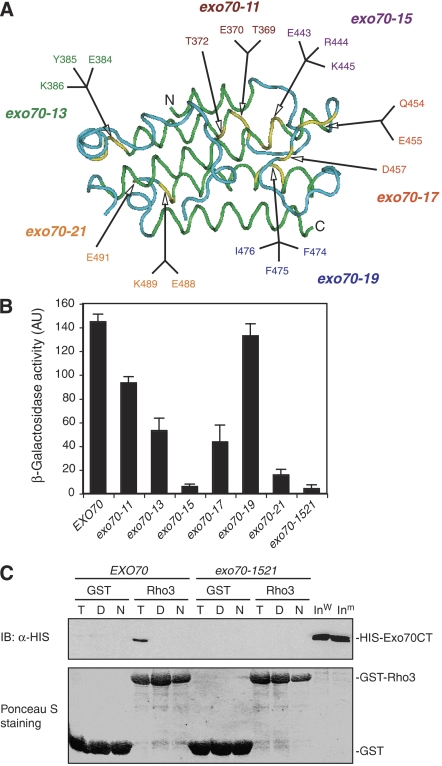
Exo70 was previously shown to interact with Rho3, a member of the Rho family of small GTPases in yeast (Adamo et al, 1999; Robinson et al, 1999), and the binding region has been mapped to Domain C of Exo70 (Dong et al, 2005). However, the functional implication of this interaction is unclear. Previous studies using rho3 mutants suggest that polarized localization of the exocyst is not controlled by Rho3 (Roumanie et al, 2005). To functionally test the role of Exo70–Rho3, we made a number of mutations on Domain C of Exo70 (Figure 8A), and the mutants were tested for their ability to interact with Rho3 through yeast two-hybrid assay. As shown in Figure 8B, the Exo70–Rho3 interaction was greatly disrupted in the exo70-15 (E443A, R444A, K445A) and exo70-21 (E488A, K489A, E491A) mutants. Furthermore, when combining the mutations in both exo70-15 and exo70-21, the resulting mutant, named exo70-1521 (E443A, R444A, K445A, E488A, K489A, E491A), completely lost its ability to bind to GTP-Rho3, as shown in the in vitro binding assay (Figure 8C). We then tested the growth of these exo70 mutants. Interestingly, none of them demonstrated any growth defect either as a single mutant or when combined with sec3ΔN (Table II). These observations suggest that, although Rho3 plays a role in polarized exocytosis (Adamo et al, 1999), its interaction with Exo70 is not essential for this process.
Figure 8.
Identification of Exo70 Domain C mutations that affect Rho3–Exo70 interaction. (A) The structure of Domain C of Exo70 that interacts with Rho3. The green and blue colors specify α-helices and loop regions in this domain. The yellow color indicates the residues mutated to alanine. (B) Yeast two-hybrid assay of the interactions between Exo70 Domain C mutants and Rho3. The average and s.d. of β-galactosidase activities were determined from three independent experiments. ‘AU', arbitrary units (n=3). (C) In vitro binding between the Exo70 Domain C mutant (exo70-1521) and Rho3. The C-termini (aa 358–623) of wild-type Exo70 and exo70-1521 (combining the mutations in both exo70-15 and exo70-21) were expressed as HISx6-tagged fusion proteins. Rho3 was expressed as a GST fusion protein and conjugated to glutathione Sepharose. These proteins were used in the in vitro binding assay in the absence of nucleotides (‘N') or in the presence of 0.05 mM GTPγS (‘T') or GDP (‘D'). GST (as control) and the GST-Rho3 fusion proteins used in the binding assay were stained by Ponceau S (lower panel). The Exo70 fusion proteins bound to GST-Rho3 glutathione Sepharose were detected by immunoblot (‘IB') using anti-HISx6 antibody. GST conjugated to glutathione Sepharose was used as a negative control in the binding reaction. ‘Inw', wild-type Exo70 input; ‘Inm', mutant Exo70 input.
Table 2.
Growth and Rho3 binding of Exo70 Domain C mutants
| Mutants | Mutation sites | Growth of exo70 | Growth of exo70 sec3ΔN | Rho3 binding (% of WT)a |
|---|---|---|---|---|
| exo70-11 | T369, E370, T372 | Normal | Normal | 64.4±3.6 |
| exo70-13 | E384, Y385, K386 | Normal | Normal | 36.6±7.1 |
| exo70-15 | E443, R444, K445 | Normal | Normal | 4.0±1.5 |
| exo70-17 | Q454, E455, P456 | Normal | Normal | 30.1±9.8 |
| exo70-19 | F474, F475, I476 | Normal | Normal | 91.7±7.1 |
| exo70-21 | E488, K489, E491 | Normal | Normal | 10.7±3.2 |
|
exo70-1521 |
E443, R444, K445, E488, K489, E491 |
Normal |
Normal |
2.9±2.1 |
| WT, wild type. | ||||
| aPercentages are based on the yeast two-hybrid β-galactosidase activity shown in Figure 8. | ||||
Discussion
Tethering proteins mediate the early interaction between secretory vesicles and their target membranes, and may promote subsequent docking and fusion catalyzed by the SNARE proteins (Pfeffer, 1999; Guo et al, 2000; Waters and Hughson, 2000; Whyte and Munro, 2002). The exocyst has been implicated in tethering post-Golgi secretory vesicles to the plasma membrane. However, despite the efforts to identify the membrane ‘receptors', how the exocyst physically associates with the plasma membrane is unclear. Here, we provide evidence that Exo70 directly interacts with PI(4,5)P2. Together with Sec3, it mediates the association of the exocyst complex with the plasma membrane.
Exo70 was reported to bind to the GTP-bound form of Rho3 (Adamo et al, 1999; Robinson et al, 1999), thus it is possible that Rho3 functions in recruiting Exo70 to the plasma membrane for polarized exocytosis. However, our genetic and biochemical analyses suggest that the membrane localization of Exo70 does not require its interaction with Rho3. Instead, we demonstrate a critical role for phospholipids in the membrane association of Exo70. Fluorescence microscopy and membrane fractionation experiments demonstrate that the exocyst components were diffused in the mss4 mutant cells shifted to the restrictive temperature (Figure 2), when the plasma membrane PI(4,5)P2 level was reduced (Desrivieres et al, 1998; Stefan et al, 2002). The lipid sedimentation assays have shown a direct interaction between the C-terminus of Exo70 and phospholipids in vitro (Figure 1). The affinity of Exo70 for PI(4,5)P2 is a few folds higher than that of its stereoisomer PI(3,5)P2, and much higher than that of monophosphorylated phosphoinositides PI(3)P and PI(4)P. Combining PI(4,5)P2 with PS in the lipid vesicles that mimic the biological membranes further increases the binding affinity. Using the Ras-rescue assay, we have further shown that the C-terminus of Exo70 was able to target the Ras protein to the plasma membrane in yeast cells. Phosphoinositides play important roles in regulating membrane traffic by recruiting and/or activating its effector proteins (for recent review see Behnia and Munro, 2005; Di Paolo and De Camilli, 2006). In yeast, PI(4,5)P2 is mostly distributed to the plasma membrane (Stefan et al, 2002; Yu et al, 2004). The binding of Exo70 with PI(4,5)P2 well corresponds to its cellular localization. Our study also indicates that Exo70 binds to phospholipids through the positively charged surface patch on Domain D at its C-terminus. It is interesting to note that these positively charged residues, while being sparsely distributed along the primary sequence, are clustered only when Exo70 is folded into the three-dimensional structure (Dong et al, 2005). We speculate that factors that change the conformation of Exo70 may regulate the association between Exo70 and the plasma membrane.
Our study also suggests that the role of Exo70 in linking vesicles to the plasma membrane is carried out in concert with another exocyst component, Sec3. The N-terminus of Sec3 interacts with Rho GTPases (Guo et al, 2001; Zhang et al, 2001). We have recently found that a group of positively charged residues adjacent to the Cdc42-binding region is crucial for Sec3 targeting to the bud tip membrane (Zhang et al, unpublished results). A mutant strain (sec3ΔN) with deletion of the Sec3 N-terminus demonstrated no secretion or growth defects under all conditions tested. Similarly, most mutations in the C-terminus of Exo70 that disrupt its association with phospholipids have no effects on the cells. Strikingly, combining sec3ΔN with these exo70 mutants leads to either severe growth and secretion defects, or inviability. The lack of apparent defects in the exo70 or sec3ΔN single mutant suggests that disrupting Exo70 or Sec3 alone is insufficient to affect the membrane targeting of the exocyst for exocytosis. On the other hand, when the targeting of Exo70 and Sec3 is both disrupted, the exocyst complex can no longer be anchored to the plasma membrane. These genetic results, combined with the biochemical analyses, suggest that Exo70 and Sec3 function in concert in exocyst targeting (Figure 9). Consistent with this model, our membrane fractionation experiments show that the same mutations that disrupt the Exo70–phospholipid binding also caused disassociation of the exocyst from the plasma membrane when the targeting of Sec3 is simultaneously disrupted; and the localization of the exocyst components to the bud tip membrane was affected in the exo70 sec3ΔN double mutants. Although caution should be taken in the interpretation of the observed synthetic effects, our results are consistent with the previous report by Boyd et al (2004) that Exo70 and Sec3 associate with the bud tip membrane more stably than the other exocyst components, and their polarization to the bud tip is independent of actin cables. Future experiments are needed to further define the relationship between Exo70 and Sec3, and confirm the potential ‘parallel targeting' mechanism. Important for this study, however, the synthetic effect between different Exo70 Domain D mutants and sec3ΔN allows us to assess the functional implications of the Exo70–lipid interaction.
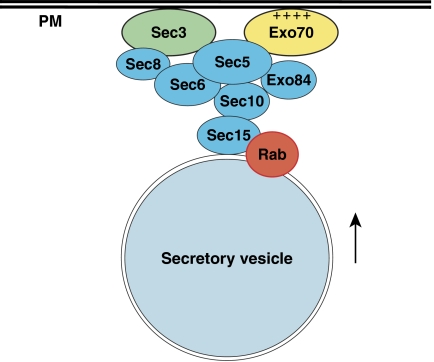
Figure 9.
Exo70 functions together with Sec3 in targeting the exocyst to the plasma membrane. Exo70 directly associates with the phospholipids in the plasma membrane (‘PM') via its positively charged residues (‘++++'). Exo70 and Sec3 interact with the rest of the exocyst subunits riding on the arriving secretory vesicles (Boyd et al, 2004). Another exocyst subunit Sec15 directly binds to the GTP-bound form of Rab GTPase Sec4 (Guo et al, 1999). The assembly of the exocyst serves to tether the vesicles to the plasma membrane site specified by Exo70 and Sec3. The exocyst is also regulated by the Rho GTPases, Cdc42 and Rho3 (not shown here).
Sequence comparison of yeast and mammalian Exo70 indicates that their C-termini are the most homologous region. Moreover, most of the positively charged residues at this region are conserved. Fluorescence microscopy study suggested that the mammalian Exo70 may associate with the plasma membrane (Matern et al, 2001; Inoue et al, 2003). It is possible that the C-termini of Exo70 from various species share a common lipid binding function, which is required for the membrane targeting of Exo70 and the subsequent recruitment of the other exocyst subunits. Further study of Exo70 in other eukaryotic cells will likely reveal a conserved mechanism for exocyst targeting and vesicle tethering.
The association of the exocyst with the plasma membrane is a pivotal step in vesicle tethering. When and where this plasma membrane-‘landing' step takes place may contribute to determining the kinetics and location of exocytosis. Since exocytosis in most eukaryotic cells is highly regulated, we speculate that the exocyst, particularly Exo70, is a target of cellular regulations. In yeast, Exo70 and other members of the exocyst complex are specifically localized to the tip of the daughter cells. Furthermore, we have recently shown that Exo70 primarily functions at the early stages of cell cycle for yeast budding (He et al, 2007). Identification of proteins that temporally and/or spatially control Exo70 may help us better understand the regulation of exocytosis in eukaryotic cells.
Materials and methods
Plasmids and yeast strains
A CEN plasmid (TRP1) containing a 2.8 kb KpnI/ScaI genomic insert that includes EXO70 was used as template for mutagenesis using QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). All of the constructs were later verified by sequencing.
Standard methods were used for yeast media and genetic manipulations (Guthrie and Fink, 1991). The strains used in this study are listed in Supplementary Table 1. To generate various exo70 sec3 double mutants, an EXO70 URA3 CEN plasmid was transformed into the sec3ΔN cells (GY2653) as a balancer, and then the endogenous EXO70 was deleted via homologues recombination with a DNA fragment containing the EXO70 promoter and terminator flanking the HIS3 gene (exo70∷HIS3), which is derived from pG376. The resulting strain (GY2811) was transformed with the TRP1 CEN plasmids harboring various exo70 mutants, and the transformants were tested for growth at different temperatures upon losing the EXO70 balancer on 5-FOA plates.
LUV sedimentation assay
LUV sedimentation assay was performed as previously described (Hokanson and Ostap, 2006). LUVs with 100-nm diameter were prepared by size extrusion. Lipid components were mixed at different molar ratios, dried with nitrogen stream and resuspended at a concentration of 2 mM in a buffer containing 12 mM Hepes (pH 7) and 176 mM sucrose. The mixed lipids were subjected to five cycles of freeze–thaw and 1-min bath sonication, before being passed through 100-nm filters using a mini-extruder (Avanti Polar Lipids Inc., Alabaster, AL). LUVs were dialyzed overnight in HNa100 buffer (10 mM Hepes, pH 7, 100 mM NaCl, 1 mM EGTA, 1 mM DTT). The percentages of phospholipids indicated in the text are the molar percentages of total PS and phosphoinositides, with the remainder being PC. Lipid concentrations are expressed as total lipid. The binding of Exo70 to LUVs was determined by sedimentation assays conducted in 200 μl total volume in an ultracentrifuge TLA-100 rotor (Beckman Coulter Inc., Fullerton, CA). The tubes were preincubated for 1 h in a 50 μM solution of PC in the HNa100 buffer to prevent nonspecific binding of Exo70 to polycarbonate centrifuge tubes. Sucrose-loaded LUVs were precipitated at 150 000 g for 30 min at 25°C. The supernatants and pellets were subjected to 12% SDS–PAGE and stained with SYPRO-red (Invitrogen Co., Carlsbad, CA) for quantification of free and bound materials.
Ras-rescue assay
Characterization of the proteins that bind to PI(4,5)P2 at the plasma membrane by Ras-rescuing assay was performed as previously described (Isakoff et al, 1998). To generate Exo70-RasQ61L fusion constructs, PCR fragments containing the N-terminus (nt 1–1050) or C-terminus (nt 996–1869) of EXO70 were inserted between the BamHI and XhoI sites of p3SOB-L2 (a gift from Dr Lemmon). The resulting constructs were transformed into the cdc25ts mutant strain. Transformants were streaked out onto SC-leucine plates and incubated at the permissive (25°C) or restrictive (37°C) temperature for 3 days. RasQ61L fused with PLCδ-PH and dynamin-PH, respectively, were used as positive and negative controls (gifts from Dr Lemmon). To test the Exo70 D-domain mutants in the Ras-rescue assay, the C-terminus (nt 996–1869) of various exo70 mutants were inserted into p3SOB-L2 and the resulting constructs were examined for their ability to rescue cdc25ts using the same method as that for the wild-type Exo70.
Light microscopy
Chromosomal tagging of the exocyst components by GFP was performed as previously described (Zhang et al, 2005a). Cells were grown to early log phase at 34°C, followed by a shift to 18°C for 6 h. The cells were then fixed as previously described (Zajac et al, 2005). The signals were scored as being mislocalized when they appeared diffused or in multiple patches in the mother cells. Immunofluorescence staining of Sec4 was carried out as previously described (Zhang et al, 2005b). The antibody was Anti-Sec4p polyclonal used at 1:1000 dilutions. The Alexa Fluoro 488-conjugated goat anti-rabbit IgG antibody (Molecular Probes Corp) was used as the secondary antibody. Actin cytoskeleton of the yeast cells was stained with Alexa Fluoro 488-conjugated phalloidin (Molecular Probes Corp.).
Subcellular membrane fractionation
Subcellular membrane fractionation of the yeast cells was performed as previously described (Goud et al, 1988). A total of 100–150 OD600 units of cells were harvested and washed with 10 mM of NaN3. Cells were converted to spheroplasts and lysed with 20 strokes in a 2 ml Wheaton tissue grinder. The lysates were centrifuged at 500 g for 10 min at 4°C. The S1 supernatant was spun at 13 000 g for 20 min at 4°C. The pellet (P2) was resuspended in the same volume of lysis buffer. Equal amounts of samples from each fraction were subjected to SDS–PAGE for Western blot analysis.
Electron microscopy
For EM, exo70-45, sec3ΔN and exo70-45 sec3ΔN cells were grown at 34°C in YPD media to early log phase and shifted to 18°C for 6 h. Cells were processed for thin section EM analysis using a Jeol-1010 Transmission electron microscope as previously described (Zhang et al, 2005a).
Bgl2 secretion assay
EXO70, exo70-45, sec3ΔN and exo70-45 sec3ΔN cells were grown at 34°C to early log phase and shifted for 6 h at 18°C. For SEC6 and sec6-4 strains, cells were grown at 25°C and shifted to 37°C for 1 h. After shift, NaN3 and NaF were added to a final concentration of 20 mM. A total of 25 ODs of cells were collected, washed with a Tris/NaN3/NaF buffer and spheroplasted. The amount of external and internal Bgl2 was determined by Western blotting with an anti-Bgl2 antibody. The amount of Adh1 from the internal pool was determined by Western blotting to show the same amount of loading.
In vitro binding between Exo70 and Rho3
Wild-type and mutant forms of Exo70 C-terminus (aa 358–623, Exo70CT) were expressed as HISx6-tagged fusion proteins. Rho3 was expressed as GST-tagged fusion protein (GST-Rho3). The purified recombinant fusion proteins (15 μg each) were used in the in vitro binding experiment as previously described (Zhang et al, 2001).
Supplementary Material
Supplementary Figure 1
Supplementary Materials
Acknowledgments
We are grateful to Drs Mark Lemmon and Michael Ostap for their advice on lipid binding experiments. We thank Dr Dan TerBush for the anti-Sec3 antibodies, and Dr Scott Emr for the mss4 strains. This work was supported by National Institutes of Health (RO1-GM64690), American Cancer Society and the Pew Scholars Program to WG.
References
- Adamo JE, Rossi G, Brennwald P (1999) The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol Biol Cell 10: 4121–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Loewith R, Parsons AB, Gao L, Tabuchi M, Zhou H, Boone C, Hall MN, Emr SD (2004) Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J 23: 3747–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T (2005) Inositol-lipid binding motifs: signal integrators through protein-lipid and protein–protein interactions. J Cell Sci 118 (Part 10): 2093–2104 [DOI] [PubMed] [Google Scholar]
- Behnia R, Munro S (2005) Organelle identity and the signposts for membrane traffic. Nature 438: 597–604. Review [DOI] [PubMed] [Google Scholar]
- Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, Gary JD, Emr SD, Weisman LS (2002) Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol 156: 1015–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser R, Muller H, Govindan B, Novick P (1992) Sec8p and Sec15p are components of a plasma membrane-associated 19.5S particle that may function downstream of Sec4p to control exocytosis. J Cell Biol 118: 1041–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C, Hughes T, Pypaert M, Novick P (2004) Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol 167: 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrivieres S, Cooke FT, Parker PJ, Hall MN (1998) Mss4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem 273: 15787–15793 [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657. Review [DOI] [PubMed] [Google Scholar]
- Dong G, Hutagalung AH, Fu C, Novick P, Reinisch KM (2005) The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat Struct Mol Biol 12: 1094–1100 [DOI] [PubMed] [Google Scholar]
- Finger FP, Hughes TE, Novick P (1998) Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell 92: 559–571 [DOI] [PubMed] [Google Scholar]
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier J, Parton RG, Stenmark H (2000) Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J 19: 4577–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ (1988) A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53: 753–768 [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P (1999) The exocyst is an effecter for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Tamanoi F, Novick P (2001) Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol 3: 353–360 [DOI] [PubMed] [Google Scholar]
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P (2000) Protein complexes in transport vesicle targeting. Trends Cell Biol 10: 251–255 [DOI] [PubMed] [Google Scholar]
- Guthrie C, Fink GR (1991) Guide to Yeast Genetics and Molecular Biology, Methods in Enzymology, Vol. 169. San Diego: Academic Press [PubMed] [Google Scholar]
- Hamburger ZA, Hamburger AE, West AP Jr, Weis WI (2006) Crystal structure of the S. cerevisiae exocyst component Exo70p. J Mol Biol 356: 9–21 [DOI] [PubMed] [Google Scholar]
- He B, Xi F, Zhang J, TerBush D, Zhang X, Guo W (2007) Exo70p mediates the secretion of specific exocytic vesicles at early stages of cell cycle for polarized cell growth. J Cell Biol 176: 771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokanson D, Ostap M (2006) Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate. Proc Natl Acad Sci USA 103: 3118–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, TerBush D, Abraham M, Guo W (2004) The exocyst complex in polarized exocytosis. Int Rev Cytol 233: 243–265 [DOI] [PubMed] [Google Scholar]
- Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR (2003) The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422: 629–633 [DOI] [PubMed] [Google Scholar]
- Isakoff SJ, Cardozo T, Andreev J, Li Z, Ferguson KM, Abagyan R, Lemmon MA, Aronheim A, Skolnik EY (1998) Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J 17: 5374–5387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DE, Lee A, Frank DW, Marks MS, Lemmon MA (1998) The Pleckstrin Homology domains of dynamin isoforms require oligomerization for high affinity phosphoinositide binding. J Biol Chem 273: 27725–27733 [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, O'Brien R, Sigler PB, Schlessinger J (1995) Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA 92: 10472–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern HT, Yeaman C, Nelson WJ, Scheller RH (2001) The Sec6/8 complex in mammalian cells: characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells. Proc Natl Acad Sci USA 17: 9648–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Murray D (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438: 605–611 [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D (2002) PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 31: 151–175 [DOI] [PubMed] [Google Scholar]
- Munson M, Novick P (2006) The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol 13: 577–581 [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V, Prehoda C, Snapper S, Taunton J, Lim WA (2005) A polybasic motif allows N-WASP to act as a sensor of PIP2 density. Mol Cell 17: 181–191 [DOI] [PubMed] [Google Scholar]
- Pfeffer SR (1999) Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol 1: E17–E22 [DOI] [PubMed] [Google Scholar]
- Robinson NG, Guo L, Imai J, Toh EA, Matsui Y, Tamanoi F (1999) Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol Cell Biol 19: 3580–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumanie O, Wu H, Molk JN, Rossi G, Bloom K, Brennwald P (2005) Rho GTPase regulation of exocytosis in yeast is independent of GTP hydrolysis and polarization of the exocyst complex. J Cell Biol 170: 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge SA, Anderson DM, Emr SD (2004) Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14–Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol Biol Cell 15: 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Audhya A, Emr SD (2002) The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol Biol Cell 13: 542–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D, Novick P (1995) Sec6, Sec8, and Sec15 are components of a multisubunit complex localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol 130: 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Hughson FM (2000) Membrane tethering and fusion in the secretory and endocytic pathways. Traffic 1: 588–597 [DOI] [PubMed] [Google Scholar]
- Whyte JR, Munro S (2002) Vesicle tethering complexes in membrane traffic. J Cell Sci 115 (Part 13): 2627–2637 [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P (2003) Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell 14: 4770–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA (2004) Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell 13: 677–688 [DOI] [PubMed] [Google Scholar]
- Zajac A, Sun X, Zhang J, Guo W (2005) Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol Biol Cell 16: 1500–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zajac A, Zhang J, Wang P, Li M, Murray J, Terbush D, Guo W (2005a) The critical role of Exo84p in the organization and polarized localization of the exocyst complex. J Biol Chem 280: 20356–20364 [DOI] [PubMed] [Google Scholar]
- Zhang X, Bi E, Novick P, Du L, Kozminski KG, Lipschultz J, Guo W (2001) Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem 276: 46745–46750 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang P, Gangar A, Zhang J, Brennwald P, Terbush D, Guo W (2005b) Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J Cell Biol 170: 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Materials